Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The act of fertilization releases the ovulated egg from a depressed metabolism and prevents its ultimate disintegration within the female reproductive tract. Immediately after fertilization, the zygote undergoes a pronounced shift in metabolism and begins several days of cleavage. During this time, the embryo, still encased in its zona pellucida, is transported down the uterine tube and into the uterus. Approximately 6 days later, the embryo sheds its zona pellucida and attaches to the uterine lining.
With intrauterine development and a placental connection between the embryo and mother, higher mammals, including humans, have evolved greatly differing modes of early development from those found in most invertebrates and lower vertebrates. The eggs of lower animals, which are typically laid outside the body, must contain all the materials required for the embryo to attain the stage of independent feeding. Two main strategies have evolved. One is to complete early development as rapidly as possible, a strategy that has been adopted by Drosophila, sea urchins, and many amphibians. This strategy involves storing a moderate amount of yolk in the oocyte and preproducing much of the molecular machinery necessary for the embryo to move rapidly through cleavage to the start of gastrulation. The oocytes of such species typically produce and store huge amounts of ribosomes, messenger RNA (mRNA), and transfer RNA. These represent maternal gene products, and this means that early development in these species is controlled predominantly by the maternal genome. The other strategy of independent development, adopted by birds and reptiles, consists of producing a very large egg containing enough yolk that early development can proceed at a slower pace. This strategy eliminates the need for the oocyte to synthesize and store large amounts of RNAs and ribosomes before fertilization.
