Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cerebellar hemorrhage has been increasingly detected in the fetus and the preterm and full-term newborn in recent years. Currently, our understanding of cerebellar hemorrhagic injury and its long-term neurological sequelae has changed significantly, in large part because of increasing survival of critically ill infants and the greater availability and application of magnetic resonance imaging (MRI). Early studies reported a prevalence of cerebellar hemorrhage as high as 25% in premature infants at autopsy, usually associated with complicated births and a striking clinical presentation. Given the relative insensitivity to posterior fossa structures of cranial ultrasonography (US) through the anterior fontanel approach, it was not until the advent of focused mastoid foramen views that an unexpectedly high incidence of cerebellar lesions was noted in living premature infants (see later).
Cerebellar hemorrhage in the fetus was previously considered a rare event. The successful application of fetal MRI has increased the frequency of prenatal diagnosis of cerebellar hemorrhages. However, the exact prevalence of prenatal cerebellar hemorrhagic lesions remains largely unknown; they are detected in about 6% of fetuses with prenatal red blood cell (RBC) transfusions for fetal anemia. The majority of fetal cerebellar hemorrhages occur between 21 and 26 weeks’ gestation. A recent study reported that fetal cerebellar hemorrhage (12.8%) was the most common cause of intracranial hemorrhage outside of the ventricular system.
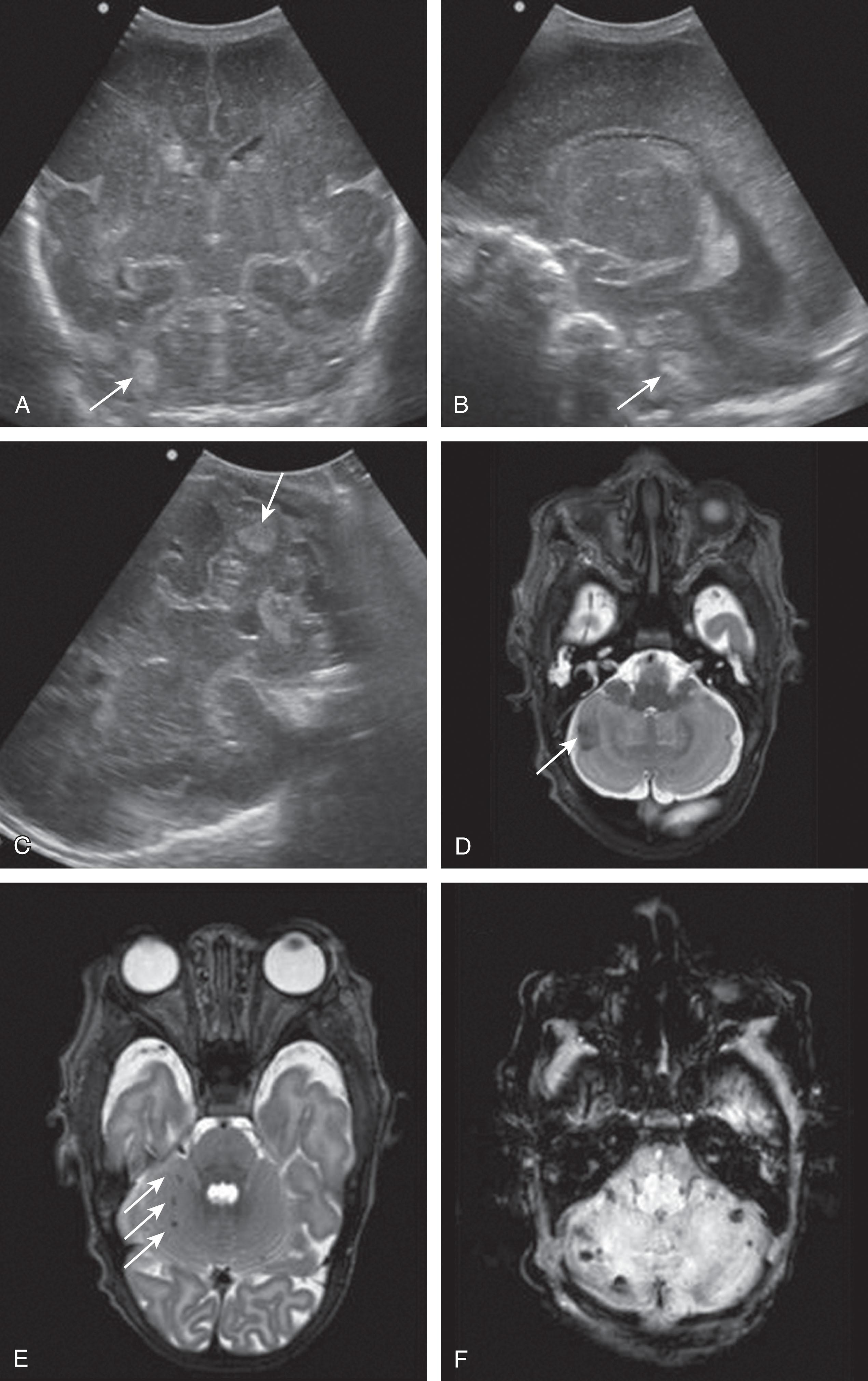
Fetal MRI studies suggest that the most prevalent location of cerebellar hemorrhage is in the lateral and caudal portions of the cerebellar hemispheres in about two-thirds of cases, with an equal frequency of occurrence of unilateral versus bilateral cerebellar involvement ( Fig. 27.1 ). Martino and colleagues have suggested a venous origin, similar to supratentorial germinal matrix hemorrhage–intraventricular hemorrhage, associated with deep venous system engorgement due to different systemic conditions. Postnatally, fetal cerebellar hemorrhage is characterized by focal volume reduction of the cerebellar hemisphere(s) and distortion, which, importantly, may also mimic cerebellar malformation. It is important to differentiate between cerebellar anomalies that are genetic (malformations) and acquired (postclastic disruptions) for clinical management of the pregnancy and prognostication.
