Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Platelet components are either prepared from whole blood donations (platelet concentrates) or are collected by apheresis (single donor platelets). In the United States, whole blood–derived platelet concentrates are produced using the platelet-rich plasma (PRP) method. First, a whole blood unit is separated by gentle centrifugation (slow spin) into red blood cells (RBCs) and PRP. The PRP is then centrifuged a second time (hard spin) to isolate one platelet concentrate plus one unit of plasma. Each platelet concentrate contains approximately 5.5 × 10 10 platelets suspended in a plasma volume of approximately 50 mL. In Europe and Canada, the alternative buffy coat method is used to produce platelet concentrates. To prepare an adult dose of platelets for transfusion, four to six platelet concentrates are pooled together.
Apheresis platelet units are collected from a single platelet donor by continuous flow centrifugation using an automated device. A high volume of whole blood is processed through the machine, and the platelets are retained in a sterile collection bag. According to AABB (formerly, the American Association of Blood Banks) standards, an apheresis platelet unit should contain a minimum of 3 × 10 11 platelets, a dose that is approximately equivalent to five pooled platelet concentrates. Current devices allow many different combinations of blood products to be collected during a single apheresis donation, such as 1 unit of platelets plus 1 unit of RBCs. Apheresis platelet units generally contain less than 1 × 10 6 white blood cells (WBCs) per unit; thus they meet the current AABB definition for leukoreduced blood products (<5 × 10 6 WBCs/unit). Although apheresis platelets cost more to produce than whole blood–derived platelet concentrates, they have become increasingly popular. In the United States in 2015, approximately 2 million therapeutic doses of platelets were provided as apheresis platelets; in contrast, only approximately 200,000 equivalent doses of whole blood–derived platelet concentrates were distributed.
Apheresis platelets provide limited advantages over whole blood–derived platelet concentrates. Radiolabeling studies indicate that apheresis platelets circulate longer in vivo than pooled concentrates, most likely reflecting gentler handling and less platelet activation during collection. Recipients of apheresis platelets are exposed to fewer donors per transfusion (one donor versus four to six as with a pool of platelet concentrates), so in principle, apheresis platelets should pose a lower risk of viral transmission than whole blood–derived platelets. However, given that the per-unit transfusion-transmission risks for human immunodeficiency virus (HIV) and hepatitis C virus have been reduced to less than 1 per 1,000,000, the viral safety advantage of apheresis platelets over whole blood–derived platelets is marginal. Data from surveillance culture studies suggest that apheresis platelets may be less likely than platelet concentrates to become contaminated with bacteria. At one time, it was predicted that apheresis platelets would be less likely than pooled concentrates to provoke platelet alloimmunization by virtue of exposing recipients to fewer unique donor human leukocyte antigens (HLAs). However, this hypothesis was not confirmed empirically. Although they express surface HLA class I antigens, platelets appear to be rather poor immunogens. HLA alloimmunization to platelets primarily appears to be triggered by contaminating WBCs within a platelet unit. Thus alloimmunization is not platelet dose dependent, and simply providing leukoreduced platelet units prevents most cases of immune-mediated platelet refractoriness. When platelet refractoriness does occur, it is often in multiparous women, who were initially sensitized to foreign (paternal) HLA antigens in previous pregnancies.
Most platelet units are transfused to prevent bleeding in nonbleeding patients, rather than to treat active bleeding. Before 1960, platelet transfusions were not widely available, and death from hemorrhage was common among patients with leukemia who received chemotherapy. In 1962, Gaydos and colleagues published a seminal study demonstrating an inverse relationship between days with bleeding and platelet count. After this study was published, prophylactic platelet transfusion rapidly became standard practice. Notably, based on their data, no specific platelet transfusion trigger was suggested by the authors. Regardless, a platelet count of 20,000/μL was widely adopted at the time as the standard prophylactic platelet transfusion trigger.
