Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Approximately 20%–30% of pediatric cancer patients with fever and neutropenia have a clinically or microbiologically proven site of infection. Table 98.1 summarizes documented infections identified in three large clinical trials of empirical antibiotic therapy administered to patients admitted to the National Cancer Institute for fever during episodes of neutropenia.
| Site or Type of Infection | Pizzo et al. No. (%) | Pizzo et al. No. (%) | Freifeld et al. No. (%) | Total No. (%) |
|---|---|---|---|---|
| Bloodstream | 81 (43) | 109 (27) | 20 (17) | 210 (29) |
| Pulmonary | 28 (15) | 88 (21) | 16 (13) | 132 (18) |
| Cutaneous | 42 (22) | 43 (10) | 19 (16) | 104 (14) |
| Head, eyes, ears, nose, throat | 11 (6) | 69 (17) | 28 a (23) | 108 (15) |
| Gastrointestinal | 4 (2) | 35 (9) | 30 (25) | 69 (10) |
| Urinary tract | 22 (11) | 29 (7) | NR | 51 (7) |
| Other | 2 (1) | 38 (9) | 7 (6) | 47 (7) |
Several series evaluating the use of central venous catheters in patients with cancer have confirmed that these devices increase the incidence of bacteremia, regardless of the level of bone marrow suppression. , Any bacteria can be responsible for catheter-associated bacteremia, with prevalence of gram-positive vs gram-negative bacteria varying by institution. Fungi, most often Candida spp., can also cause catheter-related septicemia and are difficult to eradicate without removal of the contaminated device. , Polymicrobial bacteremia should prompt an investigation to identify a causative event (e.g., swimming with the catheter unprotected, dropping of catheter tubing into bathwater, chewing on catheter tubing by young children, disconnection of catheter caps or tubing) so that appropriate education can be provided. In addition, in hematopoietic cell transplantation (HCT) patients with severe neutropenia, certain bloodstream infections may not originate from the central venous catheter, but instead result from translocation of bacteria across compromised gastrointestinal mucosa leading to a mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBSI). , Improved central venous catheter maintenance bundles are effective at reducing central-line associated bloodstream infections, but not in preventing MBI-LCBSI.
In evaluating children for presumed catheter-associated bacteremia, cultures should be obtained from all lumens of a multi-lumen device to improve diagnostic yield, as cultures may only be positive from one lumen in 30%–40% of cases.
Because of the increased risk of catheter-related bacteremia, empiric antibiotics may also be warranted in non-neutropenic patients with central venous catheters who have fever with no localizing findings. The choice of agent for empirical antibiotic therapy in children with cancer should take into consideration organisms most likely to cause catheter-related infections and should provide broad-spectrum activity until an organism is identified. Severity of illness (e.g., associated neutropenia, clinical sepsis) and the patient’s history of infection or colonization with multidrug resistant bacteria and antimicrobial prophylaxis regimens will also influence choice of antimicrobial therapy. Vancomycin may not be necessary empirically unless there is evidence of significant catheter-related soft tissue infection, clinical instability, or a gram-positive resistant organism. Therapy should be tailored to target the identified organism, based on the results of antimicrobial susceptibilities. The emergence of multidrug resistant bacteria and fungi, such as Candida auris , are a growing concern given lack of therapeutic options, high rates of morbidity, and poor outcomes in immunocompromised children. Advances in blood culture diagnostics have incorporated molecular methods allowing for faster identification of pathogens and resistance genes to inform earlier changes to antimicrobial therapy than traditional phenotypic methods. , In addition, genomic profiling and next generation sequencing are beginning to be evaluated. In a small group of 47 children with relapsed and refractory cancers, application of microbial cell-free DNA sequencing surveillance on residual blood samples was able to detect a bloodstream pathogen in 75% of samples approximately 3 days before culture-proven infection was confirmed. Further studies are needed to evaluate the clinical utility of these diagnostics in immunocompromised children.
Bacteremia can occur in conjunction with a catheter-related soft-tissue infection or without localizing findings. These infections may be confined to the skin immediately surrounding the exit site of a catheter or can involve deeper soft tissue around the subcutaneously tunneled portion of the catheter or access port. Any localizing findings in a neutropenic child should be considered evidence of infection, mandating hospitalization and administration of broad-spectrum antibiotics. Induration, erythema, tenderness, or fluctuance along the subcutaneous tunnel tract of the catheter generally requires removal of the catheter (in addition to antibiotic therapy) and debridement of infected tissue. In the setting of significant catheter-related soft-tissue infection, inclusion of an empirical antistaphylococcal agent is appropriate.