Mammalian embryogenesis employs some fundamentally different strategies from those used by the lower vertebrates. Because the placental connection to the mother obviates the need for the developing oocyte to store large amounts of yolk, the eggs of mammals are very small. Mammalian cleavage is a prolonged process that typically coincides with the time required to transport the early embryo from its site of fertilization in the uterine tube to the place of implantation in the uterus. A prominent innovation in early mammalian embryogenesis is the formation of the trophoblast , the specialized tissue that forms the trophic interface between the embryo and the mother, during the cleavage period. This innovation, which allows the embryo to tap into an external nutrient source supplied by the mother, is an evolutionary adaptation that compensates for the lack of yolk in the mammalian egg. The placenta represents the ultimate manifestation of the trophoblastic tissues.
Compared with most other species, mammalian cleavage is a leisurely process measured in days rather than hours. Development proceeds at the rate of roughly one cleavage division per day for the first 2 days ( Figures 4.1 and 4.2 ). After the two-cell stage, mammalian cleavage is asynchronous, with one of the two cells ( blastomeres ) dividing to form a three-cell embryo. When the embryo consists of approximately 16 cells, it is called a morula (derived from the Latin word meaning “mulberry”).
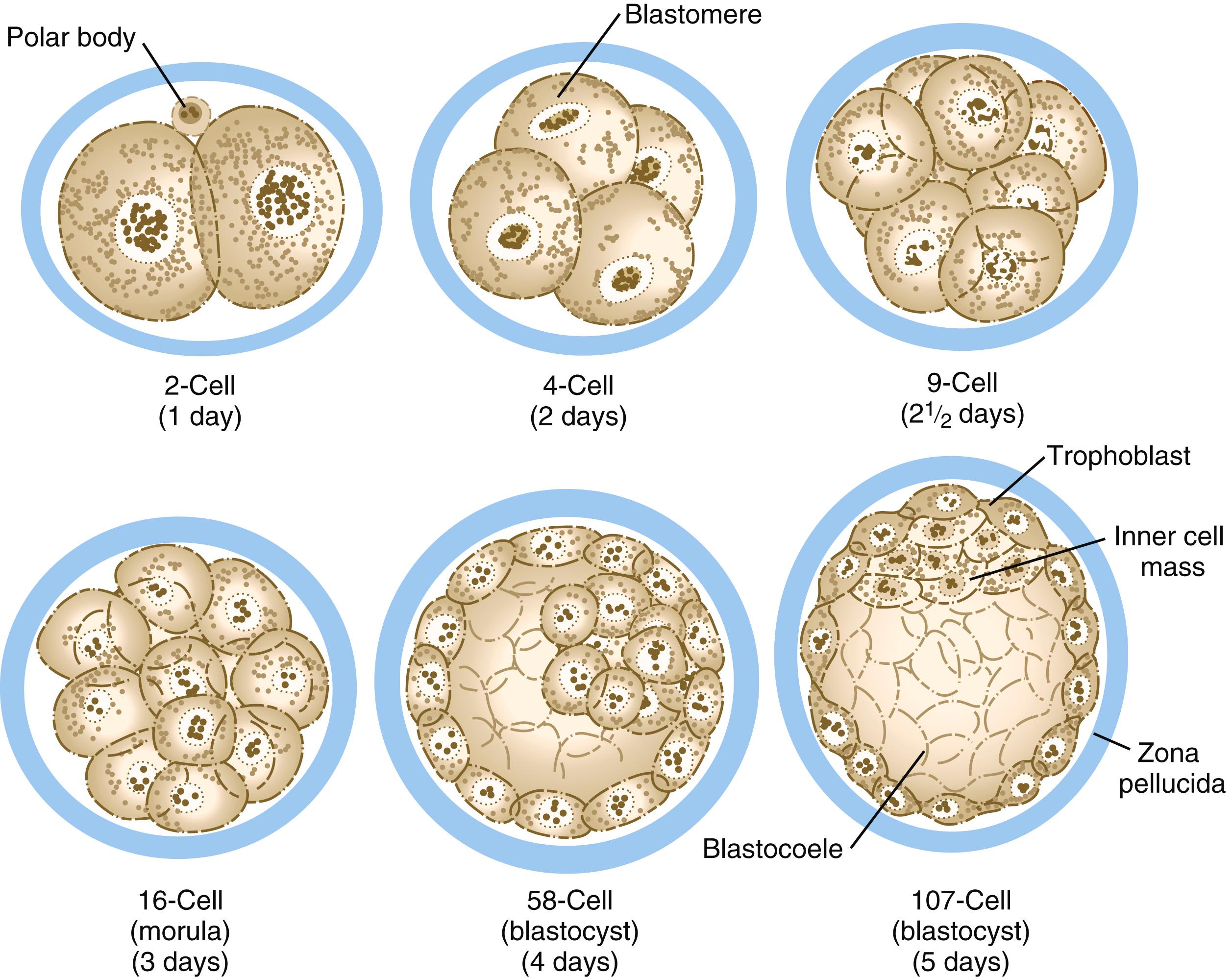
Between the 8- and 16-cell stages, the embryos of placental mammals enter into a phase called compaction , during which the individual outer blastomeres tightly adhere through gap and tight junctions and lose their individual identity when viewed from the surface. Compaction is mediated by the concentration of calcium (Ca ++ )-activated cell adhesion molecules, such as E-cadherin , in a ring around the apical surface of the blastomeres. Another factor leading to compaction is the contraction of a cortical meshwork of actomyosin, which reduces the bulging outer surface areas of the blastomeres. Through the activity of a sodium (Na + ), potassium (K + )–adenosine triphosphatase (ATPase)–based Na + transport system, Na + and water (H 2 O) move across the epithelium-like outer blastomeres and accumulate in spaces among the inner blastomeres. This process, which occurs approximately 4 days after fertilization, is called cavitation , and the fluid-filled space is known as the blastocoele (blastocyst cavity) . At this stage, the embryo as a whole is known as a blastocyst ( Figure 4.3 ).
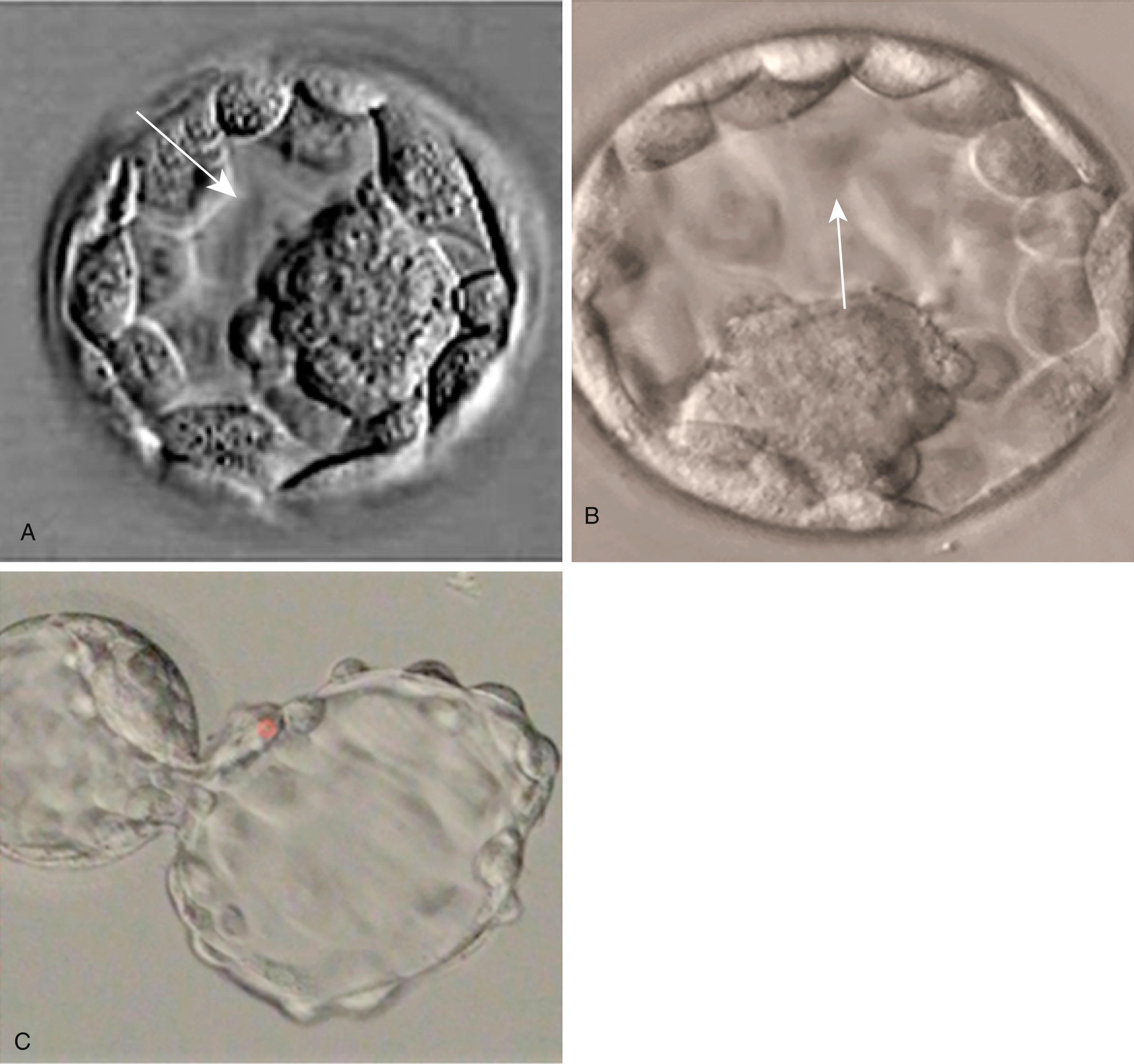
At the blastocyst stage, the embryo, which is still surrounded by the zona pellucida, consists of two types of cells: an outer epithelial layer (the trophoblast ) that surrounds a small inner group of cells called the inner cell mass (see Figure 4.1 ). Each blastomere at the two-cell and the four-cell stage contributes cells to both the inner cell mass and the trophoblast. The end of the blastocyst that contains the inner cell mass is known as the embryonic pole , and the opposite end is called the abembryonic pole . The appearance of the two cell types reflects major organizational changes that have occurred within the embryo and represents the specialization of the blastomeres into two distinct cell lineages. Cells of the inner cell mass give rise to the body of the embryo itself in addition to several extraembryonic structures, whereas cells of the trophoblast form only extraembryonic structures, including the outer layers of the placenta.
Along with the increase in cell numbers, mammalian cleavage is a period dominated by several critical developmental events. The earliest is the transition from maternally to zygotically produced gene products. A second is compaction , during which the blastomeres develop intercellular connections and begin to take on characteristics of epithelial cells. Third is the polarization of individual blastomeres, which sets the stage for the developmental decision that results in the subdivision of the cleaving embryo into two distinct types of cells: the trophoblast and the inner cell mass (see Figure 4.1 and p. 63).