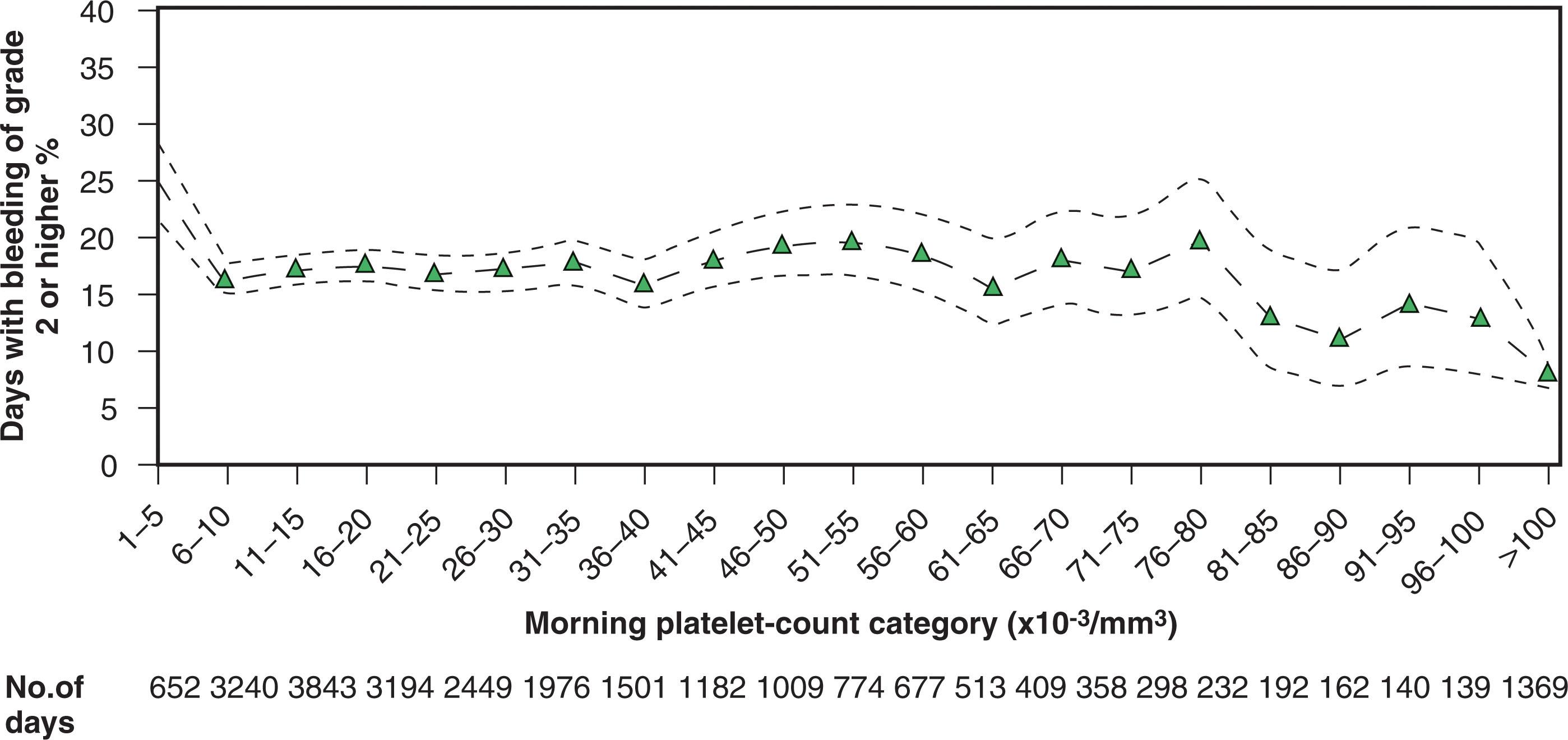
Later studies suggested that a lower transfusion trigger would be as effective as a trigger of 20,000/L. For instance, Slichter and Harker performed RBC radiolabeling studies in thrombocytopenic patients who were not receiving platelet transfusions. They demonstrated that only patients with platelet counts less than 5000/L had significantly elevated fecal RBC loss. Years later, several clinical studies directly challenged the 20,000/L trigger. Platelet transfusion triggers of 10,000/L versus 20,000/L were compared directly in three randomized prospective studies of patients with acute leukemia. These studies, as well as a nonrandomized prospective trial, did not show an increased incidence of bleeding when a trigger of 10,000/L is used. More recently, the Platelet Dosing (PLADO) trial demonstrated that the risk of bleeding among patients with hypoproliferative thrombocytopenia increased only at platelet counts less than 6000/μL. As shown in Fig. 113.1 , the risk of spontaneous bleeding was equivalent between platelet counts of 6000/μL and 80,000/μL.
Severe hemorrhage in the setting of therapy-related hypoproliferative thrombocytopenia is now quite rare. Given the substantial changes in both chemotherapy and the supportive care of patients with hematologic malignancy that have occurred over the past several decades, two randomized controlled trials examined whether routine platelet prophylaxis still provides a clinical benefit. In the study by Wandt and colleagues, patients receiving chemotherapy or undergoing autologous hematopoietic stem cell transplantation (HSCT) were randomly assigned to receive platelet transfusions only when bleeding occurred, or standard prophylactic platelet transfusions for a morning platelet count at or less than 10,000/μL. Grade 2 or higher bleeding occurred in 42% of patients receiving therapeutic platelet transfusions only, versus 19% in patients receiving platelet prophylaxis ( P < .001). There were significantly more intracerebral hemorrhages in the no-prophylaxis group (7% vs. 2%, P = .01), and there were two deaths due to bleeding in the no-prophylaxis group compared with zero in the prophylaxis group. In the Trial of Prophylactic Platelets, a similar population of patients was randomized to no prophylactic platelet transfusions or routine prophylaxis. Grade 2 or higher bleeding occurred in 50% of patients in the no-prophylaxis group versus 43% in the prophylaxis group ( P = .06 for noninferiority). In this study, there were no deaths due to bleeding. In both trials, bleeding occurred more frequently among patients being treated for acute leukemia versus autologous stem cell transplant recipients. Overall, the results of these trials support the continued use of prophylactic platelet transfusions for patients with hematologic malignancy and therapy-induced hypoproliferative thrombocytopenia. However, using a no-prophylaxis strategy specifically for adult autologous stem cell transplant recipients has been endorsed by the American Society of Clinical Oncology (ASCO).
