Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Extensive etiologic heterogeneity in ischemic stroke necessitates categorizing patients into classes with discrete phenotypic, pathophysiologic, therapeutic, and prognostic features.
The primary purpose of etiologic classification is to establish a common language in the field for better scientific communication.
Phenotypic classification provides a summary of abnormal test findings organized in major etiologic categories without weighting toward the most likely cause in the presence of multiple etiologies. Its advantage is that it retains as much patient information as possible. Its shortcoming is that it assigns strokes to a vast number of categories.
Causative classification identifies the most likely cause of stroke through a decision-making process that requires the integration of clinical, laboratory, and imaging information. Its advantage is that it assigns stroke etiologies into a small number of mutually exclusive categories. Its shortcoming is that collapsing individual etiologies into categories may result in loss of information on an individual basis.
Identification of an abnormality in stroke workup does not necessarily mean that that abnormality is the cause. Newer probabilistic classification systems take into account the relative strength of causal connections to determine the most likely etiology.
An etiology that has the potential to cause another stroke in the short term is called an unstable etiology. Infarct characteristics on brain imaging—such as size, age, location, and pattern of infarcts—can help to identify an unstable etiology.
The word etiology is derived from the Greek word aitiología, meaning “giving a reason for.” In stroke, etiology refers to cardiac, vascular, hemodynamic, and hemorheologic abnormalities that can cause a brain infarction. The terms etiology and risk factors are often used interchangeably, but they indicate different things. Risk factors do not directly cause a stroke, but they increase the probability of developing a stroke. In the causal chain, risk factors facilitate the development of etiologies, and etiologies lead to a stroke. For instance, diabetes mellitus is a risk factor but not an etiology for stroke. Diabetes mellitus promotes the development of systemic and cardiovascular abnormalities such as a stenotic plaque in the carotid artery or a microatheroma at the origin of the lenticulostriate arteries, and those abnormalities, in turn, cause a stroke. Likewise, certain cancers can induce a hypercoagulable state, which promotes thrombus formation in the chambers of the heart (cardiac embolism) or the deep venous system (paradoxical embolism), and the resultant thrombus causes a stroke. Although the line between etiology and risk factors is not always easily discernible, the nosologic distinction between the two is critical, as factors that are closer to the disease in the causal chain (i.e., etiologies) generally pose a higher disease risk than factors that are farther down (i.e., risk factors) in the chain. The clinical implication of this is that, once the risk factors have already given rise to etiologies (such as an ulcerated carotid plaque), therapeutic measures solely focusing on the risk factors would be expected to offer only limited benefit in terms of mitigating the disease risk.
The causal framework of stroke is perhaps one of the most complex in medicine. More than 100 pathologic conditions can play a role in the etiopathogenesis of ischemic stroke. Stroke etiologies often interact with each other as well as with the risk factors. Etiologic complexity of this magnitude requires an effective way of sorting patients into classes. Etiologic classification is a necessity to ensure a common language in the field for better scientific communication. The main goal of etiologic classification is to generate homogeneous subtypes with discrete phenotypic, pathophysiologic, therapeutic, and prognostic features. The etiologic subtype information can be used to select patients in clinical trials, determine the phenotype genetic and epidemiologic studies, assess treatment response, code for administrative purposes, and predict prognosis. Additionally, stroke subtypes are predictors of the future risk of recurrent stroke. Hence, in-depth evaluation to identify the etiologic mechanism of stroke is imperative for effective secondary prevention.
Stroke subtypes used to be determined chiefly on clinical grounds, relying heavily on the clinical syndrome, neurologic findings, and coexisting risk factors. For patients who died, autopsy confirmation was often the basis of the classification. With the widespread application of brain imaging, extracranial and intracranial vascular imaging, cardiac ultrasonography, long-term cardiac monitoring, and other diagnostic studies, clinical impressions have been refined and supported by laboratory confirmation of the stroke subtype. Various classification methods have been proposed over the past 60 years. Every classification system was a significant advance in the field at the time they were devised. As new diagnostic technologies became available, older systems expired and were replaced by newer systems.
