Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The clinical chemistry metabolic profile gives important quantitative information for over 25 serum analytes, often facilitating diagnosis of disease.
Serum electrolyte concentrations for sodium, potassium, chloride, and sometimes calcium are most commonly assayed using ion-selective electrodes.
Assays for other analytes, including total protein, albumin, bilirubin, creatinine, calcium, iron and iron-binding capacity, magnesium, and phosphate, are performed using spectrophotometry on complexes that each analyte forms with simple reagents. These complexes absorb light at wavelengths at which there is minimal absorbance by both the analyte itself and by the reagent. The concentration of the analyte is determined using Beer’s law.
Assays for a number of serum analytes—including ammonia, bicarbonate, cholesterol, glucose, lactate, triglycerides, urea, and uric acid—utilize enzymes that catalyze reactions in which the analytes are converted into products whose concentrations can be measured conveniently.
Spectrophotometric assays can be performed by waiting for the point at which the increase or decrease in absorbance of a complex at a particular wavelength ceases to change (end-point method) or by the initial rates of complex formation (rate method).
Assays for specific enzymes are performed using substrates that are specific for these enzymes. The concentrations of the products of the enzyme-catalyzed reactions are determined spectrophotometrically as described in Chapter 21 , allowing for direct determination of enzyme activity.
Often, the products of enzyme-catalyzed reactions are measured in coupled reactions in which the product of the enzyme-catalyzed reaction is the substrate for another enzyme added at high concentration, allowing for the rapid conversion of the product of the first reaction to another product whose concentration can be easily determined.
Interferences with spectrophotometric methods most commonly include hemolysis, bilirubinemia, lipemia, and some drugs. Hematocrit effect and volume displacement are interferences that can affect point-of-care testing as well as main laboratory chemistry analyzers, sometimes with extreme bias.
In many of the preceding chapters, we have discussed the chemical bases for the assays employed for the diagnosis of specific diseases. In this chapter, we present a consolidation of the methods that are used in the typical so-called “metabolic panel” in Clinical Chemistry, a panoply of chemical assays that are often requested for patients undergoing outpatient annual checkups or who are being admitted to the hospital. We summarize and discuss the chemical bases for these assays in a concise manner so that the reader can refer to these methods conveniently and understand the assays and their limitations. Understanding of the assays further enables an understanding of why certain substances interfere with particular assays and can give rise to misleading results. It should be kept in mind that, for many of the assays described here, more than one method can be used. Since it is impossible to cover all variations of methods used to quantitate each analyte, we describe the principles that underlie commonly employed methods and refer, where possible, to alternate approaches.
Metabolic panels consist of serum electrolytes (sodium, potassium, and chloride), bicarbonate, calcium, phosphate, magnesium, blood urea nitrogen (BUN), creatinine, glucose, uric acid, total protein, albumin, total and direct bilirubin, cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL), triglycerides, ammonia, iron and iron-binding capacity, and the enzymes: creatine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LD), γ-glutamyl transferase (GGT), amylase, and lipase. The reader may recognize various panels that measure the functioning of specific tissues, including the kidney, such as the renal “profile” (i.e., BUN, creatinine, calcium, and phosphate). These panels are of great use in diagnosing disease states, as discussed in Chapter 9 .
We divide the basic assays for these analytes functionally into four method-based groups:
Ion-selective electrodes
Spectrophotometric assays based on color complexes for specific molecules: for example, the color noncovalent complex of albumin with bromcresol green dye and bilirubin covalently reacted with diazotized sulfanilic acid to give azo-derivatized forms of the indole rings of bilirubin
Analyte concentrations using enzymes as reagents. In Chapter 21 , we discussed how small molecule substrates are used to determine serum enzyme concentrations. In a similar manner, this process can be reversed; enzymes can be used to determine the concentrations of small or large molecules (like proteins). If an analyte is a specific substrate for an enzyme and if the formation of product or disappearance of the substrate can be conveniently followed, this method can be used quite effectively.
Enzyme assays, that is, use of substrates to determine enzyme concentrations as described in Chapter 21 . Almost all of these assays are also based on spectrophotometric measurements. These depend on the substrates employed in the assays and, in many cases, the need for the use of indicator enzymes in so-called “coupled reactions.”