Prophylactic platelet transfusions are mainly given in the setting of oncology, but they are also used to prevent bleeding in thrombocytopenic neonates. Notably, a multicenter randomized controlled trial of 660 preterm infants found that using a high platelet count threshold for administering prophylactic platelet transfusions (50,000/μL) was associated with significantly higher mortality or major bleeding events than using a low threshold (25,000/μL).
There are currently thought to be two distinct clearance mechanisms for platelets. Most platelets undergo senescence after circulating in the peripheral blood for 8 to 10 days. But there is also evidence for a second clearance route in which there is a fixed daily loss of platelets that occurs independent of platelet age. Platelets exiting the circulation via this second route are postulated to function in maintaining vascular integrity. In principle, a low dose of platelets could be used to meet this daily requirement. This hypothesis was tested by the 2010 PLADO trial. A total of 1272 patients receiving HSC transplantation or chemotherapy were randomly assigned to receive low-, medium-, or high-dose platelets for a morning count of 10,000/μL or lower. The risk of spontaneous bleeding was not increased until patient platelet counts decreased to 5000/μL or lower. No differences were observed in bleeding rates among the three treatment groups, supporting the concept that few platelets are required to maintain hemostasis. Although significantly fewer total platelets were transfused to patients in the low-dose group, platelet transfusions were required more frequently.
To date, there have been no large randomized controlled trials evaluating the need for platelet prophylaxis before invasive bedside procedures such as lumbar puncture. However, retrospective data provide reassurance that moderate thrombocytopenia does not pose a serious risk for performing such procedures. Howard et al. reviewed the records of 956 consecutive pediatric patients with newly diagnosed acute lymphoblastic leukemia who underwent lumbar puncture. No serious hemorrhagic complications were observed after 5223 lumbar punctures, including 170 procedures that were done when the platelet count was 11,000 to 20,000/μL. The authors concluded that prophylactic platelet transfusion was unnecessary in patients with platelet counts greater than 10,000/μL. Lumbar punctures were performed in only 29 patients with platelet counts of 10,000/μL or less, making it difficult to assess the risk of bleeding in patients with very low platelet counts. A similar, albeit much smaller, retrospective study examined the same issue in adult patients with acute leukemia. No hemorrhagic complications were observed after 195 lumbar punctures, including 35 that were done with platelet counts of 20,000 to 30,000/μL. With very limited published data available on severely thrombocytopenic adults, AABB has suggested 50,000/μL as a minimum safe platelet count for lumbar puncture in adult patients. For central venous catheter placements, AABB has suggested 20,000/μL as a minimum safe platelet count, based on multiple observational studies. Relatively few studies have been published to date addressing the question of a safety minimum platelet count for other types of bedside procedures (thoracentesis, paracentesis, etc.)
There are currently no data from randomized trials addressing the question of what constitutes an adequate platelet count before surgery. However, retrospective studies suggest that patients with platelet counts of 50,000/μL or higher are not at excess bleeding risk during surgery. Bishop and colleagues reported a series of 95 patients with acute leukemia who underwent 130 surgical procedures with platelet counts of less than 50,000/μL. Intraoperative blood loss exceeded 500 mL in only 7% of cases. No relationship was seen between the preoperative platelet count and surgical blood loss. These data suggest that prophylactic platelet transfusions need not be administered before surgery when the preoperative platelet count is at least 50,000/μL. This rule of thumb is thought to apply to most types of surgery (cardiac, orthopedic, and so on). However, for a few types of surgeries, requiring a higher platelet count (70,000 to 100,000/μL) is traditional, although no published data currently exist either to support or refute this approach. These settings include neurosurgery, retinal surgeries, and other procedures in which the risk is not that the patient may exsanguinate but rather that even a minor bleed might cause clinically significant damage in a vulnerable vital structure such as the brain.
High-quality data to guide platelet use in the setting of active bleeding are lacking. A small randomized controlled trial in patients on antiplatelet medication who suffered intracerebral hemorrhage found worse outcomes among patients transfused with platelets compared with patients receiving standard care only.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here