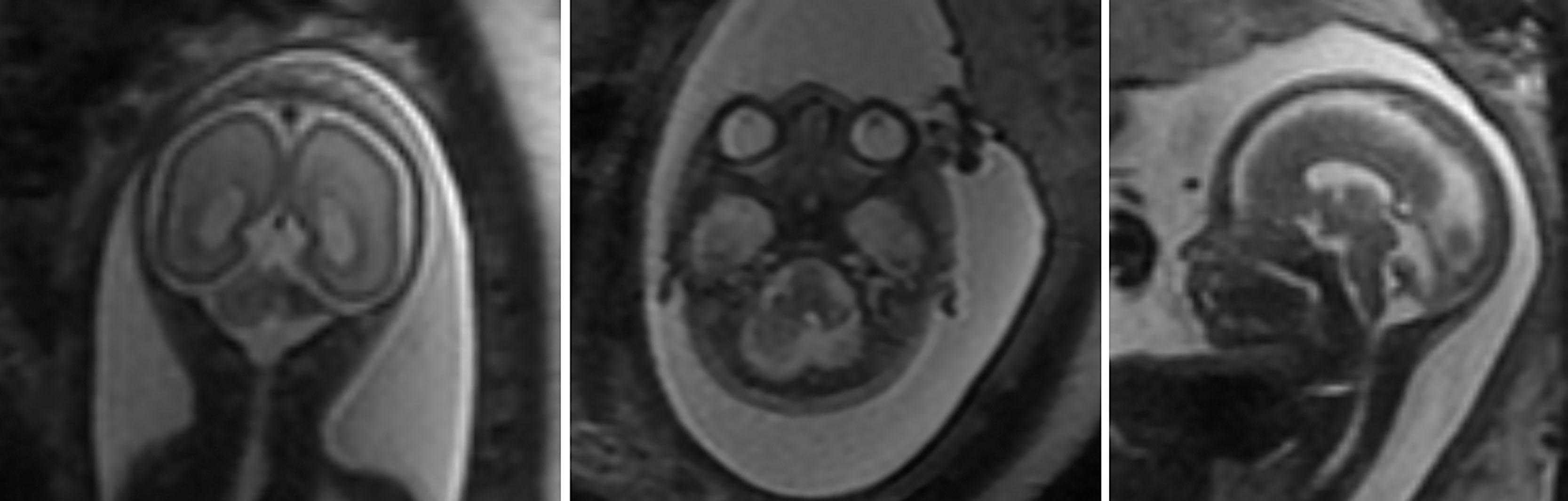
The pathogenesis of fetal cerebellar hemorrhage remains unclear, although it is likely that the fetal cerebellum shares the same maturational vulnerability as that of the premature infant. In the premature infant, immaturity of other physiological systems, especially the cardiorespiratory system, as well as premature extrauterine environmental stressors, including iatrogenic factors, appear to play a role in cerebellar hemorrhage (discussed later). Determining the maturational competency of physiological systems in the individual fetus, especially with regard to systems that compensate for circulatory insults, is not yet possible in the human fetus. However, data from fetal sheep suggest that compensatory responses only begin to emerge at approximately 0.6 to 0.7 gestation. These ages are broadly equivalent to the neural maturation of the human fetus at 26–28 and 28–32 weeks gestation respectively. If these findings apply to the human fetus, it is likely that during the second trimester, when most fetal cerebellar hemorrhages are identified, the cerebrovascular compensatory responses are immature and might leave the fetus vulnerable to cerebellar hemorrhage.
Given the relatively larger volume of amniotic fluid during the second trimester, the free-floating umbilical cord, and increasing fetal movement, it is likely that transient episodes of cord compression occur more commonly than appreciated and go undetected because of the inaccessibility of the fetus. Such circulatory fluctuations may present similar hemodynamic insults as those implicated in the pathogenesis of cerebellar hemorrhage in premature infants. Cerebellar hemorrhages have been reported after fetal RBC transfusions for severe alloimmune fetal anemia, the causes of which are likely multifactorial but may include vascular injury due to anemic hypoxia, as well as impaired cerebral vasoregulation.
The prognosis of fetal cerebellar hemorrhage, based initially on case reports with a high proportion of cases of termination of pregnancy and stillbirth/fetal demise, was considered unfavorable. More recently, available data suggest overall favorable outcomes in isolated cerebellar hypoplasia of suspected hemorrhagic etiology, with about one-third of infants presenting later with mild to moderate neurological dysfunction, including language disorders (e.g., mild dyspraxia), visuo-perceptual problems, and social-behavioral deficits, including autism spectrum disorder in one child. Long-term follow-up studies are needed and should include postnatal brain MRI and standardized neurodevelopmental assessments through school age.
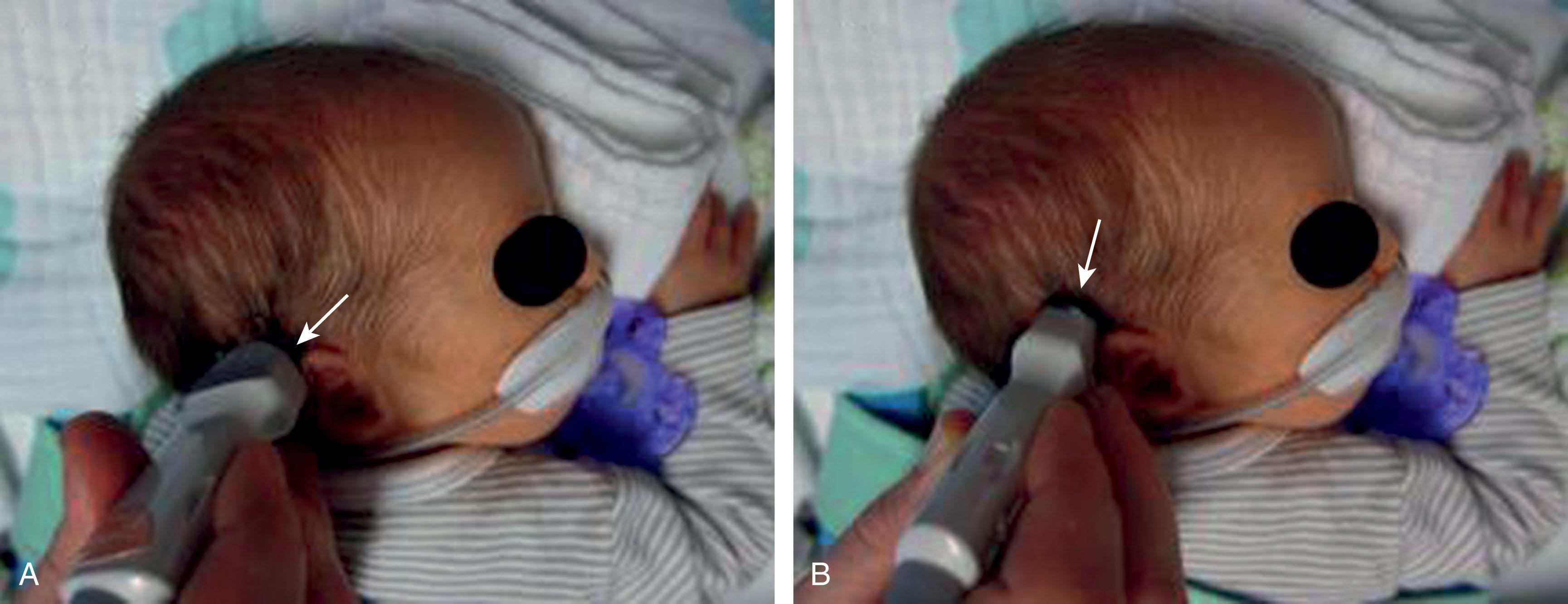
Cerebellar hemorrhage is now recognized as an important complication of premature birth and is more common among premature than term infants. Older neuropathological series reported an incidence among premature infants less than 32 weeks’ gestation or less than 1500 g birth weight, or both, ranging from 15% to 25%. Flodmark and colleagues observed 15 cases of cerebellar hemorrhage at autopsy (all in premature infants) from 79 infants studied by computed tomography (CT) for perinatal asphyxia. Among these 15 autopsy-proven cases, the hemorrhage was identified by CT in only one. (Ten of the lesions were considered to be “probably below the resolving power of the CT scanner.”) Similarly, in infants evaluated by US through the anterior fontanel, the identification of cerebellar hemorrhage is unusual. The advent of imaging through the mastoid fontanel, the thinnest region of the temporal bone at the junction of the squamosal, lambdoidal, and occipital sutures, has greatly facilitated accurate imaging of the posterior fossa in the newborn ( Figs. 27.2 and 27.3 ). Recent studies using this mastoid window report a high incidence of cerebellar hemorrhage in preterm infants. The largest reported study to date, based on use of the mastoid window in the evaluation of 1242 infants weighing less