The origin of major traditional subtypes—such as large-artery atherosclerosis, cardiac embolism, or cryptogenic stroke—is a report from an expert committee appointed by the National Institute for Neurological Disorders and Blindness (NINDB) in 1958. The specific goal of the NINDB project was to define mechanistically similar subsets to be used in an anticoagulant trial. The authors set their scope as “to place in classified form all of the known types of cerebrovascular diseases and to give meaning to such classification by defining all terms so clearly that they can be employed interchangeably by all investigators in various parts of the country,” highlighting the importance of inter-rater reliability, an essential aspect of etiologic classification. The NINDB classification had four major etiologic categories: “thrombosis with atherosclerosis,” “cerebral embolism,” “other causes,” and “cerebral infarction of undetermined origin.” The category of cerebral embolism included a subcategory, “cerebral emboli of undetermined origin,” referring to cases where the diagnosis of cerebral embolism was made at autopsy without evidence of a clear source for embolism. Stroke subtypes in the NINDB classification were primarily determined on clinical grounds. Stroke that began during sleep or within 1 hour of awakening suggested thrombosis with atherosclerosis, whereas a rapid onset of symptoms and a lack of warning symptoms indicated embolism from a cardiac source.
Heavy reliance on the clinical syndrome began to diminish in the 1960s and early 1970s owing to the increased use of computed tomography (CT) of the brain and catheter angiography. Better understanding of clinico-anatomic correlates of deep and brainstem-penetrating artery infarcts allowed for the diagnosis of lacunar infarcts. The Harvard Cooperative Stroke Registry classification devised in 1978 incorporated such advances and sorted stroke etiology into the categories of “large artery thrombosis,” “lacunar infarcts,” and “embolism.” A CT-confirmed infarction in the absence of angiographic occlusion in the clinically relevant artery suggested the diagnosis of embolism. Before the Harvard Cooperative Registry classification, the diagnosis of embolism used to be made at autopsy. The concurrent use of brain and vascular imaging led to more frequent identification of embolic strokes than previously thought based on the clinical criteria and autopsy alone (31% vs. 3%). , It is now well known that embolism is a prevalent mechanism, accounting for approximately two-thirds of strokes. ,
In the 1980s and 1990s, brain imaging, echocardiography, and Doppler ultrasonography for extracranial vessel imaging were more readily available for clinical use. These tools led to the frequent detection of lacunar infarcts, extracranial large-artery atherosclerosis, and sources of cardiac emboli. The Stroke Data Bank classification in 1986 and classification of the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) in 1993 further revised subtype definitions by incorporating findings from diagnostic technologies of the time. The Stroke Data Bank classification separated ischemic strokes into “large artery thrombosis,” “infarct with tandem arterial pathology or arterial embolism,” “embolism attributed to cardiac or transcardiac source,” “lacunar infarction,” “infarct of undetermined cause,” and “infarct with a normal angiogram.” The last category was similar to “cerebral embolism of undetermined origin” in the original NINDB classification and designated cases with imaging confirmed infarct, no obvious stroke etiology, and normal angiogram. The Causative Classification of Stroke System (CCS) devised in 2007 carried on the tradition of assigning embolic stroke of undetermined etiology as a separate category named “cryptogenic embolism.” The term cryptogenic embolism designated stroke of undetermined etiology that was associated with angiographic evidence of abrupt cutoff in an otherwise normal-looking artery or subsequent complete recanalization of a previously occluded artery. ,
Similar to the NINDB classification, TOAST was also devised to be used in an anticoagulant trial. TOAST had several innovative aspects; it stratified cardiac sources of embolism into high and moderate risk categories, set a lower bar (50% stenosis) for diagnosis of large artery stenosis, and further classified the undetermined category into three clinically relevant subsets as unknown, unclassified (multiple competing etiologies), and incomplete evaluation. Furthermore, unlike most prior systems, TOAST categorized every stroke patient into one of the five subtypes, generating mutually exclusive categories. These categories included “large artery atherosclerosis,” “cardiac embolism,” “small artery occlusion,” “stroke of other determined etiology,” and “stroke of undetermined etiology.” Although TOAST was an important step in etiologic classification, as discussed in the following section, it suffered from limited reliability and validity that restricted its utility as an efficient clinical and research tool.
Causation is difficult to infer in the absence of a gold-standard test to demonstrate the true cause of stroke. Earlier systems relied heavily on clinical criteria, but clinical grounds alone—age, risk factors, and so forth—are not sufficient to classify patients by different mechanisms of cerebral infarction. Common risk factors such as hypertension and diabetes mellitus lack specificity for stroke subtypes and are not distinctive enough to infer the cause. Likewise, the presenting clinical syndrome has little value to suggest a stroke subtype. The problem is more evident in the acute setting, where the common cognitive impairment, agitation, and poor cooperation of patients can hinder a thorough assessment of neurologic function. Relying on clinical criteria alone becomes even more problematic when risk factors or clinical syndromes are consistent with more than one etiologic subtype. Sorting such complex cases into one etiologic category based on clinical criteria alone injects uncertainty and introduces disagreement into stroke classification.
