Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Plain film radiography is the first-line imaging modality in the evaluation of pediatric chest disorders. However, computed tomography (CT) and magnetic resonance imaging (MRI) are increasingly being used and are more valuable for further characterization of pediatric chest abnormalities. Multidetector CT (MDCT) is the most important imaging modality and is more sensitive than radiographs. CT has several advantages including high spatial resolution, good temporal resolution (for cardiovascular disorders), wide field of view (FOV), isotropic resolution, and multiplanar reconstruction (MPR) capabilities, all of which make it an excellent modality for a comprehensive evaluation of pulmonary, airway, mediastinal, cardiovascular, and musculoskeletal abnormalities. Modern scanners can scan in a few seconds because of high z-axis coverage and fast gantry rotation time. This is well suited for children and obviates the need for sedation in the majority of cases. The main disadvantage of CT is ionizing radiation, which is associated with stochastic effects, including cancer. Owing to these radiation risks, CT should only be performed for a definite clinical indication if no alternative nonradiation imaging options exist. The radiation dose should be kept as low as reasonably achievable (ALARA) using several available dose-reduction strategies such as low tube voltage (80 or 100 kVp), low tube current adapted to body weight/size, automated tube current modulation, iterative reconstruction algorithms, and filters to reduce overranging effect in spiral acquisitions. The scan should be confined to the area of clinical interest.
Because of rapid image acquisition, children older than 3 to 4 years are generally compliant for CT scan, particularly if they are aware and prepared. Small children (<15 kg body weight) and those who are noncompliant are sedated for CT, typically with chloral hydrate (50 mg/kg). Ketamine, midazolam, and propofol are alternatives. General anesthesia is rarely required, particularly in noncompliant children. Older children are compliant with breath-hold instructions and are able to hold it in inspiration. Suboptimal inspiration can cause increased density in lungs. In sedated children, controlled ventilation can be used for obtaining images at optimal lung capacity. In noncompliant children, an alternative is to use respiratory gating. Intravenous (IV) contrast is necessary in several situations, particularly cardiovascular disorders. In our institution, 1 to 2 mL/kg of ioversol (Optiray 350) or iopamidol (Isovue 370) is injected, followed by 10 mL of saline bolus. Venous access is challenging in smaller children; contrast is hand injected in this subset of patients. With hand injection, the scan acquisition is initiated after the contrast bolus is administered. Power injector is preferred over hand injection to obtain homogeneous vascular opacification. An empirical delay of 12 to 15 seconds after contrast initiation for children less than 10 kg or 20 to 25 seconds for larger children can be used for most studies. Bolus tracking can be used to initiate image acquisition, particularly with cardiovascular studies. The scan acquisition is initiated when the attenuation in the structure of interest reaches a suitable threshold. However, bolus tracking is associated with higher radiation dose.
CT protocols are tailored to the specific clinical indication. Typically, lung CT is performed using a volumetric spiral acquisition at end inspiration, following administration of IV contrast. Images are reconstructed at soft tissue and bone algorithms, with thin slices (0.6 mm) and overlapping or contiguous slice reconstruction for evaluation of the mediastinum and lung. High-resolution CT (HRCT) protocols for interstitial and airway diseases include non–contrast-enhanced inspiratory acquisition (1-mm slices, 10-20 mm gap) and three expiratory images (apex, carina, base) reconstructed with high-spatial-frequency bone algorithm. In children who are unable to hold their breath, two slices are taken in right and left decubitus positions, with the dependent lung behaving in apparent expiration, whereas the nondependent lung is in apparent inspiration. Evaluation of airway abnormalities is performed using dynamic volumetric acquisition, both in inspiration and expiration. Vascular studies can be performed without any electrocardiographic (ECG) gating, but cardiac studies will require ECG gating to minimize motion artifact. Prospective ECG-gated sequences are preferred because of significantly lower radiation dose than retrospective ECG-gated images. MPRs in coronal and sagittal planes, maximum intensity projections (MIPs), minimal intensity projections (MiniP), and three-dimensional (3D) volume rendering and surface shading are the postprocessing tools used in the evaluation of chest disorders. MPRs with isotropic resolution enable visualization of structures and abnormalities in different planes, giving a better depiction for presurgical planning. MIPs are useful in evaluation of vasculature and detecting small pulmonary nodules. MiniP is useful in evaluation and quantification of air trapping and airway disorders. 3D volume rendering or surface shading is useful in evaluation of cardiovascular anatomy. Virtual bronchoscopic images are useful in the evaluation of several airway disorders, mimicking a real bronchoscopy.
MRI is used as a problem-solving tool in the evaluation of pediatric chest disorders. The advantages of MRI include wide FOV, multiplanar acquisition capabilities, good spatial and temporal resolution, inherent soft tissue contrast augmented with the use of gadolinium-based contrast agents, and the absence of ionizing radiation. MRI is well suited for the evaluation of cardiovascular, mediastinal, and pleural abnormalities. However, MRI is not widely available and acquisition times are long, as a result of which deep sedation/anesthesia is required in young children, who will not be able to lie still for prolonged periods. Evaluation of lung parenchymal and airway abnormalities is limited. MRI cannot be performed in patients with contraindications including claustrophobia. MRI protocol should be optimized for the clinical indication, and the acquisition times should be minimized to ensure patient compliance. Evaluation of the mediastinum and pleura is performed using T1-, T2-, and proton density–weighted, fat-saturated, steady-state free precession (SSFP), and diffusion sequences. ECG and respiratory gating may be required for obtaining images without respiratory and cardiac motion. Gradient echo T1-weighted images with in-phase and out-of-phase are obtained for characterizing thymic lesions. 3D volumetric contrast-enhanced T1 images (e.g., volumetric interpolated breath-hold examination [VIBE], T1 high-resolution isotropic volume excitation [THRIVE], liver acquisition volume acceleration [LAVA]) are performed to evaluate enhancement. Dynamic multiphasic contrast-enhanced sequences may help in lesion characterization. Cardiovascular studies include SSFP, perfusion, and delayed enhancement sequences. MR angiography (MRA) can be performed after administration of IV contrast (0.1-0.2 mmol/kg) and using a volumetric T1-weighted spoiled gradient echo sequence. Noncontrast MRA can be performed using volumetric SSFP, time of flight, or phase-contrast sequences.