than 1500 g, showed an overall incidence of cerebellar hemorrhage of approximately 3% ( Table 27.1 ). However, the incidence varied markedly as a function of birth weight. Infants of less than 750 g birth weight had the highest incidence (i.e., 8.7% overall, and notably, 17% in the last 2 years of the 5-year study) . Infants between 750 and 1499 g exhibited an overall incidence of approximately 2.7%. Indeed, in this large cohort, nearly 60% of all cerebellar hemorrhages were in the infants who weighed less than 750 g. Thus cerebellar hemorrhage is particularly a lesion of the most immature infants .
| BIRTH WEIGHT | INCIDENCE a |
|---|---|
| <750 g | 20/230 (8.7%) b |
| 750–999 g | 4/602 (0.7%) |
| 1000–1499 g | 11/410 (2.7%) |
| Total | 35/1242 (2.8%) |
a Incidence is expressed as n / N (%).
b Incidence in the last 2 years of the 5-year study period was 17%.
More recent data suggest that cerebellar hemorrhage in the preterm infant is even more prevalent, described in up to 19% of preterm infants of less than 32 weeks’ gestation. The addition of the mastoid window in cranial US improved the visualization and detection of cerebellar hemorrhage compared with detection by the anterior and posterior fontanel US views alone. With the mastoid view, abnormalities in the posterior fossa were detected in 71% of preterm infants but were missed using the anterior or posterior fontanel views. Notably, approximately half of small hemorrhagic cerebellar lesions (e.g., punctate hemorrhages) were undetected, even when the mastoid fontanel approach was used. Recently, a suboccipital view through the occipital foramen magnum has been proposed as this approach offers higher resolution of the cerebellar parenchyma, thereby allowing the detection of smaller lesions and the ability to visualize and compare both cerebellar hemispheres simultaneously.
When MRI is routinely performed in very preterm infants (<32 weeks’ gestational age [GA]), the incidence of cerebellar hemorrhage is still higher than with US , ranging from 15% to 37%. Moreover, some cases may be undetected if specific MRI sequences (e.g., susceptibility-weighted imaging) are not performed. With the latter approach and 3T-MRI, 37% of preterm newborns less than 33 weeks’ gestation had cerebellar hemorrhage identified ( Table 27.2 ).
| Cranial ultrasound (CUS) (with mastoid view) | 3%–9% a |
| Magnetic resonance imaging (MRI) | 15%–37% a,b |
| Sensitivity/specificity of routine CUS relative to MRI | 18%/100% c |
| Positive/negative predictive value of routine cranial ultrasound | 100%/84% c |
| Sensitivity/specificity of additional cranial ultrasound views relative to MRI | 45%/100% c |
| Positive/negative predictive value of additional cranial ultrasound views | 100%/89% |
a Data from Staggerda SJ, van Wezel-Meijler G. Cranial ultrasonography of the immature cerebellum: role and limitations. Semin Fetal Neonatal Med . 2016;21:295–304.
b Data from Gano D, Ho ML, Partridge JC, et al. Antenatal exposure to magnesium sulfate is associated with reduced cerebellar hemorrhage in preterm newborns. J Pediatr . 2016;178:68–74.
c Data from Staggerda SJ, Leijser LM, Wiggers-de Bruïne FT, van der Grond J, Walther FJ, van Wezel-Meijler G. Cerebellar injury in preterm infants: incidence and findings on US and MR imaging. Radiology . 2009;252:190–199.
Neuropathology studies have reported that the principal locus of cerebellar hemorrhage is the ventral aspect of the posterior lobe of the cerebellum and vermis. The cerebellar hemorrhages tend to be bilateral and involve the vermis (74%). Microscopically, the hemorrhages are located in the white matter or in the cerebellar cortex near the junction of the white matter and the internal granule cell layer and are associated with germinal matrix hemorrhage (95%) and pontosubicular necrosis (69%). More than half also had neuronal loss/gliosis in the inferior olivary nucleus and the dentate, which likely represents a transsynaptic degenerative process ( Fig. 27.4 ).