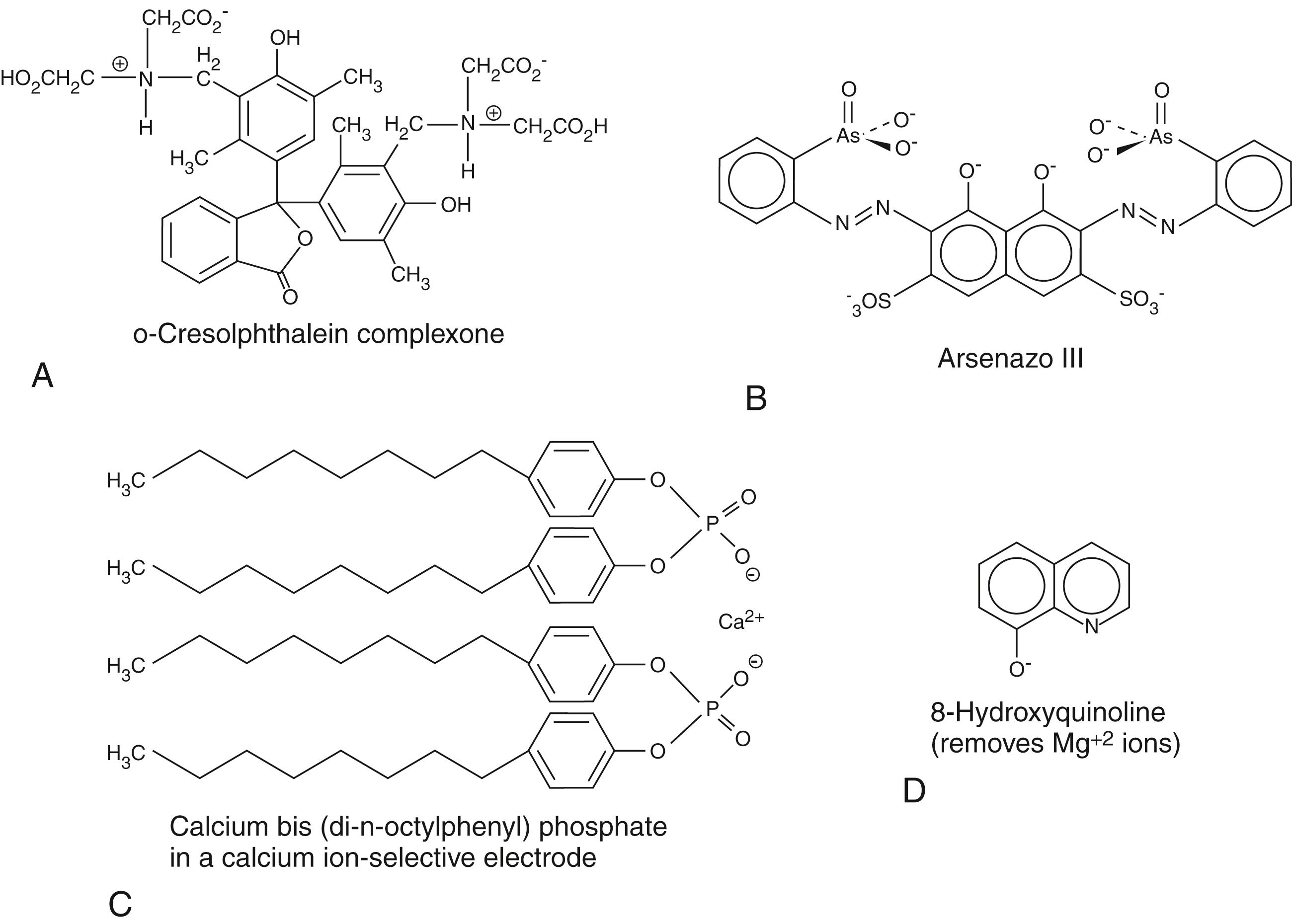
As explained in Chapter 4 , this technique is used to measure the concentrations of specific ions, using an electrochemical method. This method is nonspecific in that it does not discriminate between ions in causing voltage differences between the measuring electrode and the standard electrode. It is the presence of an ion perm-selective membrane barrier on the measuring electrode that allows only a specific ion to pass through and allow contact with the measuring electrode. Each ion requires a different material that will be selectively permeable to this ion and to this ion only. Thus, glass is perm-selective to hydrogen ion. A special polymer of the polyamino acid antibiotic, valinomycin, is perm-selective to potassium ions. A polymer resin of polyvinylchloride (PVC) is perm-selective to sodium ions. Recently, an anodized indium tin oxide membrane was found to be even more perm-selective to sodium ions ( ). Similarly, calcium-chelating organic molecules impregnated into PVC membranes serve as perm-selective membranes for calcium ions. In addition, dioctylphenyl phosphates, as shown in Figure 28.1C , have been found to chelate calcium ions selectively and to serve as effective ion-selective electrodes.