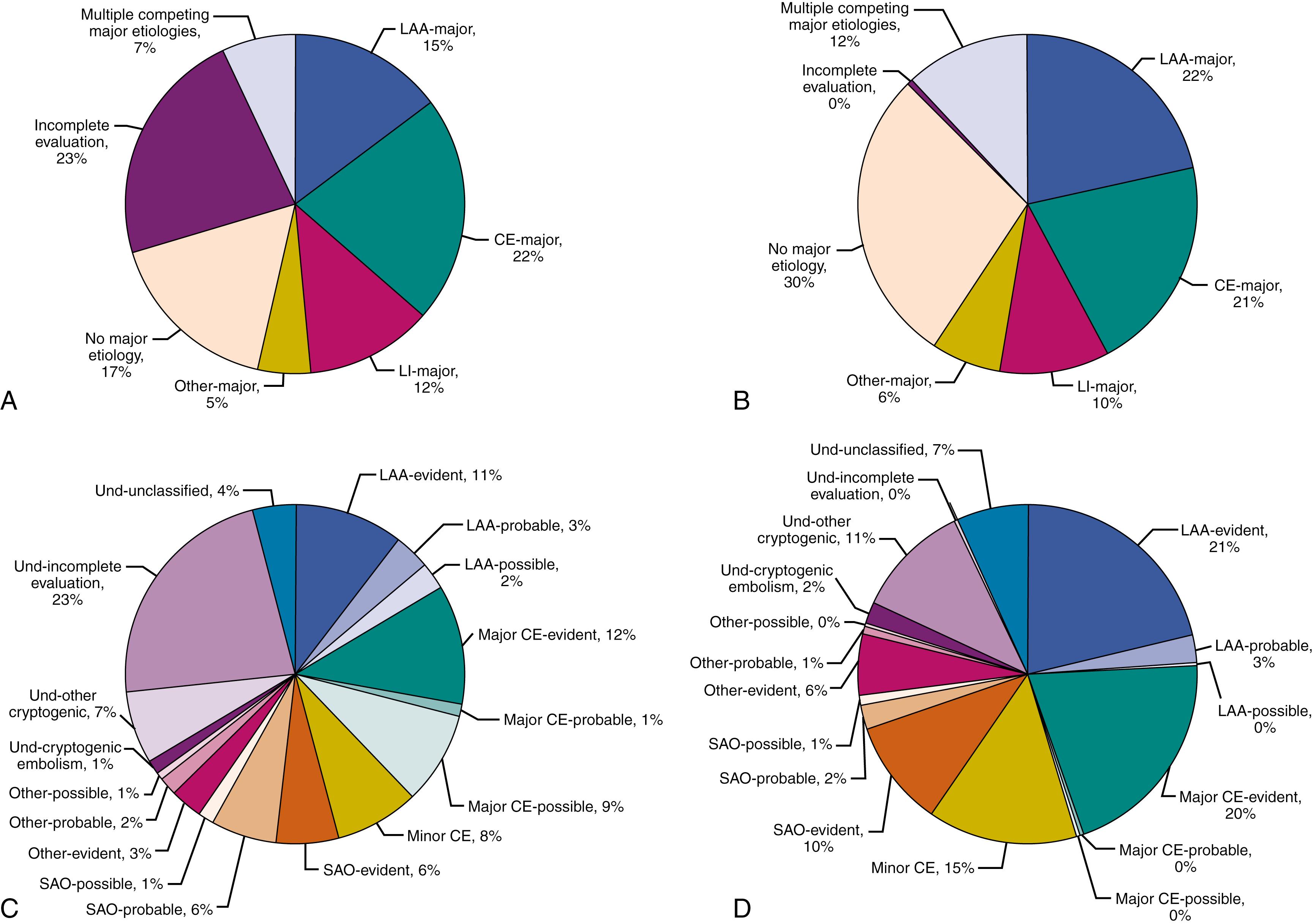
The use of CT, magnetic resonance imaging (MRI), noninvasive vascular imaging, echocardiography, and long-term cardiac monitoring has dramatically improved our ability to diagnose the etiologic subtype but has still left large issues unresolved. Even when strenuous efforts are made to establish the exact mechanism of infarction, current diagnostic technologies are far from precise. Available vascular imaging technologies cannot always differentiate whether a given arterial stenosis or occlusion is due to atherosclerosis. Also, when a significant carotid stenosis is found, judging whether the clinical syndrome arises from an embolic or hemodynamic mechanism is often tricky. Vascular imaging during the acute stage might suggest an abrupt occlusion of a large intracranial artery. Still, the presence of abrupt occlusion does not always settle the problem of the underlying source of embolus. The sensitivity of vascular imaging technologies in clinical use is not sufficient to visualize the pathology in deep, cortical, and brainstem-penetrating arteries that lead to small-artery occlusion (SAO) with high resolution. Identifying early signs of parenchymal damage and brain edema, proven to be useful as a prognostic index, is not as helpful in differentiating etiologic mechanisms. Finally, identification of an abnormality in stroke workup does not necessarily mean that that abnormality is the cause of stroke. In the National Institute of Neurological Disorders and Stroke–Stroke Genetics Network Study (NINDS–SiGN), which included approximately 17,000 patients with ischemic stroke, the cause of stroke could be attributed to large artery atherosclerosis with a high level of confidence in only 54% of patients who had an atherosclerotic lesion causing 50% or greater stenosis. Likewise, only 45% of those with a major cardiac source of embolism, and 40% of those with a typical lacunar infarct on brain imaging could be classified into the same causative category with high confidence ( Fig. 20.1 ).