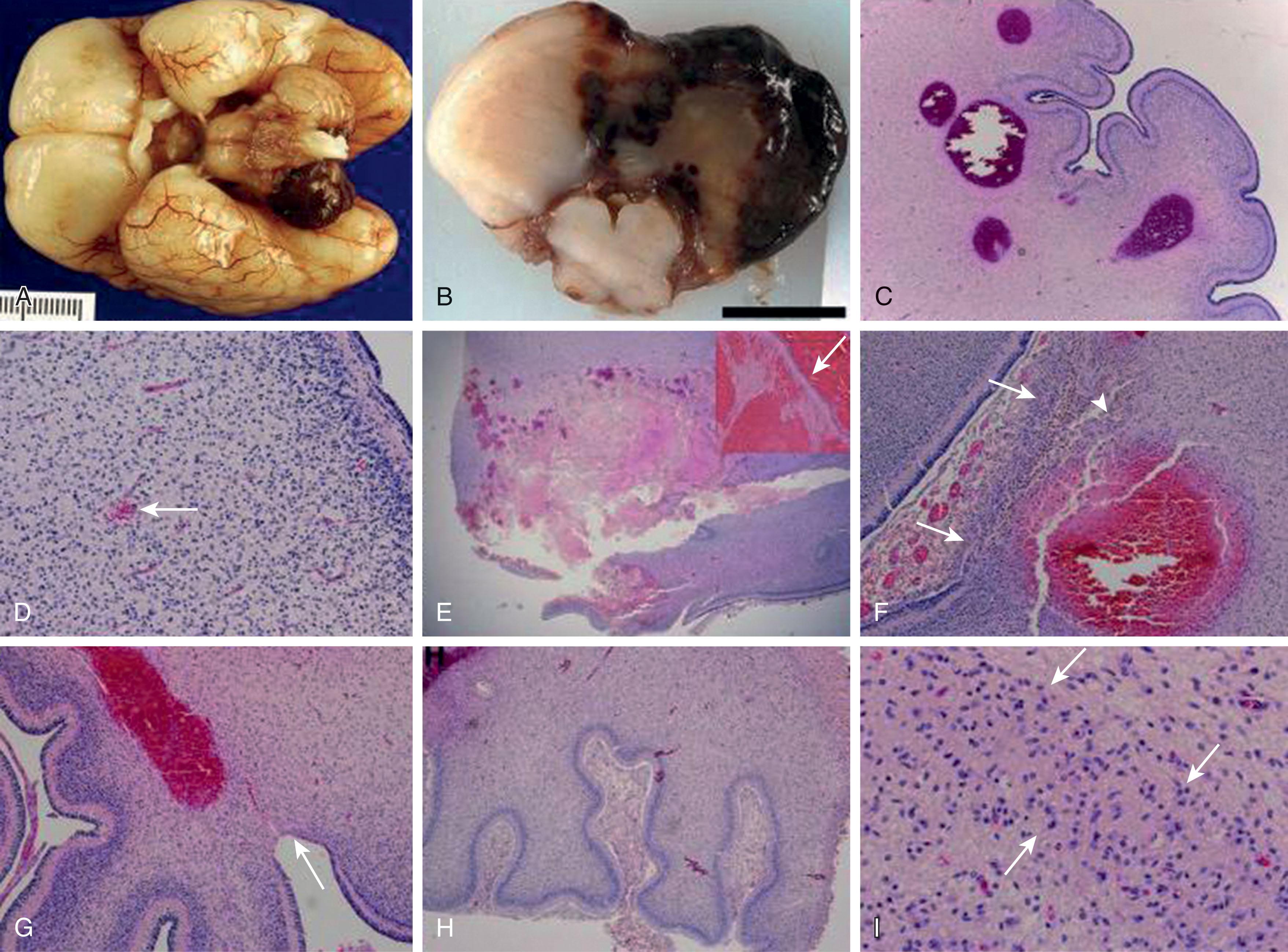
Four major categories of lesions have been described in infants with cerebellar hemorrhage ( Table 27.3 ). Primary cerebellar hemorrhage likely accounts for most cases of cerebellar hemorrhage in the preterm infant.
| Primary cerebellar hemorrhage |
| Venous (hemorrhagic) infarction |
| Extension into cerebellum of intraventricular or subarachnoid blood or both |
| Traumatic laceration of cerebellum or rupture of major veins or occipital sinus (with or without occipital diastasis) |
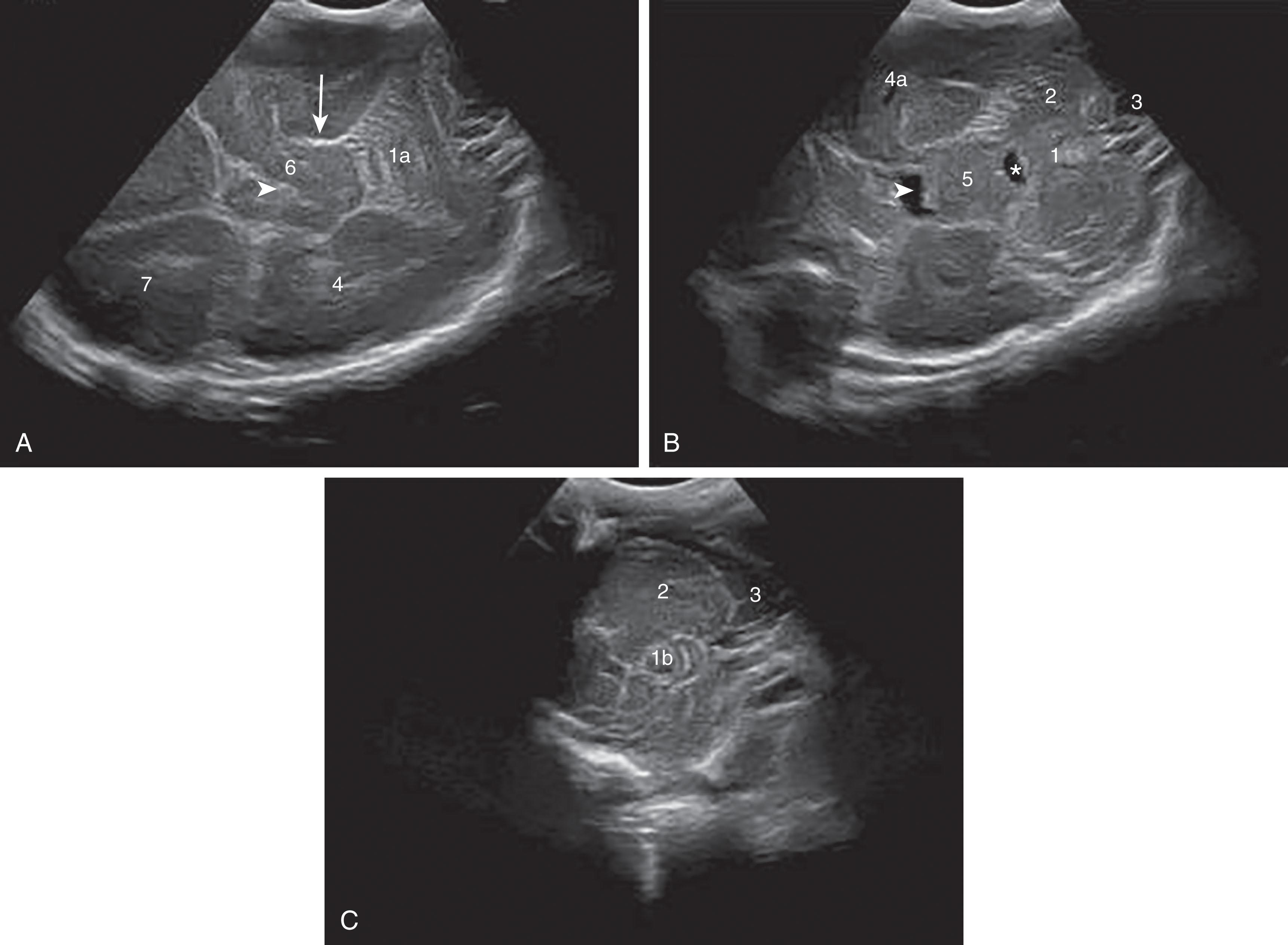
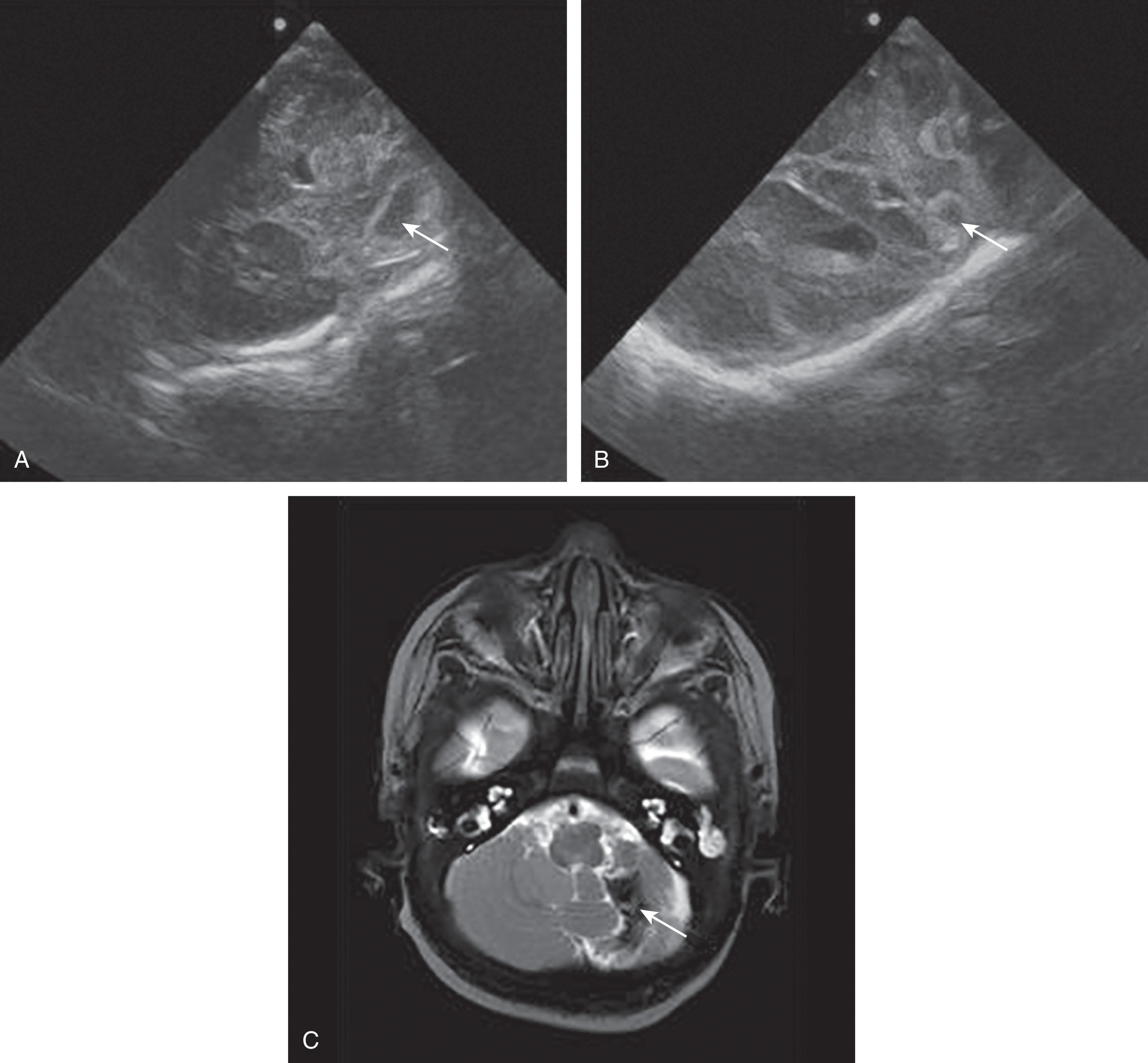
Primary cerebellar hemorrhage is the best studied of the destructive cerebellar lesions in premature infants ( Table 27.3 ). There is a broad spectrum in the severity of cerebellar hemorrhages reported, ranging from mild punctate lesions, focal unilateral lesions, to the less common and more extensive bihemispheric and vermian hemorrhages ( Figs. 27.5 to 27.7 ). The more extensive lesions range from partial inferomedial hemorrhage to near-total destruction of the cerebellum and may be associated with pontine hypoplasia on subsequent MRI ( Fig. 27.8 ). More recently, large cerebellar hemorrhages are reported less commonly. This decline may be related to improvements in neonatal intensive care, whereas punctate cerebellar hemorrhages have been reported increasingly on MRI. These punctate cerebellar hemorrhages are primarily identified by MRI and measure less than 4 mm in diameter on any given imaging plane. A cerebellar hemorrhage grading scheme has been proposed ( Table 27.4 ). The hemorrhages are followed by cerebellar atrophy, detectable about 2 months later in 37%. Atrophy is focal in the unilateral lesions and more generalized in the bilateral lesions. Notably, reductions in contralateral cerebral volumes have been defined and likely reflect impaired remote transsynaptic trophic effects (described later).