Determinations regarding catheter management (salvage or removal) will depend on the pathogen, the type of catheter, the patient’s clinical manifestations, and the presence of complications. Most episodes of catheter-associated septicemia resulting from bacterial pathogens can be treated adequately with the catheter in place, even when multiple organisms are identified. , Although current guidelines recommend the addition of antibiotic lock therapy for catheter salvage, data regarding the additional benefit of antibiotic or ethanol lock therapy in pediatric oncology patients with catheter-associated bacteremia are inconclusive and warrants prospective study. , Removal of the catheter is warranted if there is erythema or exudate at the catheter site (concerning for a catheter pocket or tunnel infection), bacteremia with certain pathogens, persistence of bacteremia despite 72 hours of appropriate antimicrobial therapy, and presence of concomitant endocarditis, suppurative thrombophlebitis, or evidence of metastatic infection. , Specific organisms that require removal of the catheter include Staphylococcus aureus , , a number of Bacillus spp., , mycobacteria, Pseudomonas aeruginosa and multidrug resistant gram-negative bacteria (e.g. Acinetobacter spp.), and fungi, most commonly Candida spp. , , ,
Cutaneous infections are common in immunocompromised patients, accounting for 22%–33% of infections in one older series and 16% of infections occur at the time of hospitalization for fever and neutropenia in another study. Infections of the skin can be caused by viruses, fungi, or bacteria.
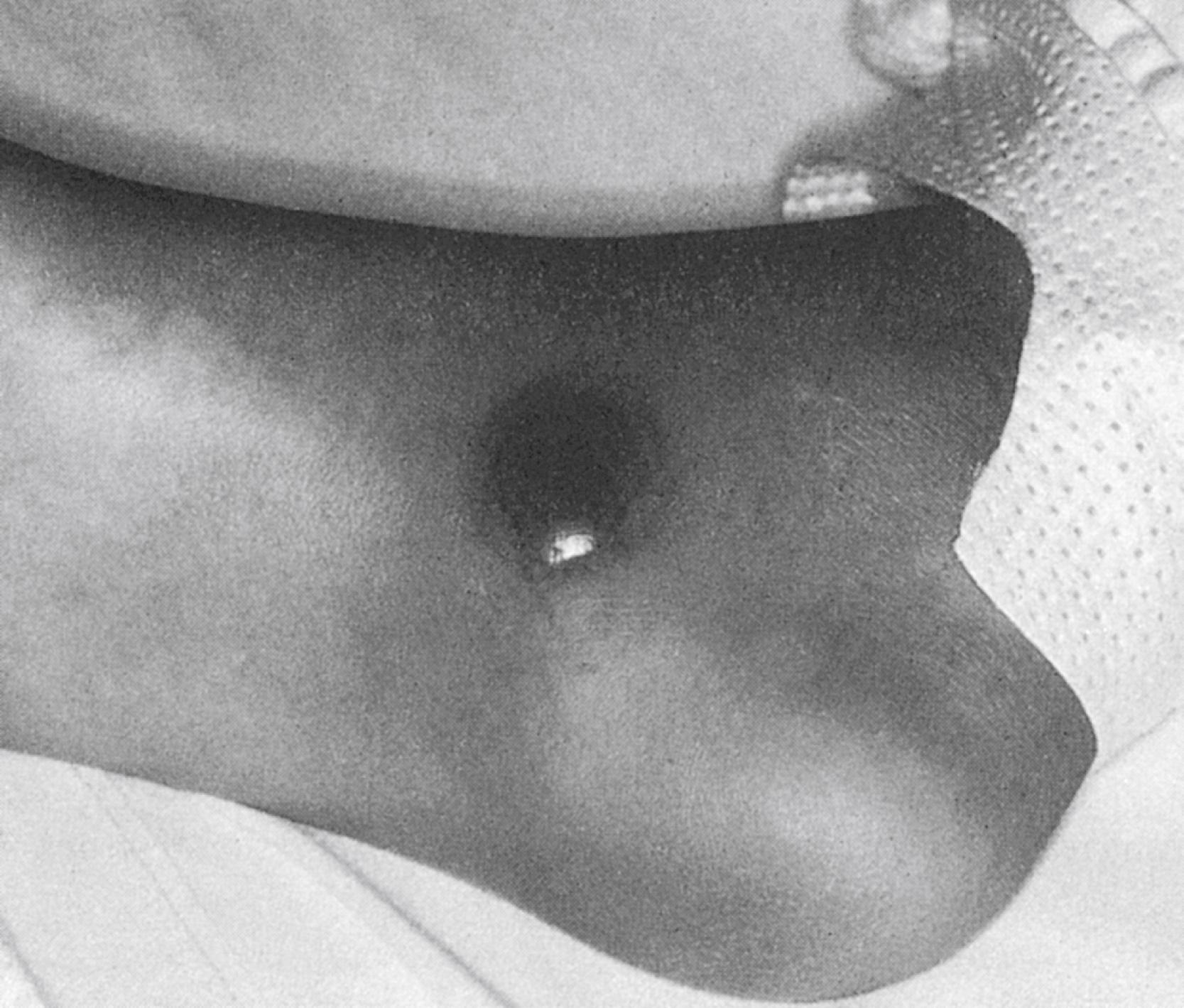
Bacterial skin infections usually are caused by staphylococci or streptococci. In immunocompromised patients, both gram-positive and gram-negative bacteria, including enteric organisms and Pseudomonas, can be isolated either from the blood or from material obtained from fine-needle aspiration of the area of cellulitis. Pseudomonas aeruginosa infection has classically been associated with the severe necrotic skin lesions of ecthyma gangrenosum ( Fig. 98.1 ), though these lesions can also be seen with invasive infections due to other bacteria (gram-negative organisms and staphylococci), as well as by mycobacteria and fungi. Treatment for presumed bacterial cellulitis should provide broad-spectrum coverage. Every effort should be made to establish a microbiologic diagnosis; new skin lesions should undergo biopsy and culture.