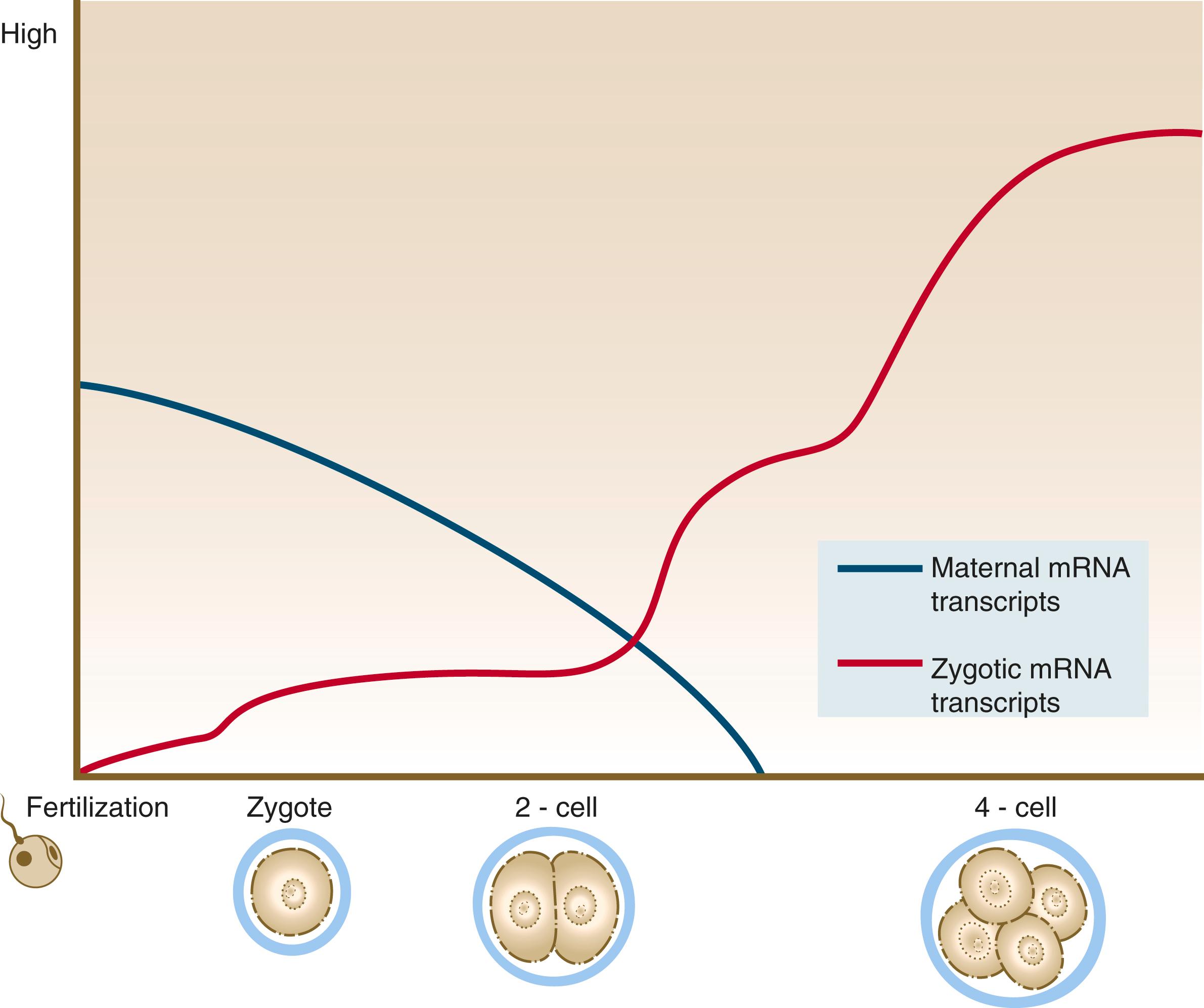
Because of the lack of massive storage of maternal ribosomes and RNAs during oogenesis, development of the mammalian embryo must rely on the activation of zygotic gene products at a very early stage. Most maternal transcription products are degraded by the two-cell stage ( Figure 4.4 ). Some of these, however, stimulate the activation of the embryonic genome, which begins producing RNAs from a significant number of genes (>1500) by the time cleavage has advanced to the four-cell stage. There does not seem to be a sharp transition from the cessation of reliance on purely maternal gene products and the initiation of transcription from the embryonic genome. Some paternal gene products appear in the embryo very early, while maternal actin and histone mRNAs are still being used for the production of corresponding proteins. As an indication of how much the early embryo relies on its own gene products, development past the two-cell stage does not occur in the mouse if mRNA transcription is inhibited. In contrast, similar treatment of amphibian embryos does not disrupt development until late cleavage, at which time the embryos begin to synthesize the mRNAs required to control morphogenetic movements and gastrulation.
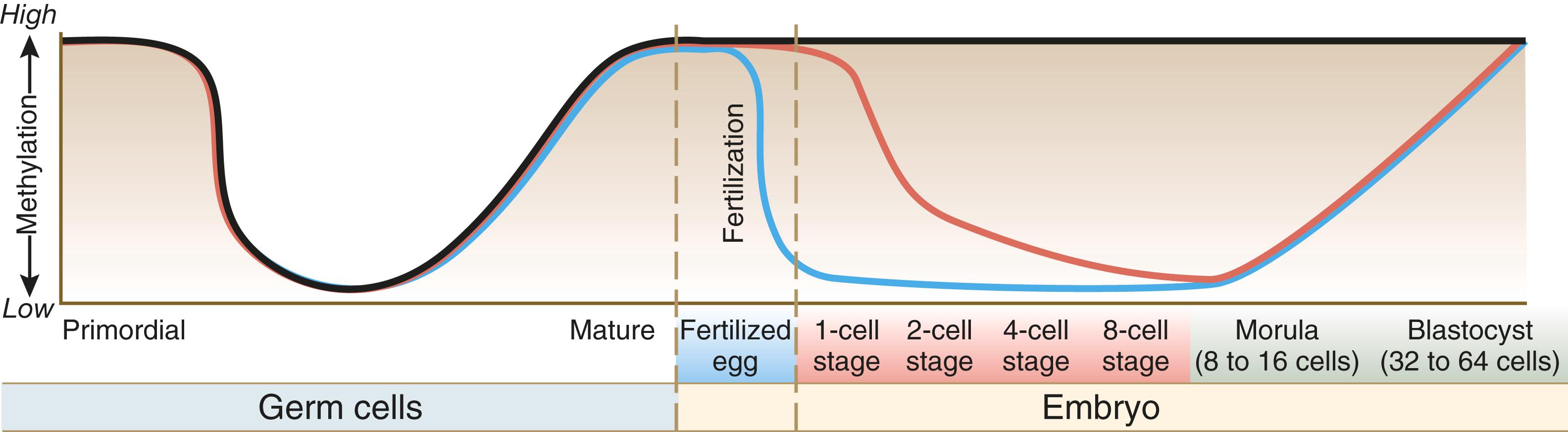
Mature eggs and sperms are transcriptionally inactive. A major reason for this is that their DNA is highly methylated. Methylation , which occurs on CpG dinucleotides, normally inactivates the associated gene. Such inactivation is often called epigenetic regulation because it does not alter the fundamental DNA sequence. Methylation can inactivate informational genes or their regulators (e.g., enhancers or promoters). Pronounced cycles of global methylation and demethylation occur during the life span of an individual ( Figure 4.5 ). Within 4 hours after fertilization, the paternally derived genome undergoes rapid, massive demethylation. Demethylation of the maternally derived genome occurs more gradually until the early morula, at