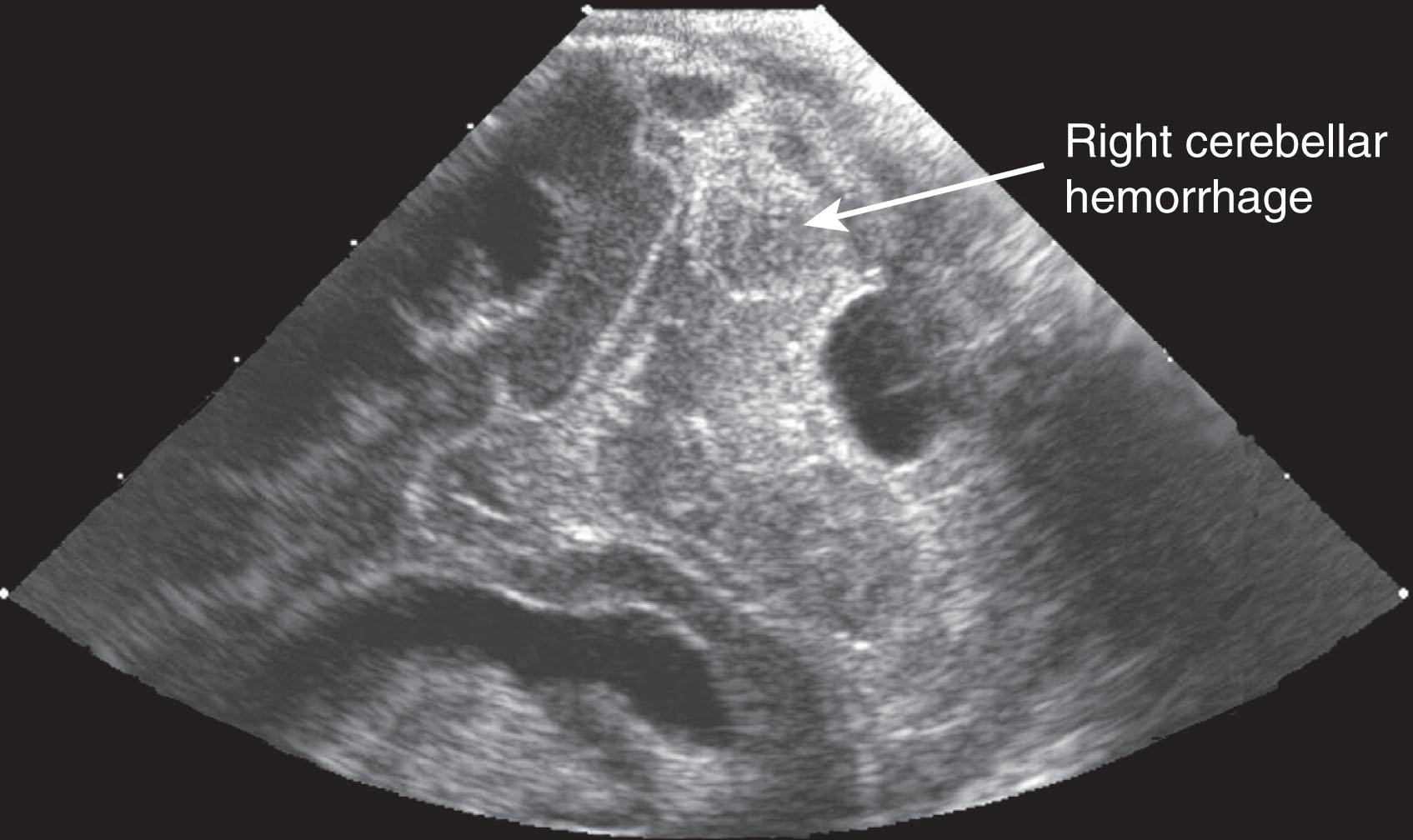
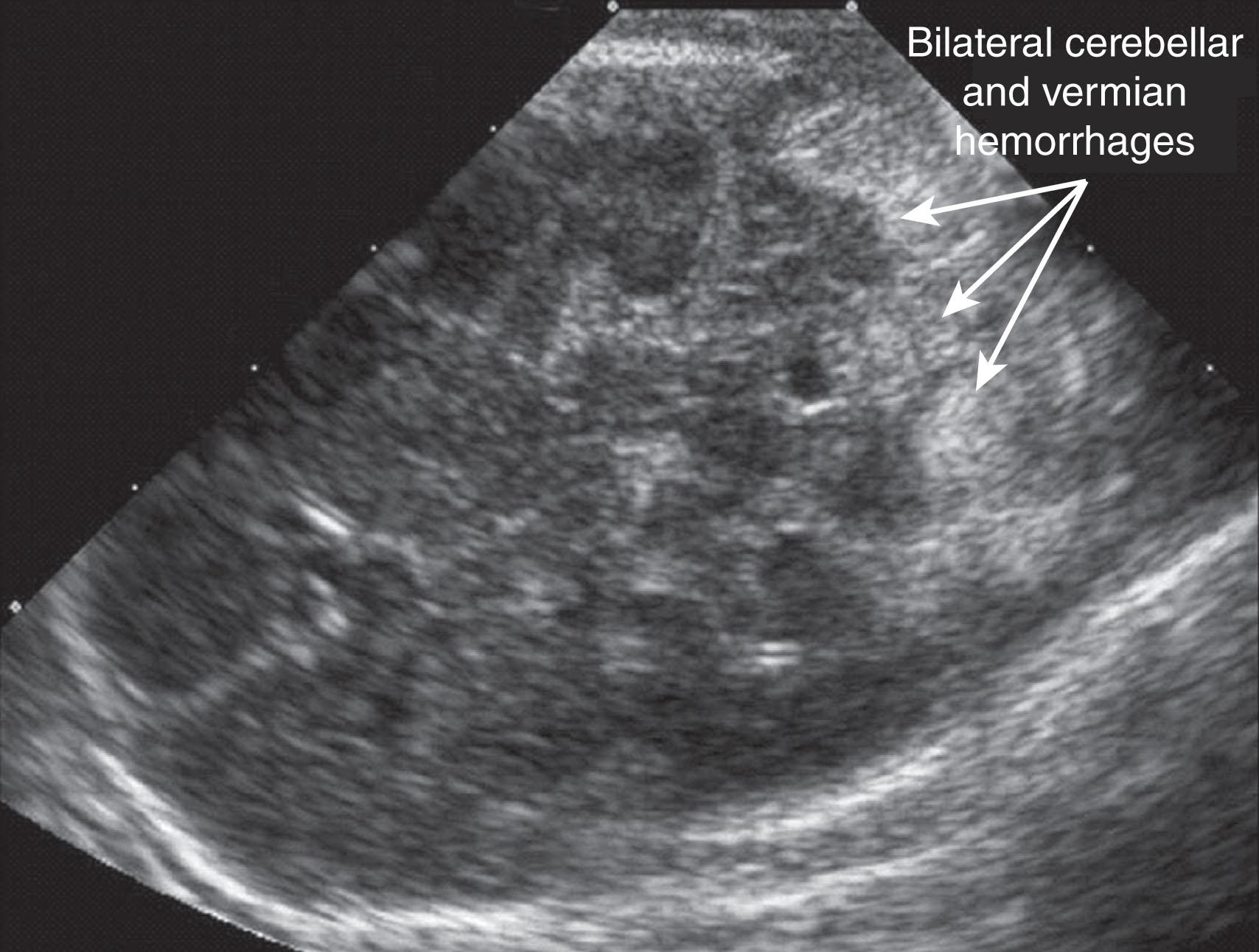
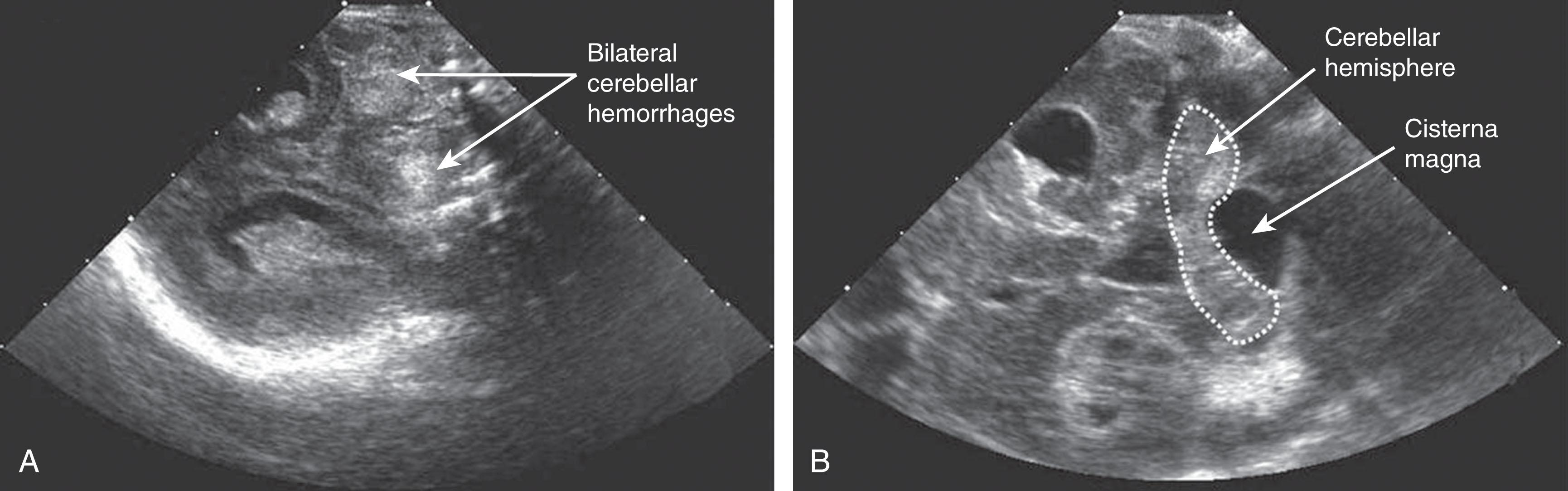
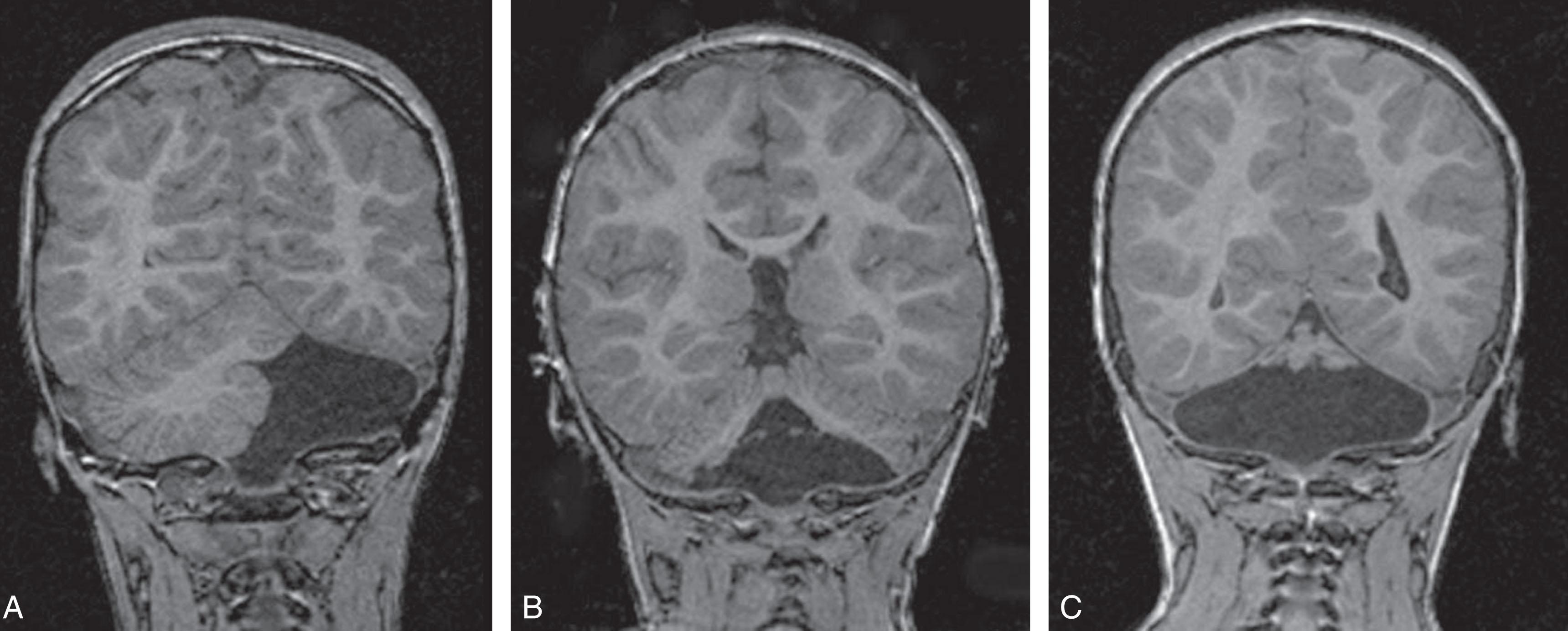
| Grade 1: Unilateral small punctate lesions (≤3 mm) |
| Grade 2: Bilateral small punctate lesions (≤3 mm) |
| Grade 3: Extensive unilateral lesions (>3 mm) |
| Grade 4: Bilateral extensive lesions (>3 mm) |
Cerebellar infarction is a recognized complication of extreme preterm birth and a potential cause for cerebellar hemorrhage ( Table 27.3 ). Distinction of hemorrhagic venous infarction from primary cerebellar hemorrhage can be very difficult, even at microscopic examination. Both entities likely share a similar pathogenetic mechanism, underlying the vessel walls’ immaturity as a result of rapid angiogenesis. Cerebellar infarcts typically involve the bilateral inferior parts of the cerebellar hemispheres, suggesting a vascular distribution in the territory of the posterior inferior cerebellar arteries. The hypoxic-ischemic insult leads to a loss of neurons in the internal granule cell layer, resulting in focal atrophy of the cerebellar parenchyma. Cerebellar infarction often occurs in combination with supratentorial white matter injury, suggesting a more generalized hypoxic-ischemic insult.