As already mentioned, neither clinical criteria nor diagnostic evaluation findings alone are sufficient to infer causation. Etiologic subtyping requires a rule-based approach that integrates clinical and diagnostic characteristics of stroke in an algorithmic manner. Such methods can identify etiologic subtypes with certain levels of confidence depending on the strength of causal evidence. Confidence in attributing an etiology to the cause of a stroke can be determined by coexisting stroke features as well as the extent to which diagnostic investigations are completed. , , For instance, the causal mechanism can be attributed to atrial fibrillation with a high level of confidence only when diagnostic examinations for alternative etiologies are performed and fail to reveal a source. Atrial fibrillation, on the other hand, can be considered the cause of stroke with low confidence when stroke workup reveals an alternative etiology or when diagnostic tests for alternative etiologies are not obtained. In a complex patient with atrial fibrillation who presents with stroke features that strongly suggest an underlying vascular problem—such as recurrent unilateral transient retinal or hemispheric ischemic symptoms or unilateral internal border-zone infarcts on MRI—atrial fibrillation cannot be attributed to the cause of stroke with high confidence without ruling out an ipsilateral vascular lesion. Although the probabilistic approach introduced in this section does not require a minimal level of investigation, one’s ability to determine the etiologic mechanism with high confidence increases with a thorough diagnostic evaluation. In clinical settings where diagnostic studies are cursory or prematurely stopped after a positive finding is obtained, the quality of etiologic subtype information can be expected to be suboptimal.
Current diagnostic technologies enable the frequent identification of multiple abnormalities. At least one cardiac source of embolism can be detected in about 50%–70% of patients with stroke by echocardiography. Likewise, nearly one-quarter of patients with a lacunar infarction harbor ipsilateral large artery atherosclerosis causing stenosis of 50% or greater. , Overall, approximately one-half of patients with stroke exhibit multiple etiologies, of which 20% harbor multiple major etiologies, such as intracardiac thrombus and severe atherosclerotic vascular stenosis. Nonprobabilistic classification systems lack the objective criteria to identify the most likely etiology in the presence of multiple competing etiologies. Hence, the strict use of such systems results in assigning multiple competing etiologies into the waste bin of the undetermined category. In practice, however, based on personal opinion, such cases are often assigned into a known subtype rather than the undetermined category. This ultimately reduces the size of the undetermined category, but the resultant decrease occurs at the expense of a loss from interrater reliability because personal opinion varies from one physician to another. Reports from independent investigators consistently demonstrate no better than moderate reliability, with agreement rates that hover around 50% for the conventional etiologic classification systems.
Newer probabilistic classification systems employ several criteria to determine the most likely etiology in the presence of multiple competing etiologies. , These criteria include the relative potential of each etiology to cause a stroke, clinical and imaging stroke features that support one mechanism over others, and the relationship between stroke etiology and the brain infarct in time and space.
Not all stroke etiologies exhibit an equal potential to cause stroke. Stroke etiologies can be ranked according to their potential to cause stroke in the absence of effective treatment. For instance, cardiac abnormalities such as left ventricular hypertrophy and mitral annulus calcification convey a low or uncertain risk of stroke and are therefore considered a minor etiology. In contrast, abnormalities such as atrial fibrillation or dilated cardiomyopathy pose a substantial risk and are considered major. Box 20.1 lists minor and major cardiac sources using a 2% annual or one-time primary stroke risk as the cutoff. Ranking of etiologies using objective criteria allows for identifying the most likely etiology in the presence of multiple competing etiologies. For instance, in a patient with a coexisting minor cardiac source and a major atherosclerotic stenosis (causing ≥50% stenosis), large artery atherosclerosis can be considered the most likely cause of stroke, as the latter poses a higher risk of stroke than the former.