which stage all the DNA is maximally demethylated. Remethylation ensues in the inner cell mass, until by the late blastocyst stage it returns to maximal levels. Within the germ cell line, the high methylation levels characteristic of the early embryo fall after the primordial germ cells have entered the genital ridge. During later gametogenesis, remethylation occurs. This remethylation imprints (see p. 58) maternal or paternal characteristics on the gametes and for some genes has profound effects on the embryos produced from these gametes. Epigenetic control is not confined to methylation patterns. Even as early as the zygote, different patterns of histone association with the chromatin account for pronounced differences in gene expression between the male and female pronuclei.
For the first couple of days after fertilization, transcriptional activity in the cleaving embryo is very low. Similarly, fertilized eggs and early mammalian embryos possess a limited capacity for the translation of mRNAs. The factor limiting translational efficiency may be the small number of ribosomes stored in the egg. As cleavage proceeds, products from maternally and paternally derived chromosomes are active in guiding development. Haploid embryos commonly die during cleavage or just after implantation. There is increasing evidence, however, that the control of early development involves more than simply having a diploid set of chromosomes in each cell.
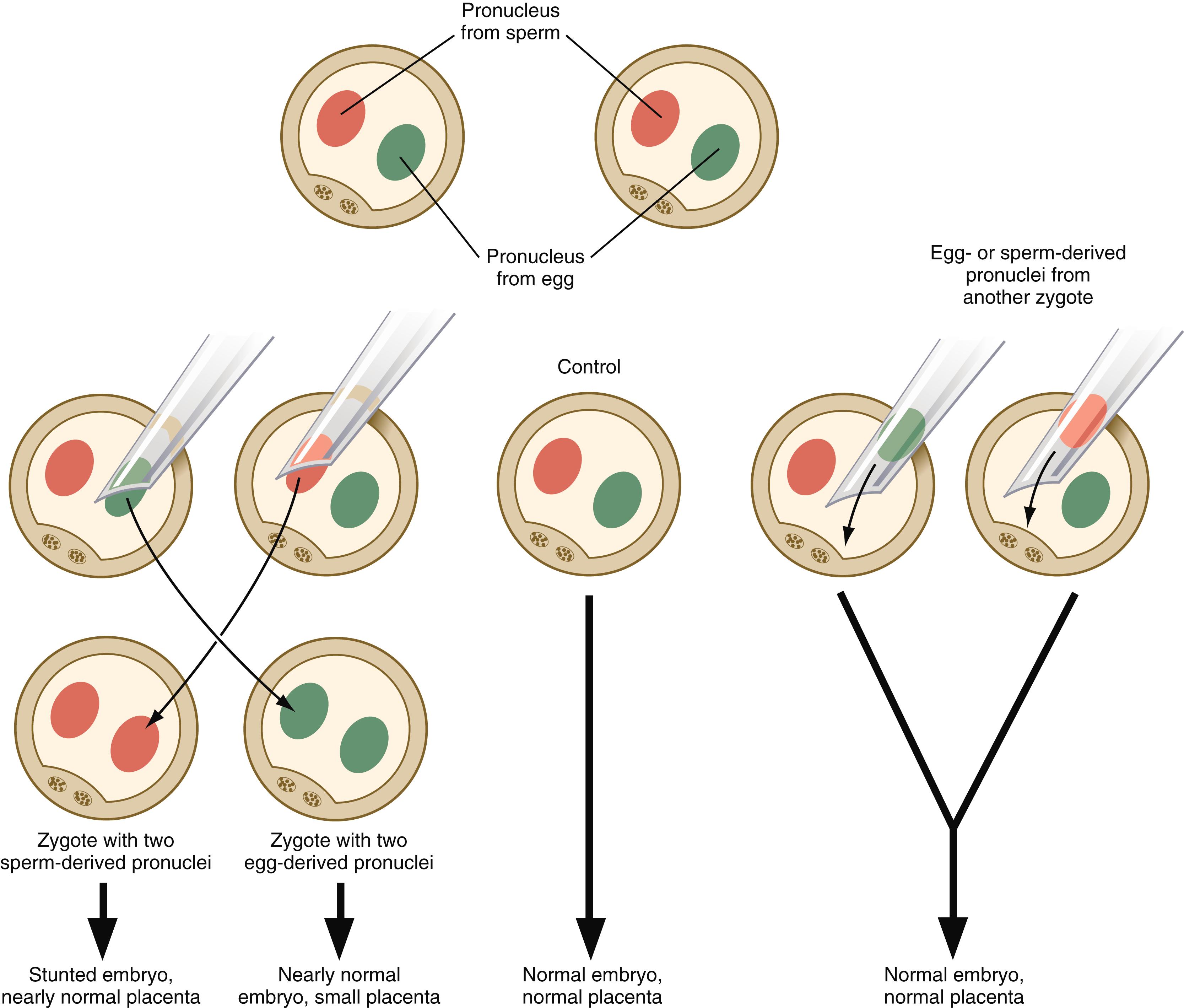
Experimentation, coupled with observations on some unusual developmental disturbances in mice and humans, has shown that the expression of certain genes derived from the egg differs from the expression of the same genes derived from the spermatozoon. Called parental (genomic) imprinting , the effects manifest in different ways. It is possible to remove a pronucleus from a newly inseminated mouse egg and replace it with a pronucleus taken from another inseminated egg at a similar stage of development ( Figure 4.6 ). If a male or female pronucleus is removed