In this chapter, we review the CT and MRI appearances of common pediatric thoracic conditions.
Congenital cystic adenomatoid malformation (CCAM) is a spectrum of malformations consisting of proliferation of distal bronchioles with suppression of alveolar development. The incidence is 1 : 25,000 to 1 : 35,000 of pregnancies. The new term congenital pulmonary airway malformation (CPAM) has been recommended, and a new classification is also proposed. There are five types of CPAMs based on pathologic classification (Stocker classification). Type 0 has a tracheal or bronchial origin and is really acinar dysgenesis or dysplasia, type 1 has a bronchial or bronchiolar origin (large cystic lesion), type 2 has a bronchiolar origin (small cystic lesion), type 3 has a bronchiolar–alveolar duct origin (adenomatoid type), and type 4 has a distal acinar origin (the “unlined” cystic lesion). The four types of CPAMs seen in imaging are large-cyst CPAM (type 1), small-cyst CPAM (type 2), microcystic (cysts < 5 mm) , solid type (type 3), and a large cyst causing mass effect, lobar expansion, and mediastinal shift that is indistinguishable from a predominantly cystic pleuropulmonary blastoma (type 4). CPAM is usually diagnosed prenatally on fetal ultrasound or MRI or early after birth.
On pathology, type 1 CPAM reveals a large (>2-cm) cyst and sometimes multiple or multiloculated cysts, occasionally with small cysts that merge with the adjacent lung. At microscopic examination, these cysts are lined by ciliated epithelium, and some contain mucigenic epithelium, highlighting the embryologic origin of the lung as a foregut derivative. Fetal ultrasound of a large-cyst CPAM (type 1) demonstrates numerous variable-sized anechoic spaces intermixed with echogenic soft tissue. On fetal MRI, large-cyst CPAMs manifest as hyperintense unilocular or multilocular lesions with discrete walls on T2-weighted images. Postnatal radiography shows variable density in the region of the mass depending on the fluid contents of the cysts and possibly mediastinal shift, depending on the size of the CPAM. In the neonatal period a large-cyst CPAM may be seen as a round soft tissue mass that gradually becomes filled with air, since there is delayed clearance of fetal lung fluid from the cysts through the abnormal airway. It may be seen as a solitary well-defined air-filled cyst with thin walls or as multiple cysts of varying size ( Fig. 41-1A ). Air-fluid levels may be identified. Postnatally, CT is optimal for detailed depiction of the lung parenchyma and airway, and these lesions are readily detectable as well-defined air-filled spaces.
On pathology, type 2 CPAM is seen as focal areas of microcystic maldevelopment of the lung parenchyma. On histologic analysis, they consist of closely apposed bronchiole-like structures with variable amounts of intervening lung parenchyma. On fetal ultrasound an echogenic mass with multiple small cysts is seen, whereas on MRI it has a variable T2 appearance that depends on the cystic and solid components. Postnatal radiography may demonstrate a heterogeneous intrapulmonary mass with small air-filled cystic areas. Some lesions can be subtle and may not be detected at postnatal radiography. CT may allow better evaluation, demonstrating a heterogeneous mass with small cysts (see Fig. 41-1B and C ) and variable surrounding consolidation. The differential diagnosis for type 1 and 2 CPAM includes congenital diaphragmatic hernia, pulmonary sequestration, bronchogenic cyst, congenital lobar emphysema, and other bronchopulmonary foregut malformations.
A microcystic (predominantly solid-appearing) CPAM (type 3) is homogeneously echogenic on prenatal ultrasound, whereas on T2-weighted MRI it manifests as a homogeneously hyperintense solid mass with normal adjacent lung parenchyma. Postnatal CT demonstrates an ill-defined area of increased attenuation. CPAMs communicate with the bronchial tree but do not typically have a systemic arterial supply. However, occasionally, systemic arterial supply may be seen, particularly in small-cyst CPAMs, and these lesions are called hybrid lesions since they demonstrate histologic and imaging features of both a CPAM and bronchopulmonary sequestration. A fast-growing CPAM may cause mediastinal shift and subsequent development of polyhydramnios and hydrops. Indicators of a poor prognosis in CPAM include large lesions, bilateral lung involvement, and hydrops. Quantitative measurements of mass size that help predict development of hydrops and poor outcome include a mass-to-thorax ratio of more than 0.56 or a CPAM volume ratio (i.e., volume of the mass divided by head circumference) greater than 1.6.