Fungal pathogens including Candida spp., Aspergillus , Fusarium , and Mucor can cause cutaneous lesions either as isolated, primary infections or as manifestations of disseminated disease. Blood and fungal cultures should be obtained as concomitant fungemia may be seen with disseminated infections caused by Candida spp, Fusarium spp, Aspergillus spp, and Scedosporium spp. Tender, erythematous skin nodules or pustules can develop during candidemia. Black, rapidly progressing, necrotic eschars should prompt immediate evaluation for fungal infection. Superficial cultures of suspicious skin lesions may not provide adequate material for diagnosis of fungal infections; biopsy generally is more useful. The presence of a single, necrotic lesion at a site of prior occlusion is highly indicative of a mold infection in immunocompromised children. Cutaneous fungal infections resulting from Aspergillus or other molds may require surgical debridement in addition to prolonged courses of antifungal therapy (e.g., amphotericin, voriconazole) because they are often a manifestation of hematogenous spread or local angioinvasive infection.
Cutaneous infections secondary to viruses may also occur in immunocompromised patients, either from primary infection or reactivation. Use of antiviral prophylaxis with acyclovir has demonstrated benefit in preventing infection in seropositive herpes simplex virus (HSV) and varicella zoster virus (VZV) allogeneic HCT recipients and in patients receiving induction therapy for acute leukemia. , HSV and VZV can cause painful or pruritic vesicular lesions that can become secondarily infected. Diagnosis is made based on clinical findings of typical skin lesions and can be confirmed by unroofing a fresh vesicle, scraping the base of the lesion, and sending the samples for HSV and VZV polymerase chain reaction (PCR). Differentiation of the two viruses is important because, although both respond to acyclovir therapy, VZV requires higher doses of acyclovir in children (500 mg/m 2 given intravenously every 8 hours) than HSV, which can be treated effectively with a lower oral dose.
Patients with cancer who develop primary varicella, especially those receiving chemotherapy, are at increased risk of serious disseminated disease, including giant cell pneumonia, encephalitis, hepatitis, and purpura fulminans. Dissemination and subsequent mortality (estimated as 7%–20% in untreated patients ) have been reduced by rapid initiation of therapy with intravenous acyclovir. Although recurrent disease in the form of zoster is rarely associated with severe complications when it remains localized to the skin, dissemination occurs in up to 25% of patients with immunocompromising conditions. Severe abdominal pain, back pain, or evidence of inappropriate antidiuretic hormone secretion may herald multisystem involvement, indicating the need for prompt use of acyclovir.