Left atrial thrombus
Left ventricular thrombus
Atrial fibrillation
Paroxysmal atrial fibrillation
Sick sinus syndrome
Sustained atrial flutter
Recent myocardial infarction (within 1 month)
Mitral stenosis or rheumatic valve disease
Bioprosthetic and mechanical heart valves
Chronic myocardial infarction together with low ejection fraction (<28%)
Dilated cardiomyopathy
Nonbacterial thrombotic endocarditis
Infective endocarditis
Papillary fibroelastoma
Left atrial myxoma
Patent foramen ovale and concurrent systemic embolism
Mitral annular calcification
Patent foramen ovale
Atrial septal aneurysm
Atrial septal aneurysm and patent foramen ovale
Left ventricular aneurysm without thrombus
Isolated left atrial smoke (no mitral stenosis or atrial fibrillation)
Complex atheroma in the ascending aorta or proximal arch
Symptomatic congestive heart failure with ejection fraction <30%
Apical akinesia without left ventricular thrombus
Wall motion abnormalities (hypokinesia, akinesia, dyskinesia) other than apical akinesia
Hypertrophic cardiomyopathy
Left ventricular hypertrophy
Left ventricular hypertrabeculation/noncompaction
Other rare sources (atrial or ventricular septal defect, preexcitation syndromes, left atrial dilation)
Certain clinical and imaging stroke features support one mechanism over others. In the presence of more than one major etiology, the causative subtype can be inferred with a moderate level of confidence based on the presence or absence of such supportive stroke features. For instance, the presence of unilateral internal watershed infarcts makes an ipsilateral atherosclerotic stenosis in the internal carotid artery (ICA) a more likely mechanism of stroke than atrial fibrillation when they coexist. A recent study identified eight features that exhibited a discriminative value ( Table 20.1 ) :
Article I. Three features of large artery atherosclerosis: prior history of one or more episodes of transient monocular blindness, transient ischemic attack (TIA), or stroke in the territory of index atherosclerotic artery within the preceding month; ipsilateral and unilateral internal watershed infarction; and multiple temporally separate infarcts exclusively within the territory of the stenotic artery
Article II. Two features of cardiac embolism: evidence of concurrent systemic embolism multiple acute and subacute ischemic lesions in the right and left anterior or anterior and posterior circulations, or both, in the absence of nonembolic occlusion or near occlusive stenosis of all relevant vessels
Article III. Three features of SAO: stereotypic lacunar TIAs that have started within the week preceding the index stroke; presentation with a lacunar syndrome; a single acute infarct within the territory of penetrating arteries in the brainstem, deep gray matter, or internal capsule that is up to 20 mm in largest diameter
| Stroke Mechanism | Level of Confidence | Criteria |
|---|---|---|
| Large artery atherosclerosis | Evident | 1. Either occlusive or stenotic (≥50% diameter reduction or <50% diameter reduction with plaque ulceration or thrombosis or plaque with <50% diameter reduction that is seated at the site of the origin of the penetrating artery supplying the region of an acute lacunar infarct) vascular disease judged to be due to atherosclerosis in the clinically relevant extracranial or intracranial arteries, and 2. The absence of acute infarction in vascular territories other than the stenotic or occluded artery |
| Probable | 1. Prior history of one or more episodes of TMB, TIA, or stroke from the territory of index artery affected by atherosclerosis within the month preceding the index stroke, or 2. Evidence of thrombosis, near-occlusive stenosis or nonchronic complete occlusion judged to be due to atherosclerosis in the clinically relevant extracranial or intracranial arteries (except for the vertebral arteries), or 3. The presence of ipsilateral and unilateral acute internal watershed infarctions or multiple, temporally separate infarctions exclusively within the territory of the affected artery |
|
| Possible | The presence of an atherosclerotic plaque protruding into the lumen and causing mild stenosis (<50%) in the absence of any detectable plaque ulceration or thrombosis in a clinically relevant extracranial or intracranial artery and prior history of two or more episodes of TMB, TIA, or stroke from the territory of index artery affected by atherosclerosis, with at least one event within the last month. | |
| Cardio-aortic embolism | Evident | 1. The presence of a high-risk cardiac source of cerebral embolism |
| Probable | 1. Evidence of systemic embolism, or 2. The presence of multiple acute infarctions that have occurred closely related in time within both right and left anterior or both anterior and posterior circulations in the absence of nonembolic occlusion or near occlusive stenosis of all relevant vessels. Other diseases that can cause multifocal ischemic brain injury such as vasculitides, vasculopathies, and hemostatic or hemodynamic disturbances must not be present. |
|
| Possible | 1. The presence of a cardiac condition with low or uncertain primary risk of cerebral embolism | |
| Small artery occlusion | Evident | 1. Imaging evidence of a single and clinically relevant acute infarction less than 20 mm in greatest diameter within the territory of basal or brainstem-penetrating arteries in the absence of any focal pathology in the parent artery at the site of the origin of the penetrating artery (focal atheroma, parent vessel dissection, vasculitis, vasospasm, etc.) or |
