Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Bone lesions lend themselves to radiographic assessment because of the fact that bone has a built-in contrast agent, the calcium hydroxyapatite crystal. Much information about bone destruction, bone production, matrix calcification and ossification, and the reactive response of the surrounding bone and periosteum is available from plain radiographs. Although other imaging techniques play a role in the diagnostic process, the plain film is still the method of choice and should never be bypassed. Imaging is an important tool for diagnosis, staging, therapeutic response evaluation, and oncologic surveillance of bone tumors and can be used to augment histopathologic findings. Imaging modalities such as radiography, computed tomography (CT), and magnetic resonance imaging (MRI) have major roles in the initial characterization and identification of bone lesions through the assessment of characteristics such as margin, bone expansion, periosteal reaction, soft tissue extension, and matrix mineralization. Additional imaging modalities such as bone scan, single-photon emission computed tomography with fused CT (SPECT/CT), 18 F-fluorodeoxyglucose positron emission tomography with fused CT (FDG PET/CT), and whole-body MRI can be used to stage patients for distant metastases. These modalities, in addition to ultrasonography, can also be used for restaging and oncologic surveillance. In this chapter, we discuss the general imaging and diagnostic features of bone tumors, assessment of therapeutic responses, and staging of both primary and metastatic bone tumors. Specific imaging features of individual bone tumors and radiographic-pathologic correlations are discussed in their respective chapters.
In general, at least 95% of bone tumors can be diagnosed with precision when the clinician, radiologist, and pathologist work in concert and share their information. The clinical history, age of the patient, location of the lesion, and radiographic appearance provide a foundation for the analytic approach to the diagnosis of bone tumors. It is important to state that the identification of a bone abnormality per se does not definitively indicate that a biopsy or indeed any surgical intervention is required. Easily recognizable fibrous cortical defects, small osteochondromas, phalangeal enchondromas, and common traumatic and avulsion lesions can be treated by observation alone. Often these are incidental findings observed on radiographs taken for other reasons. Lesions that cannot be confidently assigned to a benign radiologic category or that have aggressive or malignant appearances require biopsy.
The analytic approach begins with an appreciation that tumors of bone have different predilections for certain age groups, anatomic locations, and even specific sites within a bone. Some lesions are painless, and some present with specific pain patterns. Thus a small, painful lesion in the neck of the femur in an adolescent is far more likely to be an osteoid osteoma than a chondroblastoma. A large lytic lesion in the end of a long bone of an adult is likely to be a giant cell tumor and not a nonossifying fibroma. Computerized radiographic techniques, such as CT and MRI, facilitate analysis of bone lesions in greater detail and are indispensable techniques in planning therapy. They also provide reliable clues for the pathologist. The clinical, radiographic, and pathologic recognition patterns of the most common bone tumors and tumorlike lesions are summarized in Table 2-1 .
| Type of Tumor | Peak Age Incidence (years) | Most Frequent Sites | Radiographic Features | Microscopic Features |
|---|---|---|---|---|
| Malignant osteosarcoma | 10-20 | Metaphyseal parts of long bones (knee, proximal humerus) | Destructive lytic or blastic lesion with cloudlike opacities | Highly atypical sarcomatoid cells exhibiting tumor bone formation |
| Chondrosarcoma | >50 | Pelvis, proximal femur | Destructive lesion with punctate calcifications, cortical scalloping, or disruption | Exclusively cartilaginous tumor with atypical cartilage cells |
| Ewing's sarcoma | 10-20 | Shafts of long bones | Permeative, lytic pattern of bone destruction | Small cell tumor |
| Malignant fibrous histiocytoma | >50 | Pelvis, long bones, predominantly lower extremity (femur, tibia) | Lytic moth-eaten or permeative bone destruction with cortical disruption | Highly atypical spindle or pleomorphic cell sarcoma (no tumor bone production) |