Expectant management is appropriate for the nonhydropic fetus, whereas the survival of fetuses with hydrops improves only with fetal intervention, including aspiration of the cyst, thoracoamniotic shunt, injection of sclerosing agent, or in some cases open fetal surgery. After 32 weeks' gestation, surgical resection of the mass may be possible while the fetus is maintained on placental circulation, using an ex utero intrapartum treatment (“EXIT”) procedure. When fetal distress is not evident, the mortality rate may be as low as 5% or less. If not recognized antenatally, CPAMs are usually discovered between the neonatal period and 2 years of age, manifesting as respiratory difficulty or infection. Symptomatic infants who are diagnosed postnatally are treated with surgical resection, which generally consists of lobectomy or segmental resection. Surgical intervention for asymptomatic CPAMs remains controversial. Recurrent infection and a questionable small risk of malignancy have been cited as reasons for elective resection.
Congenital lobar emphysema (CLE) is characterized by progressive lobar overexpansion, usually associated with compression of the remaining lung. CLE can either be due to a primary intrinsic cartilaginous abnormality with weak/absent bronchial walls or secondary to intrinsic/extrinsic bronchial obstruction (e.g., large pulmonary artery or a bronchogenic cyst). In either case the collapsed airway acts as a one-way valve, resulting in air trapping. The term congenital lobar overinflation (CLO) is suggested as a substitute because in this process the alveoli expand but are not destroyed. The most commonly involved lobe is the left upper lobe (42%), followed by the right middle lobe (35%) and right upper lobe (20%). Involvement of the lower lobes is uncommon (1%). Males are more frequently affected than females. It presents with respiratory symptoms in the neonatal period but can also be detected incidentally on fetal ultrasound as a homogeneously hyperechoic mass.
In the neonatal period, CLE is seen as a radiopaque mass on radiographs, since it is filled with fluid. Gradually the fluid will be absorbed and the lesion becomes lucent. Subsequently the affected lobe becomes hyperinflated, with hyperlucency demonstrated on conventional radiography and CT scan. The vascular markings are diminished in the affected lobe ( Fig. 41-2 ). Associated findings are compressive atelectasis in the ipsilateral or even contralateral lobes, mediastinal shift, hemidiaphragm depression, and widening of the rib spaces. CT can also be helpful in identifying an abnormal bronchus or focal obstruction as an underlying etiology, as well as evaluating any associated mediastinal or cardiac abnormality. Differential diagnosis includes pneumothorax, pulmonary cysts, CPAM, and pulmonary interstitial emphysema (PIE). The pulmonary markings passing through the overinflated lung differentiate it from pneumothorax and pulmonary cysts. In CLE the septae and vascular structures are located at the periphery of expanded air spaces, whereas they are seen in the center in PIE, in which the abnormal air collection occurs in the interstitium. No associated soft tissue is seen in CLE, unlike in CPAM. Asymptomatic or mildly symptomatic patients are managed conservatively with low-volume and low-pressure ventilatory support given as needed. In patients with more severe respiratory distress, emergent surgical resection of the affected lobe might be needed.
Congenital diaphragmatic hernia (CDH) is an uncommon congenital anomaly of the diaphragm with an incidence of 1 per 2000 to 3000 births. Normally the diaphragm starts to develop at approximately 4th week of gestation and is fully formed by the 12th week of gestation. In CDH, one of the components of the diaphragm does not form properly, creating a defect that allows abdominal viscera to enter into the thoracic cavity. This impedes normal development of the lungs, resulting in severe respiratory failure soon after birth because of pulmonary hypoplasia and the presence of pulmonary hypertension. CDH can be classified based on the anatomic position of the defect into posterolateral, anterior, and central defects. The posterolateral defect (Bochdalek hernia) occurs in 70% to 75% of cases, anterior defects (Morgagni hernia) in 23% to 28%, and central defects in only 2% to 7% of cases. The posterolateral defect most often occurs on the left side (85%) but can occur on the right (13%) or even bilaterally (2%). CDH is associated with other congenital abnormalities in approximately 40% of cases. After pulmonary anomalies, cardiovascular anomalies are the most common group of congenital anomalies present in CDH infants. It has been shown to be associated with genetic syndromes such as Beckwith-Wiedemann syndrome and CHARGE syndrome and chromosomal abnormalities including trisomies of 13, 18, 21, and Turner's syndrome.
CDH can be diagnosed prenatally by ultrasonography (60% of cases) or postnatally soon after birth. Milder forms of CDH may present with mild respiratory or gastrointestinal symptoms after several months or even years. Postnatal chest and abdominal radiographs are usually diagnostic and will show an opacified hemithorax with mass effect and contralateral shift of the mediastinum with stomach and gas-filled loops of bowel in the chest. Cross-sectional imaging can be helpful in cases where the diagnosis is still not clear and to evaluate the spectrum of associated anomalies ( Fig. 41-3 ). CT is useful to demonstrate any associated lung masses and bronchopulmonary foregut malformations. CT angiography is useful for depiction of the vascular supply of lung lesions. However, oral contrast material should be avoided because it can increase the mediastinal shift and subsequent respiratory compromise. If the patient is stable, MRI can be used in the evaluation of complex lesions as well.