| Probable | 1. The presence of stereotypic lacunar transient ischemic attacks within the last week, or 2. The presence of a lacunar syndrome |
|
| Possible | 1. Presenting with a classic lacunar syndrome in the absence of imaging that is sensitive enough to detect small infarctions or 2. Imaging evidence of a single and clinically relevant acute infarction less than 20 mm in greatest diameter within the subcortical white matter in the presence of multiple chronic lacunar infarcts or scattered or confluent patches of subcortical (with or without periventricular) chronic white matter lesions (leukoaraiosis) |
|
| Other uncommon causes | Evident | 1. The presence of a specific disease process that involves clinically appropriate brain arteries |
| Probable | 1. A specific disease process that has occurred in clear and close temporal or spatial relationship to the onset of brain infarction such as arterial dissection, cardiac or arterial surgery, and cardiovascular interventions | |
| Possible | 1. Evidence for an evident other cause in the absence of complete diagnostic investigation for mechanisms listed earlier | |
| Undetermined causes | Unknown | Cryptogenic embolism: 1. Angiographic evidence of abrupt cutoff consistent with a blood clot within otherwise angiographically normal looking intracranial arteries, or 2. Imaging evidence of complete recanalization of previously occluded artery, or 3. The presence of multiple acute infarctions that have occurred closely related in time without detectable abnormality in the relevant vessels |
| Other cryptogenic: Those not fulfilling the criteria for cryptogenic embolism | ||
| Incomplete evaluation: The absence of diagnostic tests that, in the examiner’s judgment, would have been essential to uncover the underlying etiology | ||
| Unclassified | The presence of more than one possible or evident mechanism where there is either probable evidence for each or no probable evidence to be able to establish a single cause |
Confidence in attributing an abnormality to the cause of stroke requires careful consideration of the relationship between the cause (etiology) and the result (brain infarct) in time and space. Temporal relationship implies an antecedent and clearly discernible event that is closely related in time to the index stroke. Examples include stroke after acute arterial dissection, acute myocardial infarction, or vascular or cardiac procedures. Spatial relationship refers to the ability to align an etiology to the territory where the infarct has occurred. It is generally harder to establish spatial relationship than temporal relationship because it requires identification of the clinically relevant artery, which could be a challenging task in some cases with complex occlusion patterns. For instance, an ICA stenosis could be responsible for bilateral anterior circulation infarcts when the contralateral ICA is occluded or when there is an azygos A2 segment or aplasia of the contralateral A1 segment. In the presence of multiple competing etiologies, the etiology that bears a temporal or spatial relationship to the stroke is considered the more likely mechanism. For instance, acute dissection (temporal relationship) in the distal vertebral artery could be regarded as the probable causative etiology in a patient with acute infarcts in the cerebellum (spatial relationship) and concurrent chronic atrial fibrillation.
Etiologic classification is a complex multidimensional problem. It requires careful synthesis of a vast amount of information from clinical evaluation and diagnostic assessments. Automation in this area is inevitable. The CCS is a prototype automated probabilistic algorithm that was developed to improve the reliability and validity of etiologic classification. CCS establishes causal associations by harmonizing the results of stroke workup, completeness of diagnostic studies, and supportive clinical and radiographic stroke features on a case-by-case basis. CCS categorizes ischemic strokes into the same five major etiologic groups as TOAST ( Table 20.1 ). , , There are, however, several differences in definitions of subtypes between TOAST and CCS. The latter incorporates diagnostic technologies such as noninvasive intracranial angiography, diffusion- and perfusion-weighted imaging, and cardiac imaging into subtype definitions. A study that included 13,596 patients with ischemic stroke classified using both CCS and TOAST demonstrated only moderate agreement ( κ = 0.59) between the two systems. The agreement was highest for large artery atherosclerosis ( κ = 0.71) and lowest for SAO ( κ = 0.56).
Published studies show that automated classification can be done with interrater agreement rates ranging from 80% to 95%. , , , Those studies also demonstrate, however, that even with an automated evidence- and rule-based algorithm, disagreements can still occur in etiologic classification due to ambiguities in the source data and differences in the interpretation of test results. Automation improves the validity of etiologic classification as well. A recent study shows that although the conventional classification approach generates etiologic stroke subtypes with different stroke characteristics, automated classification results in considerably more distinct subtypes with discrete clinical features and hard stroke outcomes.