and replaced with a corresponding male or female pronucleus, development is normal. If a male pronucleus is removed and replaced with a female pronucleus (resulting in a zygote with two female pronuclei); however, the embryo itself develops fairly normally, but the placenta and yolk sac are poorly developed. Conversely, a zygote with two male pronuclei produces a severely stunted embryo, whereas the placenta and yolk sac are nearly normal.
Parental imprinting occurs during gametogenesis. Methylation of DNA, effected through specific imprinting control regions , is one of the major means of imprinting and results in the differential expression of paternal and maternal alleles of the imprinted genes. Imprinted genes, which are transcriptionally silenced, are not demethylated during the global demethylation event that follows fertilization. The imprinted genes are maintained during development and possibly into adulthood, but a given imprint is not passed on to that individual’s progeny. Instead, the parental imprints on the genes are erased in primordial germ cells, and new imprints, corresponding to the sex of that individual, are established in the oocytes and sperm during gametogenesis.
Not all genes are parentally imprinted. Present estimates suggest that as many as 100 human genes are imprinted. Clinical Correlation 4.1 discusses some conditions and syndromes associated with disturbances in parental imprinting.
A striking example of paternal imprinting in humans is a hydatidiform mole (see Figure 7.18 ), which is characterized by the overdevelopment of trophoblastic tissues and the extreme underdevelopment of the embryo. This condition can result from the fertilization of an egg by two spermatozoa and the consequent failure of the maternal genome of the egg to participate in development or from the duplication of a sperm pronucleus in an “empty” egg. This form of highly abnormal development is consistent with the hypothesis that paternal imprinting favors the development of the trophoblast at the expense of the embryo.
