Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Chronic pancreatitis (CP) is a rare disease in Western countries (incidence 2 to 10/100,000 per year). It ultimately leads to irreversible damage of the pancreas, with exocrine and endocrine insufficiency. In the majority of cases pain is the major clinical symptom and present early in the course of the disease. With the exception of tropical and hereditary CP, the etiology of CP has not yet been clearly elucidated. Chronic alcoholism is a precipitating factor and dramatically increases the probability of CP development; however, the disease can also develop in nonalcoholic patients without an obvious genetic background and is then referred to as “idiopathic” CP (see Chapter 52 ). Cigarette smoking plays a major role in the progression of alcoholic pancreatitis.
The pathophysiology of CP is still debated. Adherents of the “stone” theory believe that the initiating event is protein plug formation caused by a congenital lack of lithostatin, but this theory is becoming obsolete. Proponents of the “necrosis-fibrosis” theory, on the other hand, ascribe fibrosis and ductal stricture to focal inflammation and necrosis.
Pain associated with CP is multifactorial and includes increased interstitial and intraductal pressures, closed compartment syndrome, neural infiltration, ongoing acute pancreatitis, and presence of pseudocyst(s) and/or biliary obstruction. Elevated intraductal pressure caused by the presence of stones and/or stricture is one of the primary mechanisms leading to pain in CP. Because of lack of compliance of the pancreatic gland already present at the early stages of CP, elevated intraductal pressure is quickly associated with increased parenchymal pressure that impairs blood flow, leading to hypoxia, release of oxygen-derived free radicals, and further stimulation of inflammation with subsequent fibrosis. Surgical decompression of the main pancreatic duct (MPD) has been shown to be associated with pain relief in many patients and is associated with a decrease in intraductal and interstitial pressures, a similar mechanism being considered for endoscopic drainage of the MPD.
Another characteristic of pain in CP is its heterogeneous pattern, from relapsing episodic to persistent pain of varying intensity, which cannot be predicted by pancreatic morphology. The initial episodes of acute recurrent abdominal pain or acute pancreatitis often increase and may evolve into a continuous pain syndrome requiring narcotics. During the natural history of CP, pain may disappear after several years, and this is associated with the development of endocrine and/or exocrine insufficiency. This heterogeneous pain pattern is very often associated with the difficulties faced when interpreting results of clinical studies reporting the effectiveness of surgical or endoscopic drainage for pain relief in CP. In addition, ongoing smoking has a dose-dependent effect on pain relief, even after surgical treatment of CP.
The goal of endotherapy (ET) in severe CP is to decompress the MPD by removing stones and bypassing strictures. Another goal when undertaking MPD drainage is to reduce or delay the development of steatorrhea by increasing the flow of pancreatic juice to the duodenum. Significant pancreatic function improvement related to ET has not been demonstrated, although ET seems to postpone the occurrence of de novo steatorrhea for up to 10 years.
Endoscopic relief of MPD obstruction can be achieved in several ways, including endoscopic pancreatic sphincterotomy, stone removal with and without the aid of extracorporeal shock wave lithotripsy (ESWL), stricture dilation, insertion of pancreatic stents, transmural drainage of collections communicating with the MPD (see Chapter 56 ), and even direct transmural drainage of the MPD.
In addition to standard laboratory testing and plain abdominal radiographs of the pancreatic area or abdominal computed tomography (CT) scan without contrast injection for detection of pancreatic calcifications, magnetic resonance imaging (MRI) is currently the best modality for selection of patients who may benefit from endoscopic treatment and for planning endoscopic therapy ( Fig. 55.1 ).
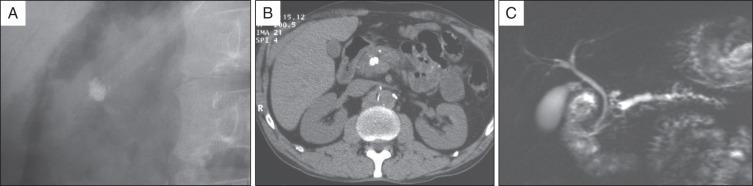
Secretin-stimulated magnetic resonance cholangiopancreatography (S-MRCP) provides information about pancreatic ductal anatomy, presence of peripancreatic fluid collection, and presence of biliary obstruction. Moreover, S-MRCP can be used to quantify pancreatic exocrine function and evaluate the short-term and long-term effects of pancreatic ductal drainage procedures ( Fig. 55.2 ).
MPD cannulation and endoscopic pancreatic sphincterotomy are the first steps of pancreatic ET (see Chapters 14 and 20 ) and provide improved access to the MPD. Minor papilla sphincterotomy (see Chapter 21 ) may be needed in up to 20% of patients in the setting of dominant dorsal duct anatomy (complete or incomplete pancreas divisum, ansa pancreatica). In a small subset of patients, endoscopic pancreatic sphincterotomy itself may resolve papillary stenosis and allow the removal of small floating MPD stones. Indeed, in some patients with MPD obstruction in the head and absence of divisum, the minor papilla can be used to bypass the obstruction and provide pain relief with sphincterotomy and stent placement.
In Western countries, calcifications in the head of the pancreas seen on routine radiographic images often imply that MPD stones are deeply impacted in the ductal wall and will be difficult to remove. In this case, ESWL should be performed before consideration of endoscopic intervention and, as will be discussed later, may be the only intervention required in selected patients.