There are three basic causes of malfunctions of ion-selective electrodes. The first and most common is defects in the perm-selective membrane itself. This allows the presence of ions other than the one whose concentration is being measured to be present at the measuring electrode. The result is ion concentrations that are falsely elevated. This defect is almost always detected in routine quality control that shows a strong upward drift in the controls despite frequent calibrations.
The second cause of inaccuracies in ion-selective electrodes is buildup of counter-voltages from liquid junction potentials present at the salt bridge connecting the measuring electrode with the reference electrode. Since these voltages oppose the voltage between the two electrodes, the voltages are diminished, giving lower-than-expected ion concentrations. Buildup of liquid junction potentials occurs over time and affects the lifetime of electrodes. Therefore, it is vital to change electrodes at the times specified by the manufacturer to avoid erroneous results.
The third cause of malfunctioning electrodes is buildup of proteins on the electrodes, resulting in alteration of the voltage between the testing and reference electrodes. This phenomenon gives rise to erratic results and lack of reproducibility of ion concentrations in a single specimen. This problem can be avoided by performing washes of the electrodes at manufacturer-specified times.
Volumetric Effect. Although it is not caused by any malfunctioning of the electrode or ion-selective membrane, the effect of serum (or other body fluid) volumes may cause inaccuracies of results. If a serum sample is directly incubated with the ion-selective membrane and electrode, the concentration of an ion can be accurately determined. This is referred to as a direct determination. However, analyzers commonly dilute the sample with buffer prior to incubation with the electrode in a process referred to as an indirect determination . If the sample contains significant amounts of lipids and/or proteins, the volume of the sample is partitioned into an aqueous phase in which the ions are dissolved and a separate lipid/protein phase. In this case, the addition of buffer to the sample will dilute the whole sample, but the ions are dissolved only in the aqueous phase. This phase has a smaller volume than the volume of the whole sample. Therefore, addition of buffer will dilute the aqueous phase of the sample more. When the ion concentration in the diluted sample is determined, the result must be multiplied by the dilution factor. If the result is multiplied by this factor, it may underestimate the ion concentration in the sample because the dilution factor was for the whole sample and not for the aqueous phase, which requires a higher factor. When this occurs for sodium ion concentration, the artifactually low concentration is referred to as pseudohyponatremia . This occurrence is an uncommon one but can affect the determinations of any analytes where volume partitioning can occur.
These are by far the most common types of assays. We discuss each analyte measured by this method.
We remind the reader that all spectrophotometric determinations are made using the Beer-Lambert Law (see Chapter 4 ), which states that the absorbance, A, of an analyte at a particular wavelength is proportional to its concentration, C, and the length of the light path, L, in the cuvette in which the analyte is dissolved in a solvent (usually water or aqueous buffer), that is,
where ε is the proportionality constant, also called the extinction coefficient .
As we now describe, the spectrophotometric determination of the concentration of an analyte requires the addition of another compound or reagent with which the analyte of interest reacts either covalently or noncovalently. The reagent is always added in excess. The resulting complex absorbs light at a wavelength at which neither the free analyte nor the unbound reagent absorbs or at which each species absorbs only minimally. If the reaction is allowed to proceed to completion and the absorbance is then determined, the method is referred to as an end-point method. In some cases, the rate of formation of the complex is slow.
To avoid long waiting periods, the rate of the reaction over a short time period is determined and is used to compute the concentration of the analyte. In Chapter 21, Chapter 24 , we discussed the order of reactions. In the case of formation of colored complexes, the analyte, A, reacts with the reagent, R, to form the measured product, that is, the color complex, or AR:
The rate equation describing the rate of formation of AR, that is, d(AR)/dt, is
where k is the rate constant for this reaction. This is referred to as a second-order rate process because the rate depends on the concentrations of both A and R. However, since R is added in excess of A, at least initially, the concentration of R does not change appreciably so that it may be considered constant. The reaction then becomes a pseudo–first-order reaction. In this case,
where R 0 is the defined added concentration of reagent so that
where k′ is kR 0 .