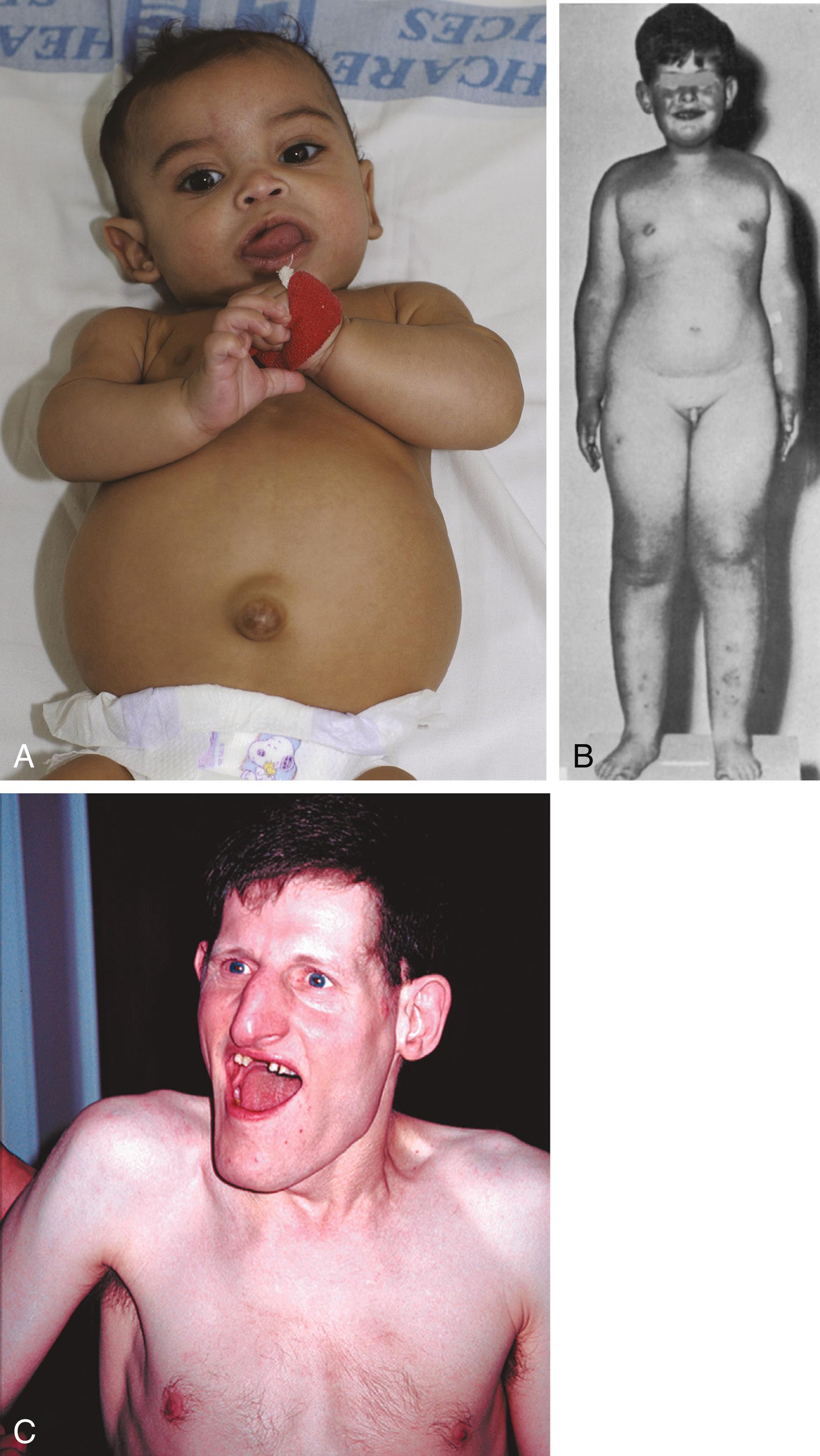
Several other syndromes are also based on parental imprinting. Beckwith-Wiedemann syndrome ( Figure 4.7A ), characterized by fetal overgrowth and an increased incidence of childhood cancers, has been mapped to the imprinted region on chromosome 11, which contains the genes for insulin-like growth factor-II (IGF-II, which promotes cell proliferation) and H19 (a growth suppressor). It occurs when both alleles of the IGF2 gene express a paternal imprinting pattern. Another instructive example involves deletion of regions in the long arm of chromosome 15, specifically involving the gene UBE3A . Children of either sex who inherit the maternal deletion develop Angelman’s syndrome ( Figure 4.7C ), which includes severe mental retardation, seizures, and ataxia. A child who inherits a paternal deletion of the same region develops Prader-Willi syndrome ( Figure 4.7B ), characterized by obesity, short stature, hypogonadism, a bowed upper lip, and mild mental retardation.
Another example of the inequality of genetic expression during early development is the pattern of X-chromosome inactivation in early female embryos. It is well known from cytogenetic studies that one of the two X chromosomes in the cells of females is inactivated by extreme condensation. This is the basis for the sex chromatin , or Barr body , which can be shown in cells of females, but not in the cells of normal males. The purpose of X-chromosome inactivation is dosage compensation, or preservation of the cells from an excess of X-chromosomal gene products.
X-chromosome inactivation is initiated at the X-inactivation center , a unique locus on the X chromosome. XIST ( X - i nactive s pecific t ranscript), one of the genes in the X-inactivation center, produces a large RNA with no protein coding potential. XIST RNA, partnering with several proteins, remains in the nucleus and coats the entire inactive X chromosome, thus not allowing any further transcription from that chromosome. One of the protein partners of XIST is Polycomb , which modifies the histone proteins that package the DNA into the condensed form known as chromatin . In the inactivated X chromosome, the XIST gene is unmethylated and expressed, whereas in the active X chromosome, this gene is methylated and silent.