A severe variant of scabies ( Sarcoptes scabiei ), called Norwegian or crusted scabies , occurs in immunodeficient patients, especially those with leukemia or lymphoma, and is characterized by the presence of 10 3 –10 6 viable mites, resulting in widespread, hyperkeratotic, crusted lesions. This form is highly contagious and may be recalcitrant to standard topical scabicidal therapy (lindane, permethrin, crotamiton, malathion, and benzyl benzoate). Ivermectin, an oral antiparasitic agent, has been shown to be highly effective in curing both routine scabies and Norwegian scabies after a single administration, although additional treatment is often employed. ,
The lungs are the most common site of localized infection in patients with neutropenia, and pulmonary infection in this population produces a wide variety of symptoms, signs, and radiographic appearances. Table 98.2 summarizes causative agents based on specific radiographic findings.
| Manifestation | Infectious Cause | Noninfectious Process |
|---|---|---|
| Focal consolidation (lobar or segmental) | Bacteria (routine and nosocomial pathogens) Legionella Oral flora (aspiration and post-obstructive) Mycobacterium tuberculosis Cryptococcus , Histoplasma, Coccidioides |
Pulmonary hemorrhage Pulmonary infarction Atelectasis Radiation pneumonitis Drug-related pneumonitis Tumor |
| Diffuse interstitial infiltrate | Viruses Pneumocystis jirovecii Mycobacteria, including miliary tuberculosis Disseminated fungi ( Cryptococcus, Histoplasma, Coccidioides) Mycoplasma Chlamydophila |
Pulmonary edema Adult respiratory distress syndrome Drug-related pneumonitis Radiation pneumonitis Lymphangitic metastasis Lymphocytic interstitial pneumonitis (HIV) |
| Nodular infiltrate (with or without cavitation) | Molds: Aspergillus, Mucor, Fusarium Bacteria (especially Staphylococcus aureus , Pseudomonas , Klebsiella , anaerobic bacteria), Nocardia Mycobacteria, including M. tuberculosis Viruses (e.g., CMV, HSV, VZV, EBV, RSV) |
Tumor |
Acute localized infiltrates at the onset of fever in a patient with cancer, with or without neutropenia, are most often due to bacterial pathogens. Because both community-acquired and nosocomial pathogens can be responsible for pneumonia in neutropenic patients, initial antibiotic therapy should provide coverage for organisms such as Streptococcus pneumoniae and Haemophilus influenzae, as well as Pseudomonas spp. and other gram-negative bacteria. Legionella also should be considered in febrile, immunocompromised patients with patchy infiltrates; antigen test on urine is sensitive and specific for L. pneumophila, serogroup 1 . Legionella organisms have been identified in air conditioning equipment and showerheads and can be spread by aerosolization of contaminated water. Patients with pulmonary metastases also may be at increased risk for post obstructive pneumonia with a variety of pathogens, including anaerobic bacteria. The antibiotic regimens used for empiric therapy of fever without localizing findings in a patient with neutropenia (e.g., fourth-generation cephalosporin, extended-spectrum penicillin or carbapenem) generally are appropriate for initial management of pneumonia. In some cases, the addition of a macrolide (i.e., erythromycin, clarithromycin or azithromycin) for possible Mycoplasma or Legionella infections is warranted.
Viruses, such as influenza and parainfluenza viruses, respiratory syncytial virus, and adenovirus, also can cause localized infiltrates in immunocompromised children, although a diffuse process is more common. Respiratory viruses affecting the lower respiratory tract can lead to hospitalizations for fever and neutropenia and may be associated with significant morbidity and mortality in children with underlying malignancy. , In children who had respiratory symptoms and were candidates for transplantation, detection of a respiratory virus pre-HCT was associated with increased overall mortality; thus, temporary delay of HCT may need to be considered in symptomatic children, if feasible. Consideration of a viral process does not obviate the need for broad-spectrum antibiotic therapy in a patient with neutropenia, fever, and a pulmonary infiltrate. Detection of influenza would necessitate antiviral therapy in this population.
Localized infiltrates in patients who fail to respond to broad-spectrum antibiotics have a broad differential; as such, guidelines recommend sampling of lung fluid by bronchoalveolar lavage (BAL) in patients with fever and neutropenia with an infiltrate of uncertain etiology. Timely bronchoscopy and BAL allows for an accurate infectious diagnosis, provides guidance for targeted antimicrobial therapies, and has led to improved patient outcomes. Infection resulting from fungi, Nocardia , mycobacteria (including Mycobacteria tuberculosis ), or antibiotic-resistant, hospital-acquired bacteria can present as localized infiltrates. Endemic fungi, Histoplasma , Cryptococcus , and Coccidioides also can cause localized pneumonitis in endemic geographic regions and, in compromised hosts, can be associated with extra pulmonary infection as well. The presence of Candida in respiratory tract secretions, even in patients with pulmonary infiltrates, correlates poorly with causation because of the high frequency of colonization of the oral cavity and tracheobronchial tree. Blood cultures positive for Candida or typical retinal lesions of endophthalmitis are associated with disseminated infection, which can include pneumonia. In the absence of these findings, definitive diagnosis usually requires histopathologic confirmation.