Thus, the rate of formation of AR is proportional to the concentration of A. Plots of AR versus time should be linear for the initial time periods (which can range from seconds to minutes). The slope is k′ = kR 0 , whose value is known. Therefore, A must be equal to the slope of the initial straight line divided by k′. This process is quite similar to the one described to compute enzyme concentrations in serum at low substrate concentrations, as described in Chapter 21 .
In the same manner in which we determine the concentration of enzymes in serum by observing the initial rate of the enzyme-catalyzed reaction in the presence of saturating concentrations of substrate, where the concentration of the substrate is known, we can likewise determine the initial concentration of A by measuring the initial rate at which AR forms. This rate is proportional to the initial concentration of A, the desired quantity.
Compounds that interfere with assays for analytes can cause either significantly higher or lower values than the true value. There is great variability in the reasons for interference. For example, for analytes that bind to dyes to form complexes that absorb at unique wavelengths, the presence of exogenous molecules—such as drugs, especially antibiotics, that may also absorb at the same unique wavelengths as for the complexes—will render higher values than for the analyte being assayed alone. The presence of exogenous agents—such as immunoglobulins, that can bind to an analyte being assayed for—may block the binding of the analyte to a dye or to an enzyme in the assay, resulting in artifactually lower values. In some assays involving electron transfers from the compound being assayed to an acceptor molecule, the presence of high levels of even endogenous analytes that can serve as electron acceptors can cause spuriously lower values for the level of the assayed analyte. Fortunately, these types of occurrence are not common. For example, in the case of small molecule interference of the absorbances of color complexes, many of the color complex absorbances are determined at wavelengths higher than 500 nm, where most small molecules—such as drugs, cofactors such as NADH, FADH, pyridoxal phosphate, and so on—do not absorb. However, because these interferences sometimes do occur, they must be acknowledged.
More common as causes of interferences are three types of nonspecific agents: hemolysis, resulting in the release of hemoglobin from lysed red blood cells that can affect the absorbances in certain assays; icteremia from both direct and indirect bilirubin; and lipemia from elevated levels of triglycerides and/or lipoprotein complexes. In general, these three agents cause spuriously elevated results. In the case of lipemia, this elevation is due to light scattering that increases the absorbance of a sample over many wavelengths. As discussed earlier in the ion-selective electrode section and further later, lipemia can also cause a spurious decrease in absorbance due to the fact that most assays involve dilution of serum or plasma. Since elevated levels of lipids in serum or plasma consume significant volumes, errors in estimation of the actual dilutions occur such that the dilution factor is lower than it should be, resulting in computation of spuriously lower values.
Because these three latter agents—hemoglobin, bilirubin, and lipids—are the most common causes of spurious assay results, most commercial assays evaluate each to determine at what concentration interference of a given assay can occur. This involves the addition of different concentrations of the agent to serum that has been assayed for a given analyte in the presence of increasing concentrations of each potential interfering agent. The agents are hemoglobin for hemolysis, bilirubin for icterus, and a lipid preparation called Intralipid, a 20% fat emulsion used to simulate extremely turbid samples. At its highest concentration, Intralipid is the equivalent of approximately 1500 mg/dL serum triglyceride, for lipemia. The results are reported as the highest concentration of each agent assayed for which there was no change in the assay result. No change in the result is usually defined as an assay result that is within ±10% to 12% of the value determined in the absence of this compound.
It should be noted that multiple methods are used to determine analyte levels in body fluids; these methods may differ in sensitivity to potential interfering agents. Thus, it is not possible to discuss interferences for all methods for each analyte. In general, we discuss interferences for the most commonly used methods for each analyte. In addition, there are numerous possible interferences from drugs used to treat different conditions, all of which would consume voluminous discussions. Since these interferences are rare but voluminous, we refer the reader to a compendium of potential drug interferences for further references ( ) and discuss only those that are commonly observed.
The most common point-of-care testing (POCT) is for glucose, done both in hospitals and more extensively for self-monitoring of outpatients. Because medical personnel and patients themselves naturally have a strong belief that the results obtained with their own hands are correct despite potential bias, it is valuable for laboratorians to understand how some common interferences can lead to discrepant and inaccurate results by POCT.
Biologically and medically important measurements of chemical constituents in blood are largely made in serum or plasma as representative of concentrations that perfuse the organs and tissues of the body. POCT typically utilizes specimens of whole blood, which consists of almost one-half cellular elements. The impact of erythrocytes on measurements can be enormous, as they occlude the surface of dipstick-style systems. In this hematocrit effect , higher hematocrits let less plasma pass to react with POCT reagents, leading to falsely low results. Lower hematocrits (more typical of hospitalized patients) allow more plasma to pass, leading to falsely elevated results. Only systems that separate cells from plasma before the plasma moves on to reagent strips can avoid the hematocrit effect.