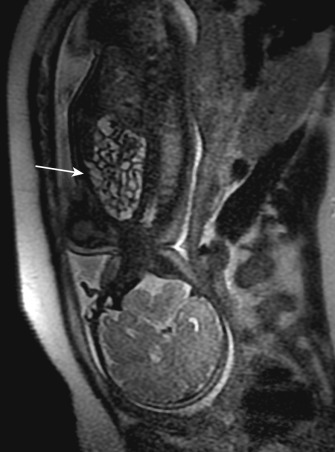
In recent years there has been an increase in the use of fetal surgery as an experimental treatment approach. The postnatal management of CDH has evolved in recent decades and now includes gentle ventilation with permissive hypercapnia and delayed surgical repair after stabilization. Prognosis of CDH is related to the degree of pulmonary hypoplasia and need for extracorporeal membrane oxygenation (ECMO) in the neonatal period. Follow-up chest imaging after surgical repair might be useful to evaluate the functional consequences of the underlying pulmonary hypoplasia and its complications. Pulmonary hypoplasia will manifest as a smaller lung with diminished pulmonary vascularity.
Pulmonary sequestration is defined as a segment of lung tissue that is separate from the tracheobronchial tree and receives its blood supply from a systemic artery. There are two types of pulmonary sequestration: (1) intralobar, which is covered by pleura of the adjacent lung ( Fig. 41-4 ), and (2) extralobar, which has its own separate pleural covering ( Fig. 41-5 ). Intralobar sequestrations account for 75% of cases of sequestration. Both intra- and extralobar forms receive systemic arterial supply. In 80% to 90% of sequestration, the arterial supply is from the thoracic aorta (see Fig. 41-4B and C ) coursing through the inferior pulmonary ligament, and in the remaining cases it is from the abdominal aorta. Venous drainage of the intralobar form is to the pulmonary vein (see Fig. 41-4C ), whereas the extralobar form drains into systemic veins (see Fig. 41-5E ). The extralobar form is most commonly diagnosed in the prenatal/neonatal period, and is often associated with other congenital systemic anomalies, such as CDH, cardiac abnormalities, pulmonary hypoplasia, or foregut duplication cysts. The intralobar form is usually diagnosed later in childhood or adulthood, usually with recurrent infections or hemoptysis. Association of intralobar sequestration with other congenital anomalies is rare. The intralobar form typically affects the posterior basal segment of the left lung in 60% of cases. Rarely upper lobe and bilateral lesions have been reported.
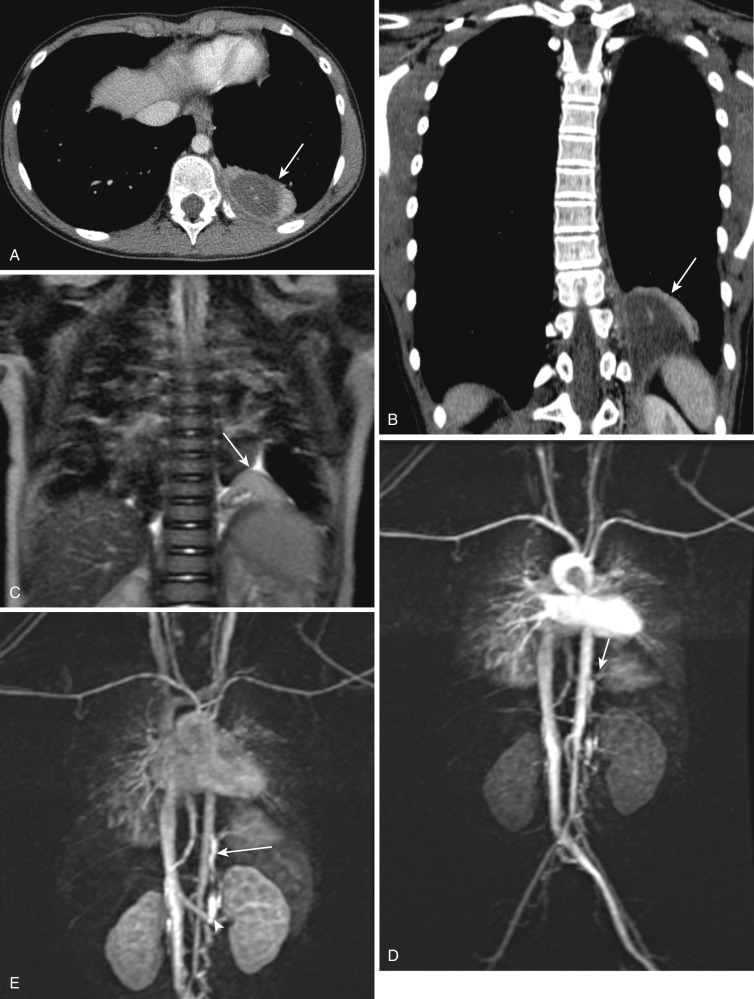
The radiographic appearance is nonspecific and depends on the degree of aeration and the presence of infection within the sequestered lung. Most pulmonary sequestrations appear as a triangular or oval mass in the medial and basal portion of a lung, more commonly on the left (see Fig. 41-5A, B and C ). The lack of communication of the sequestered lung with the normal tracheobronchial tree is associated with continued accumulation of secretions accompanied by acute inflammation, infection, and air trapping. Repeated infection may establish an abnormal communication with the tracheobronchial tree and cystic bronchiectasis may ensue. In older patients the sequestration will show the effects of recurrent infections. Occasionally, calcifications occur in pulmonary sequestration and are readily detected by CT. MDCT angiography and MRA are the imaging methods of choice in the noninvasive assessment of pulmonary sequestration, showing in excellent detail the anomalous systemic vessel. Systemic arterial supply originates from the lower thoracic/upper abdominal aorta and is an essential feature of pulmonary sequestration that differentiates it from other focal lesions with similar characteristics, such as CPAM (see Fig. 41-5D ). Venous drainage is also identified, with the intralobar form draining into pulmonary veins (see Fig. 41-4C ) and the extralobar form draining into systemic veins (see Fig. 41-5E ).