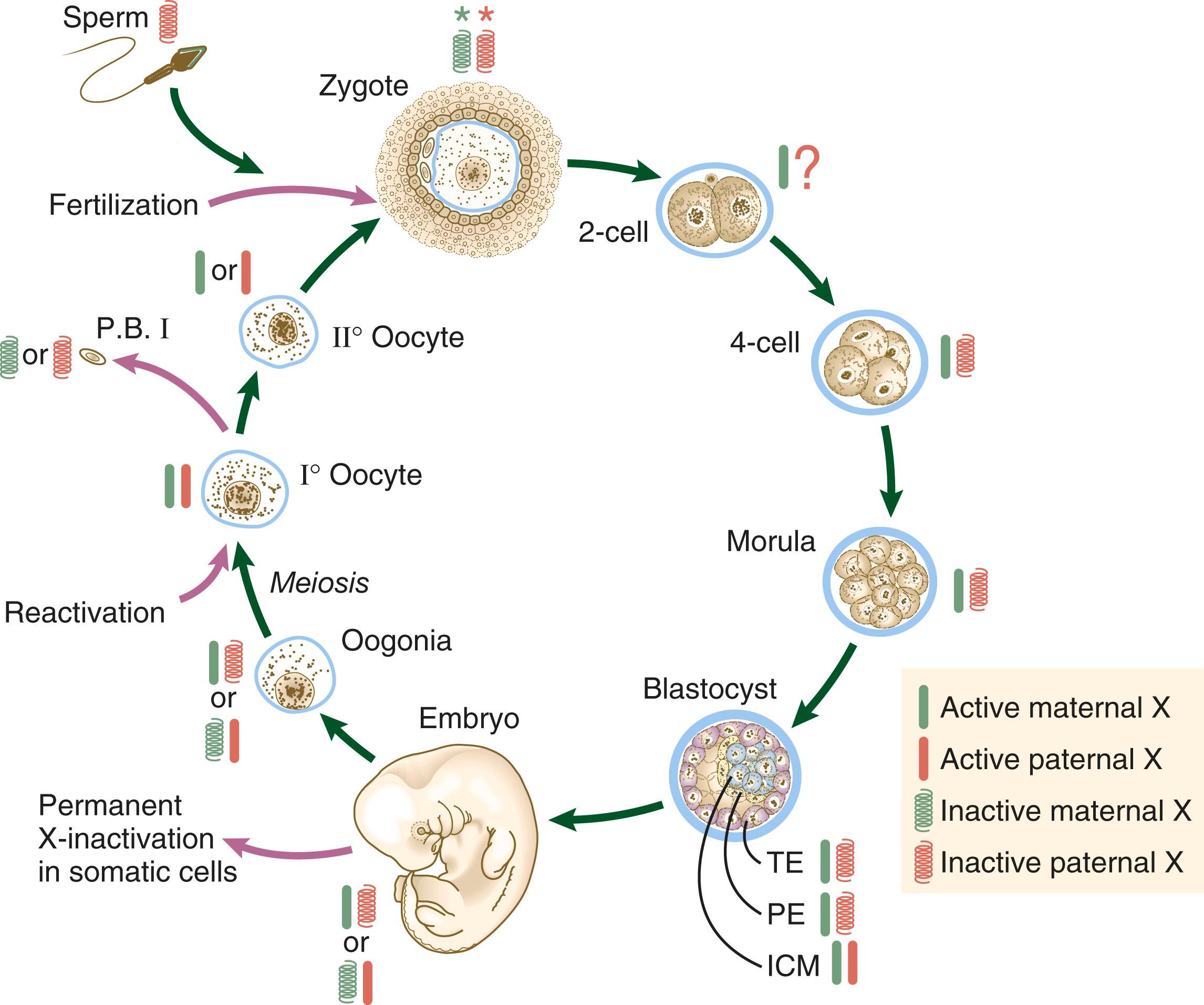
Genetic studies show a complex ontogenetic history of X-chromosome inactivation ( Figure 4.8 ). In the female zygote, both X chromosomes are transcriptionally inactive, although not through the actions of XIST because of the global inactivation of transcription in the early cleaving embryo. By the four-cell stage and into the morula stage, the paternally derived X chromosome is inactivated as the result of parental imprinting. Then, while the embryo forms the blastocyst, the paternally derived X chromosomes in the trophoblast and the hypoblast (see Figure 5.1 ) remain inactivated, but within the cells of the inner cell mass both X chromosomes become active. While the cells of the inner cell mass begin to differentiate, the somatic cells undergo random permanent XIST-based X-chromosome inactivation of either the maternal or the paternal X chromosome. Within the germ cell line, activation of both X chromosomes occurs during the first meiotic division.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here