Patients should calibrate results from their own POCT meter with simultaneous measurements on a plasma or serum specimen performed with standard laboratory analyzers. Any major discrepancy due to hematocrit effect or other phenomenon should be taken into account for a patient to use POCT for long-term self-monitoring.
As stated earlier, another potential source or error in POCT and even main laboratory testing is volume displacement of plasma in a specimen due to extremely high triglycerides. The physiologically important measurement is that in plasma, but high levels of triglycerides can displace the plasma on its path to reagent strips, leading to falsely low results. Similarly high triglycerides can displace plasma or serum in samples aspirated for analysis on a main laboratory instrument. A conventional approach to this problem has been to separate plasma or serum from high triglycerides by ultracentrifugation, although this additional maneuver might not be available at all institutions.
A very successful way to solve the problem of high triglycerides for measurement of electrolytes, including calcium, is to utilize ion-selective electrodes, which directly measure the physiologically active ions. Some instrument configurations use a standard volume aspirate of sample that is diluted in a larger quantity of buffer prior to measurement with ion-selective electrodes. This latter approach is inadequate for specimens with high triglycerides because, as described above, plasma containing the analytes to be measured is displaced in part during the sample aspiration, leading to falsely low results. Especially for calcium measurements, it is important to have available an ion-selective electrode method for use in undiluted specimens.
A new development in diabetes care is continuous glucose monitoring (CGM) for subcutaneous sampling of interstitial fluid every 15 minutes using devices that are mounted on the skin. Results are used for controlling glucose with an insulin pump (e.g., artificial pancreas). These electrochemical devices are still undergoing widespread evaluation for oxidation-reduction interferences, such as from acetaminophen, which can cause markedly false-positive results ( ).
As an internal control for presence of excess reducing substances that can interfere with urine dipstick measurement of glucose, a pad for detecting ascorbic acid has been introduced by some manufacturers. The use of this result is not to determine that the patient has been taking high levels of vitamin C but rather to detect what might be a serious false negative for glucose due to high level of the reducing substance that reacts with hydrogen peroxide at the chromogen step of the glucose detection reagents.
We will now discuss commonly used methods to determine the concentrations of analytes by formation of color complexes.
As noted in Chapter 20 , albumin is the most anionic of the major serum proteins and migrates toward the anode on serum protein electrophoresis. It binds with high affinity to bromcresol green (BCG), a derivative of triphenyl methane, and bromcresol purple (BCP). The reaction with BCG is shown in Equation 28.6 .
The amount of albumin present in the sample is measured as an end-point reaction at 628 nm. A similar reaction with BCP gives a color complex whose absorbance is read at 603 nm. BCG reacts also to some extent with α-1 and α-2 proteins, sometimes tending to overestimate the concentration of albumin. BCP does not react with these proteins; thus, it is considered to be more specific in quantitation of albumin.
Interferences. Lipemic samples (triglycerides >10 g/L), also can result in higher values. Hyperbilirubinemia or hemolysis does not affect the assay since the absorption maximum of the complex is at a wavelength distinct from those at which bilirubin and hemoglobin interfere. Heparin interferes positively with the BCG method. This effect does not occur at therapeutic levels of heparin but rather with specimens collected through a line containing heparin, resulting in high concentration of the drug in the blood specimen. Specimens containing dextran should also be avoided.
As discussed in Chapter 20 , all proteins are polyamino acids linked by peptide (CONH) bonds. These peptide bonds form complexes with cupric ions in cupric sulfate to form a color complex in alkaline solution, the so-called biuret reaction as shown in Equation 28.7 .
Note that this occurs for every four consecutive peptide units. Since most proteins contain at least 150 to 200 amino acid residues, and many of them more than 300 amino acid residues, many of these complexes are formed for each protein. Since there are large numbers of proteins in serum, this reaction is multiplied manyfold, making the biuret method quite sensitive. The absorbance of the colored complex is determined at 540 nm.
Interferences. Hemolysis causes no significant interference. Bilirubinemia has been reported not to cause positive interference at levels of ≤30 mg/dL, although in dog sera, this level was found to be 10 mg/dL. Lipemia causes negative interference. The antibiotics carbenicillin and methylbenzethonium have been found to cause positive and negative interferences, respectively.