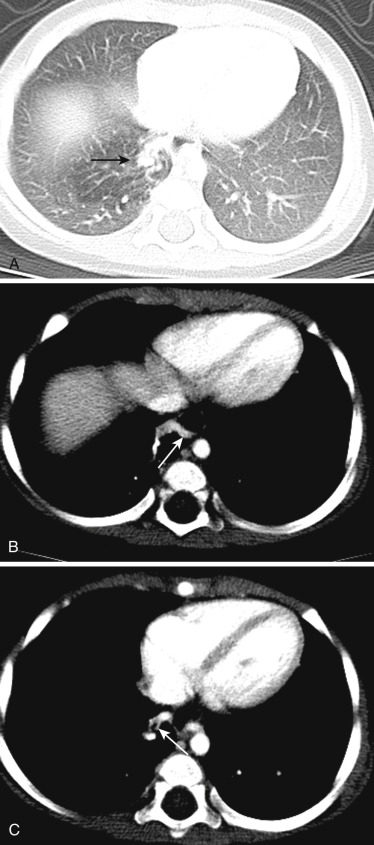
Scimitar syndrome (hypogenetic lung syndrome, congenital venolobar syndrome) is characterized by partial anomalous pulmonary venous drainage of the lung associated with hypoplasia of the lung and pulmonary artery. It is caused by failure of integration of the primitive pulmonary venous plexus with the common pulmonary vein, resulting in communication with cardinal or umbilical veins; it is almost always seen on the right. The anomalous vein drains part or the entire right lung into the inferior vena cava ( Fig. 41-6 ), coronary sinus, right atrium, azygos vein, portal vein, or hepatic vein. Cardiac dextroposition and systemic arterial supply to the right lung are also seen. Occasionally not all these features are present in an individual patient. Anomalies of bronchial and pulmonary arterial branching, lobes, and fissures are commonly seen, including horseshoe or crossover lung. Other associations include pulmonary sequestration, aortopulmonary collateral vessels, congenital cardiac defects (e.g., atrial septal defect, hypoplastic left heart), CDH, and musculoskeletal abnormalities. Scimitar syndrome results in a left-to-right shunt, which if large or associated with pulmonary hypertension may require further treatment with surgery or embolization.
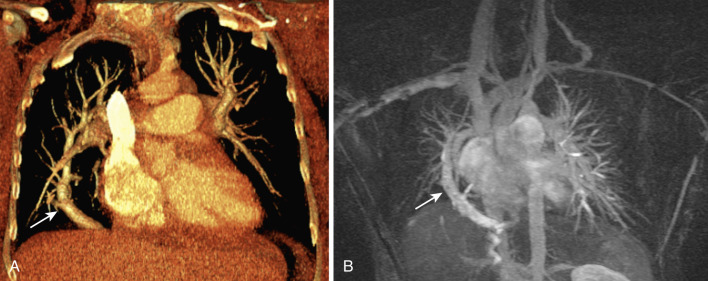
On radiographs a characteristic scimitar sign is seen, which is caused by the anomalous pulmonary vein curving medially toward the diaphragm and inferior vena cava and progressively widening, giving the appearance of a scimitar (Turkish/Persian sword with a curved blade). CT and MRA are useful in accurate depiction of this anomalous venous drainage (see Fig. 41-6 ). MRI also helps quantification of the left-to-right shunt using velocity-encoded phase-contrast imaging. Abnormalities of the lung, systemic arterial supply, and other associated abnormalities can also be evaluated using CT and MRI. Scimitar sign can also be seen in another entity called anomalous single pulmonary vein (ASPV) (anomalous unilateral single pulmonary vein, meandering pulmonary vein, scimitar variant, pseudoscimitar syndrome, or pulmonary varix), in which the entire lung is drained by a single pulmonary vein that has an anomalous course, but the main difference from scimitar syndrome is that this vein drains normally into the left atrium. It shares several common features with scimitar syndrome, including right sidedness, right lung hypoplasia, cardiac dextroposition, systemic arterial supply, branching abnormalities, and other congenital defects. ASPV is caused by hypoplasia/atresia of one of the pulmonary veins before pulmonary segmentation, as a result of which drainage of the entire lung occurs through the remaining vein. Since ASPV is not a shunt, it does not require further investigation or treatment. Other differential diagnoses for scimitar sign include pulmonary sequestration, pulmonary varix, and pulmonary nodule/mass.
Pulmonary arteriovenous malformations (AVMs) are vascular communications between a pulmonary artery and vein (95%) bypassing the capillary bed. Pulmonary AVMs are usually congenital but may also be idiopathic, occur as a result of trauma and infection, or be secondary to hepatopulmonary syndrome and bidirectional cavopulmonary shunting. About 60% of pulmonary AVMs are associated with Rendu-Osler-Weber syndrome (hereditary hemorrhagic telangiectasia), and 15% of hereditary hemorrhagic telangiectasia patients have pulmonary AVM. This is an autosomal dominant disorder caused by genes that encode transforming growth factor binding protein, with associated abnormalities in liver, skin, brain, and gastrointestinal tract. Owing to this high association, screening for pulmonary AVM is indicated in this subgroup. Physiologic consequences of pulmonary AVM depend on the degree of right-to-left shunt and include hypoxemia, dyspnea, and cyanosis. Hereditary pulmonary AVMs tend to increase in size over time, with clinical manifestations usually presenting by the fifth decade. These patients are also prone to paradoxical embolization complications including stroke and brain abscess.