Extension of blood from intraventricular or subarachnoid spaces has been suggested as a cause of cerebellar hemorrhage ( Table 27.3 ). This notion was raised particularly by the studies of Donat and colleagues. In 10 of their 20 cases of cerebellar hemorrhage, secondary dissection of blood into the cerebellum appeared to occur either from the fourth ventricle into the vermis or, less frequently, from the subarachnoid space into the cerebellar hemispheres. In these cases, massive hemorrhage into the lateral ventricles was the original source of the blood. This notion is supported by observations of a strong association of cerebellar hemorrhage with cerebral intraventricular hemorrhage (IVH). (Indeed, in our aforementioned study of 1242 premature infants who were less than 1500 g of birth weight and studied in vivo, about two-thirds of cases were associated with IVH.) Similarly, neuropathological studies also report that cerebellar hemorrhage is accompanied by IVH in 95% of cases. Hemosiderin deposition on the cerebellar surface from extraaxial blood can have toxic effects and result in injury to underlying structures (e.g., the external granule cell layer) and may impair the immature and rapidly developing cerebellum ( Chapter 4 ). However, a neuropathology report found no evidence for dissection of blood from the fourth ventricle or from the subarachnoid space that originates from IVH. Similarly, in a detailed MRI study of 73 preterm newborns with cerebellar hemorrhages, 70% (51/73) had no IVH and only 10% (7/73) had severe IVH.
The fourth potential mechanism of cerebellar hemorrhage, traumatic injury , with laceration of cerebellum or with rupture of cerebellar bridging veins or occipital sinus, often occurring with occipital osteodiastasis ( Table 27.3 ), was discussed in Chapter 26 . In the preterm infant, the ventral posterior loci of cerebellar hemorrhage is in a distribution that corresponds to the posterior inferior veins that drain the inferior cerebellar hemispheres into the transverse sinus, and the inferior vermis into the confluens. The compliant skull of the preterm infant would render it vulnerable to compression of the occipital region by external forces, displacing the squamous portion of the occipital bone and distorting the venous sinuses at their confluence, thereby increasing venous pressure, and preferentially affecting the ventral cerebellum. This mechanism of injury raises the possibility that cerebellar hemorrhage can arise from a venous source.
The neuroimaging of the loci of the hemorrhages within the cerebellum in the small premature infant indicates origins in both the hemisphere and the vermis. The lesions tend to be focal and localized. Approximately 70% of the lesions are localized to one cerebellar hemisphere, and 20% are localized to the vermis ( Table 27.5 and Table 27.6 ). Smaller lesions have included both subpial and subependymal locations, which are the sites of the germinal matrices in the external granule cell layer and subependymal zones, respectively. More recently, very small or punctate cerebellar hemorrhagic lesions have been reported on MRI ( Figs. 27.9 and 27.10 ). The more widespread use of MRI and newer MRI techniques, such as susceptibility-weighted imaging, have increased the detection of these punctate lesions, which are difficult to detect by cranial US. In large lesions, the cerebellar cortex and underlying white matter are destroyed. Notably, the neuropathological study described earlier by Haines and colleagues showed that cerebellar hemorrhage was multifocal with lesions of variable size and histopathological age, findings suggesting that cerebellar hemorrhage may develop as a series of recurrent hemorrhagic episodes occurring over a period of time.
| LOCUS | OCCURRENCE (%) |
|---|---|
| Unilateral hemisphere | 71 |
| Vermis | 20 |
| Both hemispheres and vermis | 9 |
| Isolated a | 23 |
| Intravascular factors |
| Pressure-passive cerebellar circulation/ischemia |
| Increased venous pressure (compliant skull) |
| Disturbed coagulation (e.g., maternal cocaine abuse, fetal alloimmune thrombocytopenia) |
| Vascular factors |
| Tenuous vascular integrity |
| Involuting vessels: subependymal and subpial germinal matrices |
| Vessels in the internal granule cell layer |
| Hypoxia-ischemia |
| Extravascular factors |
| Direct external effects on cerebellar parenchyma and vessels (compliant skull) |
| Poor vascular support: subependymal and subpial germinal matrices |
| Extension from intraventricular hemorrhage |
The pathogenesis of cerebellar hemorrhage is undoubtedly multifactorial but can be considered in three broad categories involving intravascular, vascular, and extravascular factors ( Table 27.6 ). Although there may be considerable overlap of these factors in the pathogenesis of cerebellar hemorrhage in the fetus and the premature and term infant, it is likely that maturational factors are more prominent in the fetus and premature newborn. In the cerebellum of the fetus and preterm infant, the pathogenesis bears similarities to that of IVH ( Chapter 28 ). In the term infant, the pathogenesis appears to relate principally to hypoxic-ischemic, traumatic, or coagulopathic factors. Those factors playing a more prominent role in term cerebellar hemorrhage are discussed later.
As discussed in Chapters 16 and 19 , the cerebral circulation of the newborn, especially the sick preterm newborn, is pressure passive. A pressure-passive cerebral circulation potentially exposes the newborn brain to injury with the risk of vascular rupture during periods of hypertension and after periods of hypotension. If the pressure-passive state of the cerebral circulation also affects the cerebellar circulation, which is likely, then vulnerable capillaries (see Vascular Factors section later) can be exposed to bursts of arterial pressure flow, caused by hypertensive spikes, infusions of colloid, and so forth (see also Chapter 28 ). Rupture is a potential result. Notably, the autoregulatory capacity of the cerebellum is even narrower than that of the cerebrum, and ischemia may develop. Episodes of cerebral ischemia might also cause vascular injury ultimately leading to hemorrhage upon reperfusion. In one large series, several pathogenetic factors that are potentially consistent with the occurrence of ischemia included a relation to fetal distress, the need for an emergency cesarean section, the requirement of pressor support, patent ductus arteriosus (PDA), and low pH in the early neonatal period (relative to matched controls without cerebellar hemorrhage). More contemporary studies and a recent meta-analysis have identified similar risk factors, including fetal heart rate abnormalities, intubation at birth, high-frequency oscillation ventilation, mechanical ventilation greater than or equal to 7 days, hypotension, the need for volume expanders, high-dose caffeine, sepsis, PDA, necrotizing enterocolitis, bronchopulmonary dysplasia, and grade 3 to 4 IVH. Together, these factors reflect increased cardiorespiratory morbidity as a broad predisposing factor for cerebellar hemorrhage in preterm infants. Unique risk factors that appear to predispose to cerebellar hemorrhage versus IVH include multiple gestation, chorioamnionitis, and emergent delivery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here