| Adamantinoma of long bone | >20 | Predominantly tibia with synchronous involvement of fibula | Lytic lesion with predominant involvement of anterolateral cortex | Nests of epithelioid cells in fibrous stroma |
| Borderline giant cell tumor | 20-40 | Ends of long tubular bones (knee regions, distal radius) | Eccentric epiphyseal lytic lesion | Multiple osteoclast-like giant cells with mononuclear stromal cells |
| Epithelioid hemangioendothelioma | >30 | Lower extremity, multifocal | Multifocal, sharply demarcated lytic lesions involving bones of one extremity (predominantly lower extremity) | Vascular neoplasm with epithelioid endothelial cells |
| Benign osteoid osteoma | 10-30 | Long bones of lower extremity (femoral neck) | Well-demarcated intracortical lytic lesion with sclerosis of adjacent cortex | Interlacing network of bone trabeculae in vascular stroma |
| Osteoblastoma | 5-45 | Posterior neural arch of vertebral column and craniofacial bones | Similar to osteoid osteoma (radius >1.5 cm), less sclerosis | Same as osteoid osteoma |
| Enchondroma | Wide-range | Small bones of hands and feet, long tubular bones | Radiolucent defect with punctate or stippled calcifications (<2 cm) | Mature cartilage (low cellularity) |
| Chondroblastoma | 10-20 | Epiphyses of long tubular bones in skeletally immature patients | Sharply demarcated epiphyseal defect | Tumor composed of chondroblasts with focal immature cartilage formation (chicken wire calcifications) |
| Chondromyxoid fibroma | 10-30 | Metaphyses of long tubular bones | Eccentric cortical-based lytic defect with scalloped margin | Myxoid tissue with septa containing chondroblasts and giant cells |
| Hemangioma | >40 | Craniofacial bones and vertebral bodies | Lytic defect with prominent striations; honeycomb appearance | Vascular lesion with capillary or cavernous vessels |
| Nonneoplastic fibrous dysplasia | 0-20 | Craniofacial bones, ribs, femur | Central, lytic defect with ground-glass appearance | Woven bone trabeculae in fibrous stoma |
| Nonossifying fibroma | 0-20 | Metaphyses of long tubular bones (femur, tibia) | Eccentric cortex-based lytic defect with sharp scalloped margins | Fibrohistiocytic lesion with giant cells |
| Aneurysmal bone cyst | 10-25 | Metaphyses of long tubular bones | Expansile lytic defect | Cystic lesion with hemorrhage; septa containing histiocytic and giant cells |
| Synovial chondromatosis | >40 | Major joints of lower extremity | Punctate or stippled calcifications in periarticular soft tissue | Island of atypical cartilage in synovial membrane |
| Pigmented villonodular tenosynovitis | >40 | Periarticular synovial tissue and tendons | Ill-defined, multinodular periarticular mass | Histiocytic cells, giant cells, hemosiderin deposits |
| Osteofibrous dysplasia | 0-15 | Tibia, fibula | Multilocular lytic defects of anterolateral tibial cortex associated with sclerosis and tibial bowing; synchronous fibular lesions | Fibroosseous lesion with zonal architecture |
| Giant cell reparative granuloma | Craniofacial bones, acral skeleton | Central lytic defect | Giant cell lesion with fibrohistiocytic cells and reactive bone | |
| Myositis ossificans | >20 | Parosteal or periosteal soft tissue | Zonal maturation, peripheral shell of bone | Histiocytic cells; reactive bone formation maturing toward periphery |
| Florid reactive periostitis | 20-40 | Acral skeleton | Ill-defined bone surface lesion | Metaplastic cartilage; reactive bone and giant cells |
| Avulsion/stress fracture | 20-40 | Attachment of major tendons, long bones, and pelvis | Ill-defined surface bone lesion; presence of fracture line | Reactive process with metaplastic cartilage and reactive bone |
| Pubic osteolysis | Females >50 | Pubic bones | Lytic lesion with florid periosteal reaction | Reparative process with prominent metaplastic cartilage and reactive bone |
Most benign tumors and tumorlike lesions, including osteoid osteoma, chondroblastoma, chondromyxoid fibroma, solitary bone cysts, aneurysmal bone cysts, and nonossifying fibromas, occur in adolescents and young adults (second and third decades of life). Giant cell tumor is a lesion that almost exclusively occurs in skeletally mature patients (i.e., those with growth plates fused) between ages 20 and 50 years. Of the malignant bone tumors, osteosarcoma and Ewing's sarcoma are tumors of children and young adults. On the other hand, chondrosarcoma, malignant fibrous histiocytoma, plasma cell myeloma, metastatic tumors to the skeleton, and secondary malignancies in Paget's disease of bone, after radiation injury, and adjacent to bone infarcts usually develop during the sixth through the eighth decades of life.