The typical CT features of pulmonary AVM are: smooth lobulated oval/round mass ( Fig. 41-7A ) or even serpiginous nodule, less than 1 cm to several centimeters in size, associated with a visible enlarged feeding artery and an enlarged draining vein (see Fig. 41-7B ). The feeding artery usually has less than half the diameter of the nodule. Complex AVMs having more than one feeding artery are rare. The most common location is the lower lobe. CT or MR pulmonary angiography can be used to confirm the vascular nature of the lesion. Multiple AVMs can be confused with metastases if the enlarged feeding vessels are overlooked. MIP reconstructions can also be obtained to scrutinize the pulmonary parenchyma for malformations and depict the size and number of the supplying arteries. Pulmonary AVMs can be successfully treated with embolotherapy.
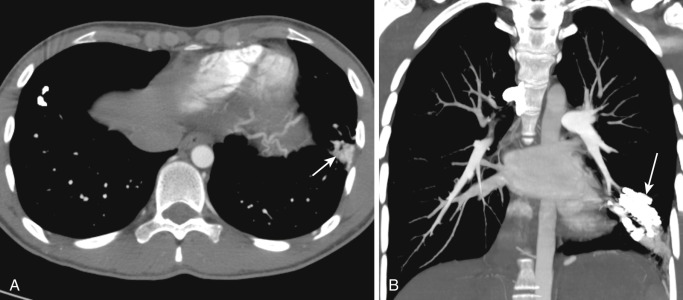
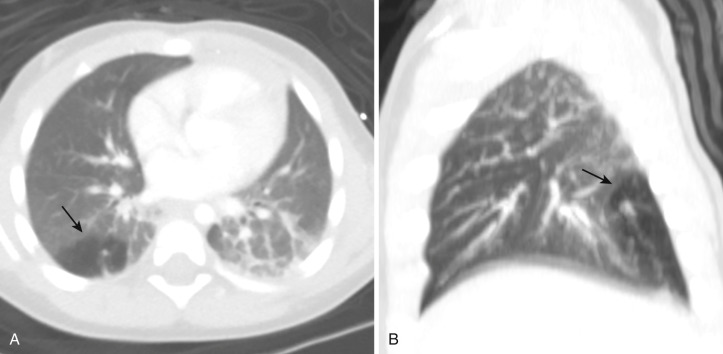
Congenital bronchial atresia is a rare anomaly resulting from focal obliteration of a segmental (less commonly a subsegmental) or lobar bronchus that lacks communication with the central airways. The bronchi distal to the stenosis are dilated and filled with mucus, forming a bronchocele. The alveoli supplied by these bronchi are ventilated by collateral pathways and show features of mild air trapping, resulting in a region of hyperinflation around the dilated bronchi. The upper lobe bronchi are more frequently affected, particularly the apicoposterior segment of the left upper lobe; middle and lower lobes are rarely affected. In some cases, bronchial atresia may be acquired postnatally owing to traumatic or postinflammatory insult to the bronchus. Bronchial atresia is usually asymptomatic and is found incidentally in chest radiographs; however, dyspnea, pneumonia, and bronchial asthma have been reported. In bronchial atresia the airway is occluded rather than narrowed; consequently there is no ball-valve effect as seen in CLE. Hence the lobe or segment does not become nearly as hyperinflated as with CLE.
The characteristic chest radiographic findings consist of a bronchocele, seen as rounded branching opacities radiating from the hilum, that may contain an air-fluid level. The distal lung is emphysematous and produces an area of hyperlucency around the affected bronchi. In newborns the affected segment contains fetal lung liquid trapped behind the atresia and may be seen as a fluid-filled mass. CT is a sensitive modality for demonstrating the typical features of bronchial atresia. It shows a round opacity at the site of the atresia, medial to the air trapping caused by a mucus plug, which can be clearly depicted by performing expiratory CT 15 ( Fig. 41-8 ). CT is also excellent for excluding the presence of a hilar mass and precisely determining the location of lesions. In doubtful cases, multiplanar reformation helps distinguish mucoid impaction from nodular lesions. CT also shows the obstruction, associated segmental hypoattenuation, decreased vascularity, and possible proximal bronchocele or broncholith. When the location is basal, spiral CT with contrast can easily exclude a vascular component that could indicate pulmonary sequestration. MRI shows the bronchocele as a branching structure radiating from the hilum, with high signal intensity on T1- and T2-weighted images; however, MRI cannot depict regional air trapping. The major indication for surgery in these patients is recurrent infection.