The fungal pathogens of most concern in the patient with neutropenia include Aspergillus , Fusarium , Mucor , Scedosporium , and Trichosporon because these organisms cause rapidly progressive, extensively destructive infection. The epidemiology of invasive mold infections in children varies by geographic location. Unlikely to be identified at the onset of neutropenia and fever, the finding of a progressive or new infiltrate, accompanied by fever, nonproductive cough or hemoptysis, and pleuritic chest pain, in a persistently neutropenic patient during broad-spectrum antibiotic therapy suggests the diagnosis of invasive fungal pneumonia (most frequently with Aspergillus ). CT may reveal multiple pulmonary nodules, sometimes with cavitation, that are not readily apparent on routine chest radiographs. The classic histopathologic finding in these infections is invasion of the blood vessels with thrombosis and resulting infarction and hemorrhage. Whereas recovery of Aspergillus from the respiratory tract of patients without neutropenia can represent colonization, isolation of the organism in the setting of a patient with prolonged neutropenia and pulmonary infiltrate is highly predictive of invasive disease and is considered sufficient evidence for initiation of antifungal therapy. Neither BAL, which has a recovery rate of about 50% in biopsy-proven Aspergillus pneumonia, nor transbronchial biopsy, which has a yield of about 20%, is highly sensitive for the diagnosis; open lung biopsy may be required. , Testing by a nonculture-based, enzyme-linked immunosorbent assay (ELISA) for Aspergillus galactomannan of blood and BAL specimens has facilitated the diagnosis of invasive pulmonary aspergillosis in patients with hematologic diseases. Overall sensitivity and specificity for proven invasive aspergillosis in adults is 71% (95% CI of 68%–74%) and 89% (95% CI 88%–90%), respectively. In children, the negative predictive value of the Aspergillus galactomannan for screening was 85%–100% and 70%–100% for diagnosis of invasive pulmonary aspergillosis. A positive galactomannan assay coupled with suggestive CT findings supports the diagnosis of probable invasive aspergillosis in neutropenic cancer and HCT transplant patients ; a positive culture remains the gold standard. The performance characteristics of fungal PCR and other nonculture-based fungal assays such as β-D-glucan vary by the pediatric population studied, tissue tested, and clinical indication for testing; as such, further study is needed to optimize their application as adjuncts to diagnosis. Next generation sequencing for cell-free fungal DNA in plasma has been evaluated as a diagnostic adjunct for invasive infection in at-risk children with high clinical suspicion when tissue-based testing cannot safely be performed, but requires further study.
Historically, the treatment of choice for Aspergillus pneumonia had been amphotericin B. Voriconazole is the drug of choice for primary treatment of invasive aspergillosis based on findings of an international, randomized, open-label trial demonstrating that voriconazole conferred a significant patient survival benefit and overall therapeutic response compared with amphotericin. Liposomal amphotericin may be considered as alternative or salvage therapy. Echinocandins (caspofungin and micafungin) should not be utilized as monotherapy for treatment of invasive aspergillosis but may be considered for use in cases of refractory aspergillosis or in patients intolerant of amphotericin and triazoles. Combination therapy with voriconazole and caspofungin for invasive aspergillosis has been reported to be successful in isolated cases. This may prove to be a good choice, given the reported few adverse effects of the combination and theoretical lack of antagonism with other antifungal agents. Results of a recent randomized, double-blind, placebo-controlled trial has demonstrated improved survival rates in adults with hematologic malignancies and after HCT with suspected or documented invasive aspergillosis who received combination therapy with voriconazole and an echinocandin compared with those who received voriconazole monotherapy. Other fungal pathogens, such as H. capsulatum , C. immitis , and C. neoformans , can also cause focal infiltrates in patients with neutropenia who are receiving corticosteroid therapy or with epidemiologic exposures. Amphotericin remains the therapy of choice for severe disease due to these fungi.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here