ASCOD (Atherothrombosis–Small vessel disease–Cardiac causes–Other uncommon causes–Dissection) is another commonly used algorithmic system. It categorizes stroke patients based on their phenotypic characteristics in five domains (A, S, C, O, D), where each domain can be defined in five possible states: 0 for no abnormality, 1 for a definite cause, 2 for an uncertain cause, 3 for conditions that are not likely direct causes, and 9 for incomplete investigation. ASCOD incorporates completeness and quality of diagnostic evaluation into three levels of confidence as a definite cause, causality uncertain, and unlikely a direct cause. Published data show that the predecessor of ASCOD, the ASCO system, , which shares a similar framework with ASCOD, exhibits moderate to good interrater reliability and high discriminative validity.
There are two major types of etiologic classification in stroke: phenotypic and causative. Phenotypic classification documents abnormal test findings without weighting toward the most likely cause in the presence of multiple etiologies. There are no tradeoffs among different etiologies. Abnormal test findings are mapped onto one or more of the four following major etiologic categories: large artery atherosclerosis, cardiac embolism, lacunar infarction, and other uncommon causes. Thus a patient can be placed into more than one etiologic category. For instance, a patient with a typical lacunar infarction, 50% or greater atherosclerotic stenosis, and dilated cardiomyopathy is classified as “lacunar infarction plus large artery atherosclerosis plus cardiac embolism.” The Baltimore–Washington Classification, CCS, , and ASCOD systems are phenotypic classification systems. Phenotypic classification can be useful for selecting patients in large-scale epidemiologic and genetic studies and coding for administrative purposes. Its main advantage is that it retains as much patient information as possible. Its shortcoming is that it assigns strokes into a vast number of categories. For example, a three-category phenotypic system where each category is defined in four possible states (such as major, minor, absent, and unknown) could result in 81 possible subtypes (4th power of 3). There are 257 subtypes in Baltimore–Washington Classification, 96 in the phenotypic version of CCS, and 3125 in ASCOD.
The number of etiologic categories correlates inversely with the statistical power in research studies dealing with etiologic subtypes. Hence it is imperative to reduce the number of phenotypic subtypes, and this can be done with a causative classification . Unlike phenotypic classification, where etiologies can be mapped on more than one etiologic category, causative classification assigns etiologies into mutually exclusive categories. As outlined in the prior section, the designation of the causative subtype is a decision-making process that requires the integration of multiple aspects of ischemic stroke evaluation, including clinical and imaging stroke characteristics. TOAST and CCS are causative systems. Because of the lack of a gold standard, causative classification’s ability to unambiguously assign the cause of stroke is limited. Collapsing individual etiologies into categories results in loss of information on an individual basis, but, as previously mentioned, this loss is compensated by the gain in statistical power in research studies.
Approximately half of the recurrent strokes occurring within the first year occur within the first 90 days. The risk of recurrent stroke is highest during the first few days after stroke and declines rapidly after that, reaching a steady low rate after 90 days. It is imperative to differentiate the causative etiology that has the potential to cause another stroke (unstable etiology) in the short term (90 days) from the etiology that does not (stable etiology). The concept of unstable etiology is an important one; it describes a population that may benefit from the timely institution of preventive treatments, such as carotid endarterectomy or acute anticoagulation, at specialized stroke centers that have the infrastructure for prompt evaluation and treatment. Several prognostic models have been developed to identify high-risk populations such as the CHADS2-VASC score for atrial fibrillation and ECST and SCAIL scores for symptomatic carotid stenosis. Although etiology-based tools are widely used in clinical practice, acquiring the necessary predictor variables can sometimes require additional investigation, time, resources, and prolonged hospitalization. Further increasing the complexity is that stroke etiologies can be found in isolation in only half of the patients; the remaining half suffer from multiple concurrent etiologies.
Accumulating evidence suggests that etiologic stroke subtypes convey important prognostic information. According to a recent study, 90-day stroke recurrence rates differ by etiologic stroke subtypes. Large artery atherosclerosis and other uncommon causes are associated with the highest 90-day risk for recurrence (11%–14%), followed by cardiac embolism (6%), undetermined category (3%–5%), and SAO (1%). The high risk in the category of other uncommon causes is mediated by acute nonatherosclerotic arteriopathies such as arterial dissection, active vasculitis, and iatrogenic factors.