As discussed in Chapter 22 , the most common method for measuring serum bilirubin is the Jendrassik-Grof method. In this method, diazotized sulfanilic acid reacts with two pyrrole rings of bilirubin to give two phenylazo adducts, as shown in Equation 28.8 ( ).
In this equation, Me is the methyl group, V is the vinyl group, and P is the 3-substituted propionic acid moiety. Note that, interestingly, electrophilic aromatic substitution occurs on the pyrrole rings such that the central methylene carbon is removed altogether, giving the two sets of phenylazo adducts, which absorb at 600 nm.
As discussed in Chapter 22 , bilirubin is conjugated to glucuronic acid at either or both propionic acid (P) moieties. Unconjugated bilirubin is much less soluble than are the conjugated forms. Most of the unconjugated bilirubin is bound tightly to albumin, making it largely unavailable for reaction with the diazotized sulfanilic acid reagent. However, addition of agents such as ethanol, methanol, or caffeine-benzoate induces the dissociation of unconjugated bilirubin from albumin, allowing it to react with the reagent. Caffeine-benzoate is the preferable agent since methanol and ethanol can induce protein precipitation and increased turbidity. Interference by hemoglobin resulting from hemolysis can also occur. The soluble bilirubins (mostly conjugated, but see later discussion) that react in the absence of dissociating reagents are termed direct bilirubin , while the bilirubin that reacts in the presence of the dissociating agent is total bilirubin . So-called indirect bilirubin (mostly unconjugated bilirubin bound to albumin) is obtained by subtraction of direct bilirubin from total bilirubin ( ). As noted in Chapter 22 , not all conjugated bilirubin is detected as direct bilirubin, and a small amount of unconjugated bilirubin may be detected as direct bilirubin.
Interferences. Studies on Beckman AU analyzers indicate that there is no interference on total bilirubin levels as a result of hemolysis up to 25 mg/dL hemoglobin in serum and, as a result of lipidemia, up to 500 mg/dL of Intralipid.
Most methods rely on the formation of a color complex of Ca +2 ions with an appropriate chelating agent with which it forms a color complex. Since approximately half of total calcium is bound to protein, mainly to albumin, lowering the pH of the sample liberates calcium ions. The calcium ions are then reacted with a strong chelating compound with which they form a color complex. The two major calcium chelating compounds that are currently used are ortho-cresolphthalein (forms a red-colored complex) and Arsenazo III ( ; ), whose structures are shown in Figures 28.1A and 28.1B , respectively. The reaction is shown in the following equation:
where dye is either ortho-cresolphthalein or Arsenazo III.
Use of ortho-cresolphthalein is often accompanied by a further step in which the reaction solution is treated with the compound 8-hydroxyquinoline, whose structure is also shown in Figure 28.1D , at alkaline pH to prevent any possible complex from forming with magnesium ion. The amount of complex that forms is rather strongly dependent on the temperature, which must be carefully maintained. At low calcium concentrations, a one-to-one complex forms, whereas at higher calcium concentrations, two moles of calcium bind to one mole of dye. Since the extinction coefficients of mono- and di-calcium dye complexes differ, the absorbance is nonlinear with respect to calcium concentration. However, use of a modified Beer’s law equation can be employed to compute concentration from absorbance; nonlinear methods can likewise be used for this purpose. The only major potential interfering compound for this assay is magnesium ion, which can be removed with 8-hydroxyquinoline, as noted earlier.
Arsenazo III has a high affinity for calcium ions, but the amount of complex formed is strongly pH dependent, Usually, imidazole buffers at pH 6.0 are employed for this reaction. Importantly, citrate, which is a tricarboxylic acid that can chelate calcium ions, has been found to interfere with this reaction. It has been found that determination of absorbance at 650 nm is the optimal wavelength for determination of the concentration of calcium ion complex with Arsenazo III at which there is little interference by other analytes, other than in hemolyzed samples.
Since calcium can be released from its normally bound states by acidification, addition of acid to a serum sample will convert all calcium to readily measurable ionic forms. Thus, total calcium can be measured voltammetrically. The electrode is a specific calcium ion exchanger of which there are several types, including ETH 1001 and bis-di-(n-octylphenyl) phosphate. The structure of the latter is shown in Figure 28.1C ( ). Ionized calcium (i.e., calcium that is not bound to protein or in complex formation with strong chelators) can be measured directly with ion-selective electrodes without the prior acid treatment step. As with other ion-selective electrode methods, protein that can coat the electrodes can cause interference.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here