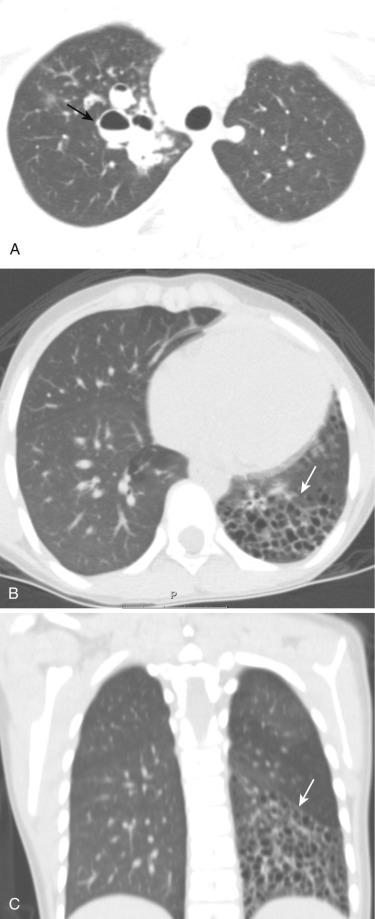
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the CFTR gene and the most common lethal genetic disease in Caucasians. CF is a multisystem disorder of exocrine gland function, with resultant abnormal viscosity of mucus and decreased mucociliary clearance. In the respiratory tract these conditions result in recurrent lung infection and chronic sinusitis with nasal polyps. Lung involvement is the main cause of morbidity among patients with CF. Improvements in therapeutic options have substantially increased the life expectancy of CF patients, with a current median survival of approximately 40 years, which is expected to increase even further.
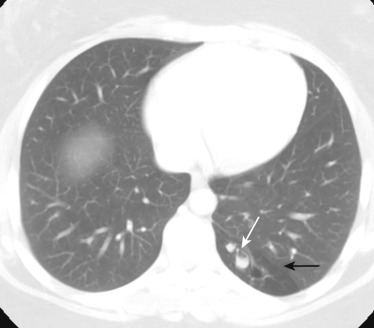
Until 2 decades ago, chest radiograph was the most widely used imaging to monitor morphologic changes in the CF lung. Although chest radiograph correlates significantly with pulmonary function test parameters, it has a low sensitivity to detect early changes in the CF lung. Currently, HRCT is recognized as the gold standard for assessment of morphologic changes of the airways and lung parenchyma for both early diagnosis and monitoring of CF lung disease with higher sensitivity than chest radiograph and lung function testing. Therefore, multiple disease-scoring systems based on HRCT findings have been proposed.
An HRCT finding in early stages of lung involvement in CF is a mosaic attenuation pattern resulting from air trapping related to small airway disease. Other typical features that may be seen during the course of the disease include bronchiectasis ( Fig. 41-9 ) and peribronchial thickening, mainly involving the upper lobes, and centrilobular nodules and a tree-in-bud pattern secondary to mucous plugs. Development of bronchiectasis in CF is almost universal, detected in approximately 30% of children with normal findings at lung function testing. Bronchiectasis alone or in combination with mucus plugging is given the highest weighting in most scoring systems and is thus commonly considered to be the most significant finding when evaluating CF lung disease. Pneumothorax is a rare complication with an incidence of 0.64% per year among CF patients.
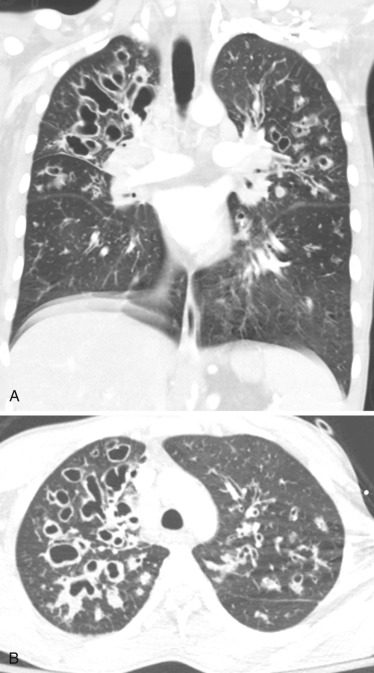
Considering the necessity of lifelong repeated imaging studies, MRI has been proposed as a radiation-free alternative to CT. Although spatial resolution is lower as compared to CT, MRI has the advantage of higher soft tissue characterization and visualizing different regional functional aspects of the lung parenchyma (pulmonary hemodynamics, perfusion, and ventilation). Using common MRI sequences, it is possible to visualize bronchiectasis/bronchial wall thickening, mucus plugging, air-fluid levels, consolidation, and segmental/lobar destruction.
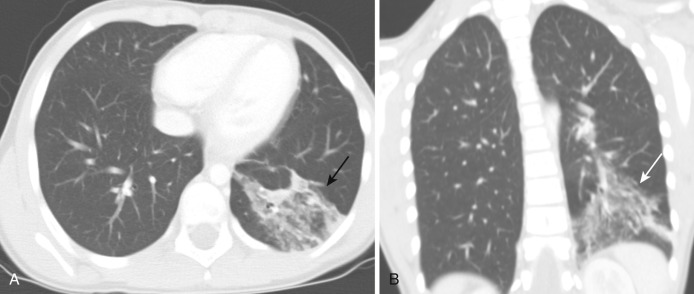
Pediatric pneumonia is most commonly caused by Streptococcus pneumoniae. Other organisms include Mycoplasma pneumoniae and Chlamydophila pneumoniae and viruses. In newborns, group B Streptococcus, Listeria monocytogenes, or gram-negative rods are common bacterial agents. In children aged 1 to 3 years, the most common organisms are S. pneumoniae, Staphylococcus aureus, and Haemophilus influenzae. In infants, toddlers, and preschool-aged children, viruses are the most common pathogens, with respiratory syncytial virus (RSV) followed by parainfluenza type 1, 2, 3 and influenza A and B. S. pneumoniae is the most common bacterial pathogen. In school-aged children and young adolescents, Mycoplasma is the most common organism, with others being S. pneumoniae, S. aureus, and Streptococcus pyogenes. In older adolescents, Mycoplasma is the commonest, but C. pneumoniae and fungal infections can be seen. Pneumonia is seen on radiographs, but CT is performed when (1) there are persistent signs, symptoms, and radiographic findings not responding to treatment; (2) to exclude underlying lesions such as sequestration; and (3) to evaluate complications such as pleural effusion, abscess, and empyema. CT is performed when radiographic findings are normal, equivocal, or nonspecific. CT is more sensitive than radiographs in the detection of pneumonia. CT findings of pneumonia include air space disease ( Fig. 41-10 ), consolidation, ground-glass opacities, air bronchograms, air space nodules, and centrilobular or perilobular distribution.