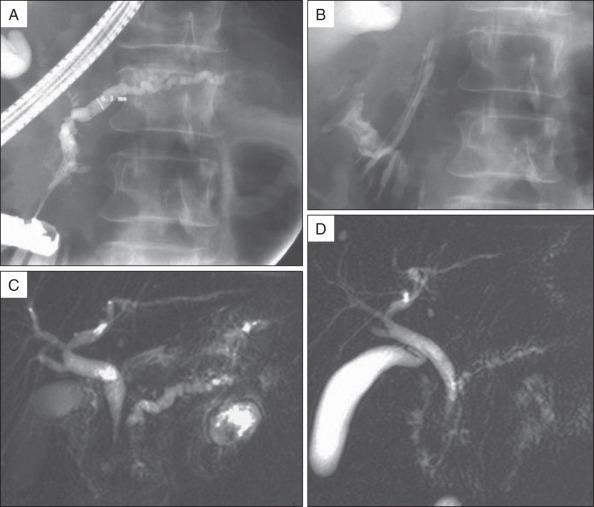
Biliary sphincterotomy is performed before endoscopic pancreatic sphincterotomy in the setting of cholangitis, obstructive jaundice, or associated cholestasis, or when it is technically necessary to facilitate access to the MPD. If a biliary sphincterotomy is performed, the MPD orifice is located between the 3 and 6 o'clock positions on the right margin of the sphincterotomy. After pancreatic opacification, a hydrophilic guide wire (Terumo Inc. [Tokyo, Japan]; Glidewire [Olympus Corp., Center Valley, PA]) can be maneuvered through the stricture or alongside the stones, using a torque device under radiologic guidance ( Fig. 55.3 ). Pancreatic sphincterotomy is then performed over the guidewire after deep cannulation with a standard or tapered pull-type sphincterotome. We prefer to use pure cutting current, extending the incision to the duodenal wall. The same technique can be used for minor papilla sphincterotomy. Alternatively, a stent can be inserted into the MPD and sphincterotomy performed using a needle knife over the stent.
In tertiary referral centers that specialize in endoscopic management of severe pancreatitis, ESWL is often performed before endoscopic therapy is undertaken. Its role was clarified in recent European Society for Gastrointestinal Endoscopy guidelines : “ESWL consistently provides stone fragmentation in 90% of patients (Evidence level 1+); it facilitates endoscopic extraction of MPD stones (Evidence level 2+). Spontaneous elimination of stone fragments following ESWL occurs in approximately 80% of patients. ESWL alone is more cost-effective than routinely combining ESWL with ERCP (Evidence level 1+).”
A recent meta-analysis (27 studies, including 6 prospective; 3189 patients) reported pancreatic ESWL to be safe and effective for the treatment of patients who have MPD stones larger than 5 mm, and do not experience pain relief after conservative management.
Technically, it is important to use a lithotriptor with a bidimensional x-ray focusing system and a high-power generator. Ultrasonic localization of pancreatic stones lacks precision. When performed under general anesthesia or deep sedation, 3000 to 6000 shock waves can be applied at an intensity of 0.33 to 0.54 mJ/mm 2 , which provides complete fragmentation of the stones after a median of 1 session, though in our experience up to 5 sessions are rarely needed. Intravenous administration of secretin during ESWL may facilitate subsequent endoscopic extraction.
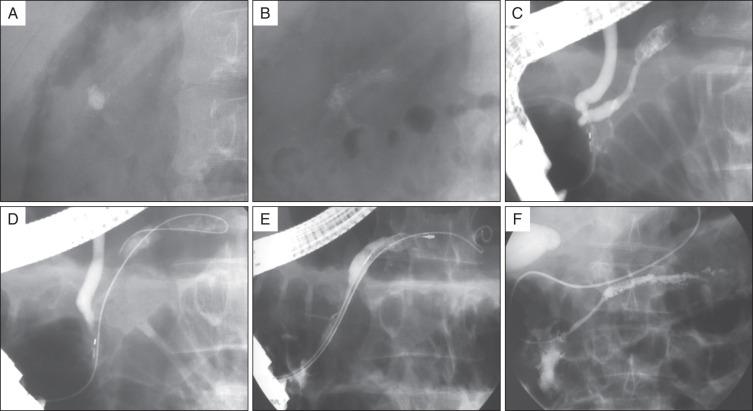
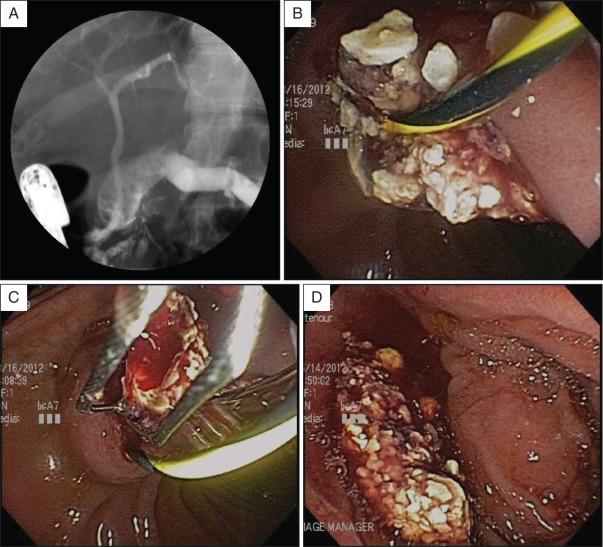
With the patient in the prone position, the shock wave generator is placed to the patient's right side when stones are located in the head of the pancreas, and to the patient's left side when stones are located in the body or tail of the pancreas. ESWL is much more effective for fragmentation of calcium carbonate pancreatic stones than for biliary stones. Stones fragmented into millimeter size can usually be easily removed during endoscopic retrograde cholangiopancreatography (ERCP) ( Figs. 55.4 and 55.5 ). When the lithotriptor is located within or close to the endoscopy unit, ESWL and therapeutic ERCP may be performed consecutively during one general anesthetic session.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here