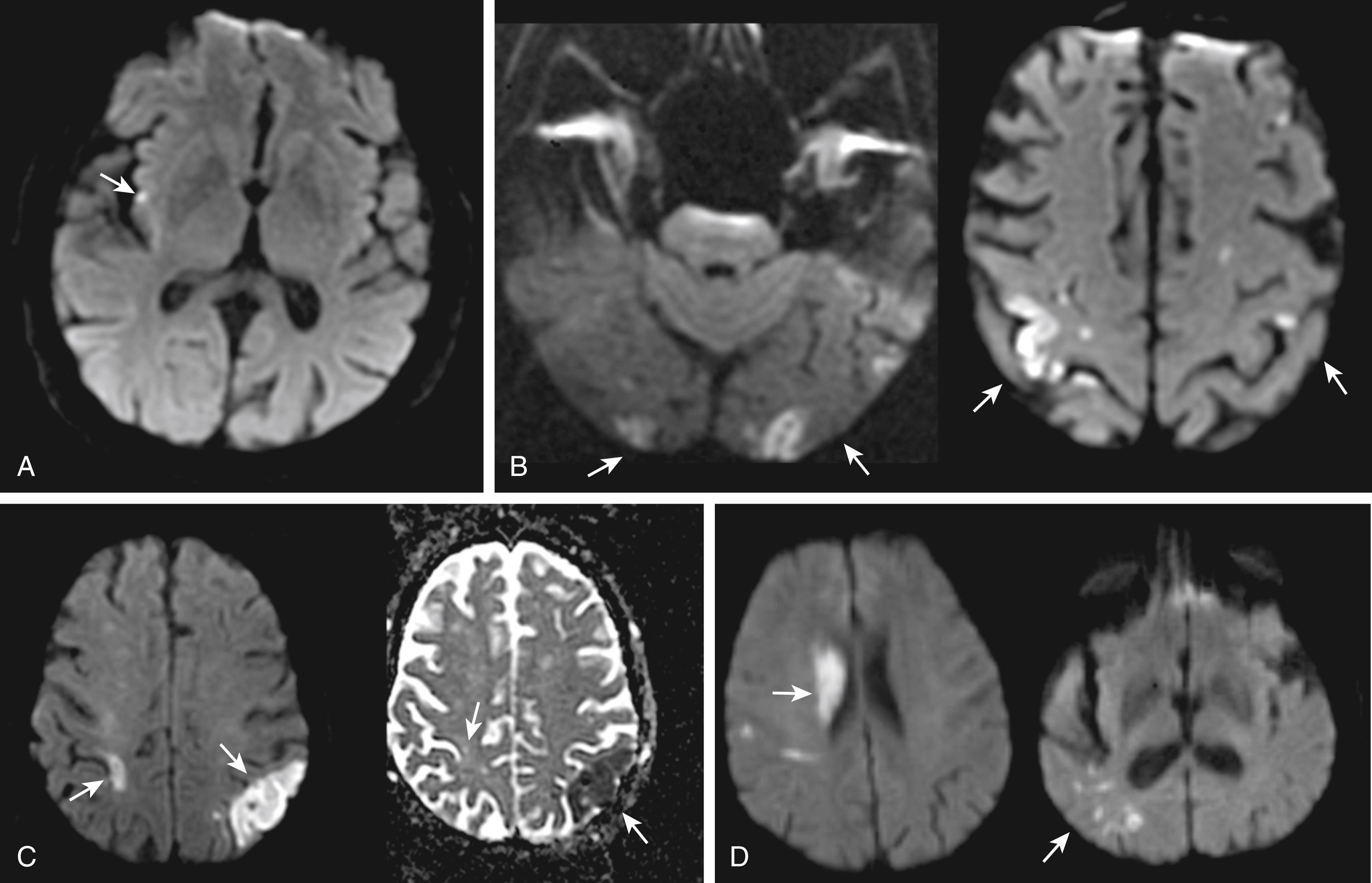
Stroke etiologies appear to leave specific footprints in the brain, depending on their potential to cause another stroke in the short term. Infarct characteristics on brain imaging that correlate with increased risk for recurrent stroke include the presence of simultaneous acute infarcts in both hemispheres or both anterior and posterior circulations, multiple acute or subacute infarcts, and isolated cortical location ( Fig. 20.2 ). , An automated instrument that incorporates such imaging footprints of unstable etiology with etiologic subtype information as well as with a clinical predictor, the history of prior TIA or stroke within the preceding month of index stroke, , provides 90-day stroke risk estimates that range from 1% to 40% depending on the number of predictors. Future tools that integrate further structural, proteomic, and genetic characteristics of stroke subtypes could be expected to further enhance the precision for diagnosis of unstable etiology.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here