In this text the age distribution of various tumors and tumorlike lesions is discussed under the separate diagnostic headings, and the peak incidence for most is presented graphically. Although exceptional cases of individual bone tumors occur at unusual ages (e.g., chondrosarcomas in children or Ewing's sarcoma in middle-aged patients), it is best to be circumspect with regard to these statistical guidelines.
The fact that tumors of bone have predilections for certain bones and even for characteristic locations in an individual bone provides an additional clue about the nature of the lesion. Figure 2-1 shows the sites of predilection of the more common benign bone tumors. In some instances, the location alone strongly suggests the diagnosis, as in a heavily ossified tumor on the posterior surface of the distal femur. That will most likely prove to be a parosteal osteosarcoma. Adamantinoma and osteofibrous dysplasia almost exclusively involve the tibia or fibula. Chondroblastoma is most often seen in the epiphyses of long bones in children or in tarsal or carpal (epiphysioid) bones. Periosteal chondroma has a strong predilection for the surface of the proximal humeral metaphysis. Solitary bone cysts in childhood are almost always found in the proximal humeral shaft or upper end of the femur. Nonossifying fibromas in childhood are lesions that favor the metaphyses of long bones of the lower extremities.
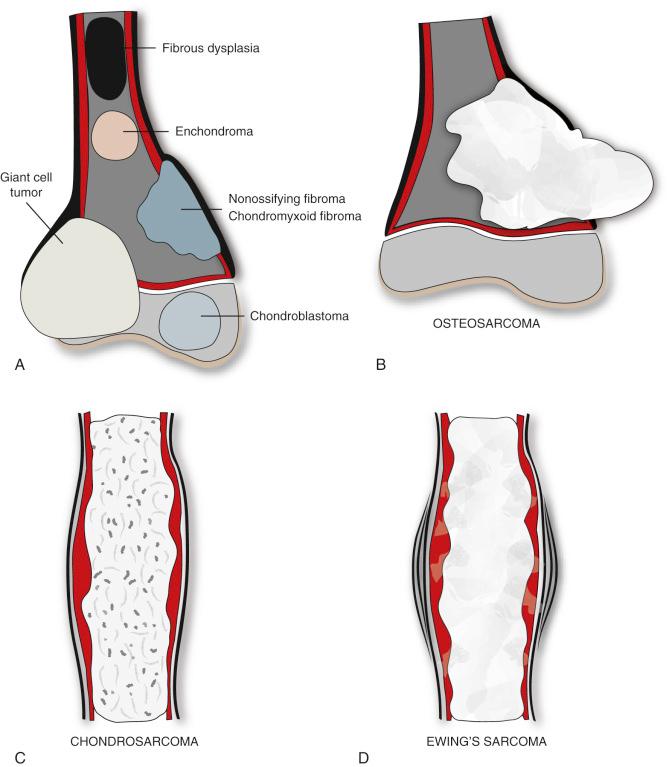
Similar generalizations can be made for bone sarcomas. Chondrosarcomas have a predilection for the femur, pelvis, ribs, and sternum and are almost never found in the spine or short tubular bones. Osteosarcomas arise most often in the distal femoral shaft or proximal tibial shaft before closure of the growth plates, and they seldom penetrate the cartilaginous physis to involve the epiphysis. Ewing's sarcoma tends to have a diaphyseal point of origin, although it may also involve the metaphysis. Ewing's sarcoma seldom affects the epiphyseal ends of bones. Figure 2-1 shows the growth patterns of the most frequent primary malignant bone tumors, osteosarcoma, chondrosarcoma, and Ewing's sarcoma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here