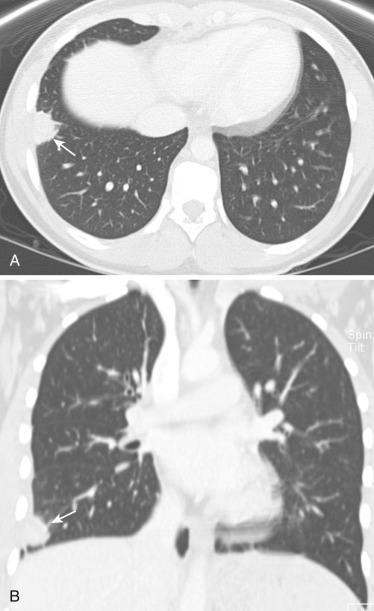
Round pneumonia is a community-acquired pneumonia manifesting as a rounded opacity on imaging. It is more common in children younger than 12 years. It is usually caused by bacterial infection, most commonly S. pneumoniae (90%). In older children, especially those with immunodeficiency, fungal or mycobacterial infections are occasionally encountered. Smaller alveoli, closely apposed septa, and underdeveloped interalveolar connections constrain the spread of infection to adjacent alveolar sacs in young children, resulting in a round opacity on imaging. Patients present with clinical signs and symptoms of pneumonia. The characteristic finding is a solitary circumscribed spherical opacity, with predilection for the posterior segment of the lower lobes. The lesion is usually in contact with the pleura or hilum. Air bronchograms are seen in 20% of cases. Satellite lesions are sometimes present, but multiple round pneumonias are rare (1%). Calcification, cavitation, lymphadenopathy, or pleural effusion are not characteristically present. In a child presenting with clinical symptoms of pneumonia, a rounded opacity on radiograph or CT scan ( Fig. 41-11 ) is indicative of round pneumonia. A rounded opacity in adults could be neoplastic, but in children it is almost always benign. There are other entities mimicking round pneumonia, such as inflammatory masses (e.g., lung abscess, tuberculosis, fungal infection), congenital anomalies, neoplasms, and pseudomasses. CT should be considered for further evaluation of a radiographic round opacity in the following settings: clinical features not consistent with a pneumonic process, persistent opacity after appropriate antibiotic treatment, radiographic signs of nonpulmonary origin, or immunocompromised patient with suspicion of complicated infection.
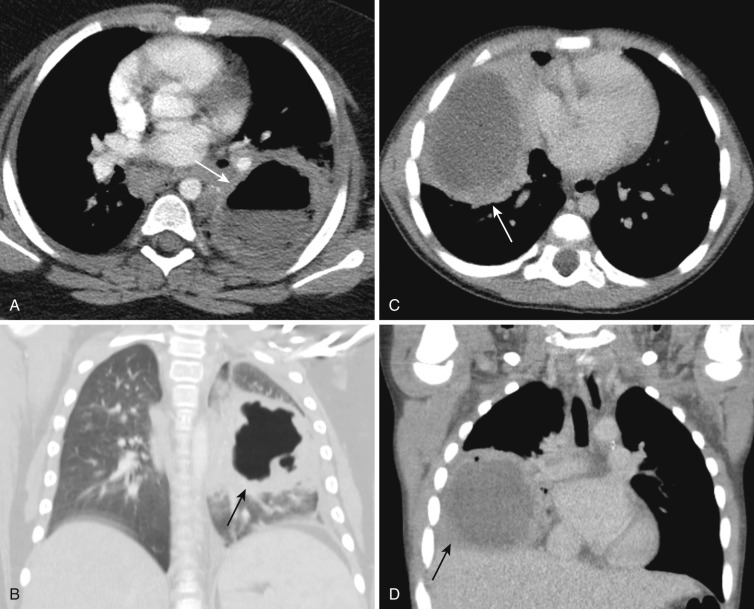
Cavitary necrosis (necrotizing pneumonia) is one of the parenchymal complications of severe pneumonia. The spectrum of parenchymal complications of pneumonia also includes lung abscess, pneumatocele, and bronchopleural fistula. In adults, necrosis and cavitation are usually associated with S. aureus, Pseudomonas aeruginosa, Klebsiella, and anaerobic infection, but in children S. aureus or S. pneumoniae are most commonly involved. Cavitary necrosis is demonstrated as a dominant area of nonenhancing necrosis surrounded by a thin wall or without a wall within a parenchymal consolidation. Lung abscess is characterized as a solitary cavity with a thick enhancing wall ( Fig. 41-12 ). Pneumatocele presents at later stages of healing necrosis. Although children with necrotizing pneumonia typically have a prolonged and intense course of illness, most will ultimately recover without the need for surgical intervention.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here