Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The cerebellum, a unique brain structure with distinctly organized neuronal circuits, is critical for motor and cognitive functions. The cerebellum has dense neuronal connections with almost all regions of the cerebral cortex and brainstem, and can serve as a “hub” to regulate the many brain functions. While cerebellar dysfunction has been implicated in tremor, dystonia, and autism, the prototypical disorder of the cerebellum is cerebellar ataxia, a clinical sign that can have a variety of causes, including nutritional deficiency, immunological dysfunction, vascular and degenerative etiologies, and genetic mutations. Searching for genetic causes for ataxia is particularly relevant because the genetic mutations for ataxia often have very high penetrance; therefore, genetic identification for cerebellar ataxia is often diagnostic. In addition, there are many genetic mutations associated with cerebellar ataxia, which indicates that these genetic mutations converge at the dysfunction of the cerebellar circuitry, highlighting the complex biological processes required to maintain the integrity of this brain structure. The diagnosis of cerebellar ataxia is often regarded as very complicated by neurologists. To simplify and streamline the search for the causes of cerebellar ataxia, this chapter aims to provide a step-by-step approach. In brief, the first step is to recognize the signs and symptoms for ataxia and associated neurological features. The second step is to search for the structural, nutritional, and immunological causes of ataxia. If genetic ataxias are considered, repeat expansions needed to be determined before genetic sequencing for mutations because repeat expansion–associated ataxias are much more common, and more difficult to detect using conventional sequencing technologies. Finally, degenerative etiologies are likely the causes for ataxia onset at an old age. Of note, a significant portion of ataxia patients might eventually have no identifiable causes during life; these patients usually follow a slowly progressive clinical course. Complex environmental and genetic interactions, epigenetic alterations, or regional genetic somatic mosaicism might explain some of these cases; these remain underexplored areas in cerebellar ataxia.
This chapter describes clinical features, imaging findings, and genetics for the differential diagnoses of cerebellar ataxia, providing a guide for clinicians. However, the detailed genetic diagnosis of cerebellar ataxia can be very extensive, and is beyond the scope of this chapter. Instead, this chapter only includes the common causes of genetic ataxia.
The motor part of the cerebellum receives sensory inputs from the outside environment to calculate the proper movements in response. These sensory inputs could be either from tactile sensory nerves or from the vestibular system; therefore, the dysfunction of these systems is sometimes difficult to distinguish from the primary problems of the cerebellum. The current understanding of how the cerebellum integrates sensorimotor information is based on Marr-Albus-Ito theory, in which the cerebellum can function as a neuronal learning machine ( ). This theory is based on the physiological recording and anatomical connections of the cerebellar circuitry that are capable of altering synaptic strength in responses to motor learning. Dysfunction of the cerebellar circuitry thus results in erroneous motor learning and improper motor predictions and commands, leading to symptoms of ataxia and/or tremor.
The same principles that hold for the motor cerebellum can be applied to nonmotor regions of the cerebellum, which have many connections to frontal, parietal, and temporal areas of the cerebral cortex. Therefore, dysfunction of the nonmotor cerebellum has been postulated to cause inappropriate prediction of emotional and cognitive responses; this is known as cerebellar cognitive affective syndrome ( ). Further studies in physiology and anatomy of the cerebellum will provide a more comprehensive understanding of cerebellar function and will accelerate therapy development for cerebellar ataxia.
Recognizing the early symptoms of cerebellar ataxia is an important first step in establishing the symptom onset and the chronicity of the disease. Table 23.1 lists the common early symptoms of cerebellar ataxia. The first symptom is usually gait difficulty ( ), which can manifest as “walking as if one is drunk,” difficulty in running, turning, walking on high heels, and walking up or down stairs without holding on to the railings. These symptoms could be intermittent in the very early stage and patients might experience these symptoms only after ingestion of small amount of alcohol. Later on, these symptoms could become constant. Beyond abnormal gait, slurred speech, from occasional word pronunciation difficulty to persistent speech problems, is often encountered.
Early Signs and Symptoms
Late Signs and Symptoms
|
Tremor of the hands is also commonly experienced by patients with ataxia ( ). Clumsiness and bad handwriting are sometimes described by patients. Dizziness (vertiginous or nonvertiginous) is another symptom associated with cerebellar ataxia. Double vision, particularly when patients turn their heads quickly, is also another common symptom.
In the later stages of cerebellar ataxia, patients might experience falls, swallowing difficulty, blurry vision, and loss of hand dexterity in performing daily activities such as dressing and using utensils.
After establishing the symptoms of cerebellar ataxia, the next step is to determine the chronicity of these symptoms (acute vs. subacute vs. chronic) and the rate of progression, which will be important for the differential diagnosis ( Table 23.2 ). In acute-onset cerebellar ataxia, infectious, vascular, and toxic causes need to be considered. For subacute-onset cerebellar ataxia, immune-mediated etiology would be on top of the differential diagnoses. Genetic and degenerative cerebellar ataxias usually have insidious onset with progressive clinical courses. Another category is episodic cerebellar ataxia, which encompasses various causes (see Table 23.2 ).
Acute Causes of Cerebellar Ataxia (Minutes to Few Days)
Subacute Causes of Cerebellar Ataxia (Weeks to Months)
Chronic Causes of Cerebellar Ataxia (Months to Years)
Episodic Causes of Cerebellar Ataxia
|
Besides recognizing the symptoms of cerebellar ataxia, identification of associated neurological signs is equally important, because these additional symptoms can often provide diagnostic clues. The commonly associated symptoms are peripheral neuropathy, parkinsonism, dystonia, tremor, sleep dysfunction, autonomic symptoms, seizures, and hearing loss.
The neurological examination of cerebellar ataxia constitutes five domains: eyes, speech, hands, legs, and gait. Scale for Assessment and Rating of Ataxia (SARA) is a commonly used clinical scale to assess different domains of cerebellar ataxia, except for the eye movements ( ). The SARA scale encompasses the following: (1) gait, (2) stance, (3) sitting, (4) speech, (5) finger chase, (6) nose-finger, (7) fast alternating hand movements, and (8) heel-shin slide. ![]() demonstrates a complete examination of the SARA scale. For the gait examination, ataxia patients are asked to walk normally. Variable stride length and/or veering toward one side are common gait patterns in the early stage of cerebellar ataxia. A wide-based gait usually develops as a compensatory mechanism in the moderate and severe stage of cerebellar ataxia (
demonstrates a complete examination of the SARA scale. For the gait examination, ataxia patients are asked to walk normally. Variable stride length and/or veering toward one side are common gait patterns in the early stage of cerebellar ataxia. A wide-based gait usually develops as a compensatory mechanism in the moderate and severe stage of cerebellar ataxia ( ![]() ). In other words, depending on the disease stage and individual compensatory strategy, the ataxic gait might differ. To detect subtle difficulty in gait, observing patients running or walking up or down stairs can be useful.
). In other words, depending on the disease stage and individual compensatory strategy, the ataxic gait might differ. To detect subtle difficulty in gait, observing patients running or walking up or down stairs can be useful.
Neurological Examinations of a Case of SCA1. While walking, the patient exhibits variable stride length and walking directions. In the stance examination, she has increased body sways and difficulty in tandem stance. She has mild truncal sways while sitting and dysarthria while talking. She has overshoot in her finger-chase examination and intention tremor in her finger-nose examination. Her rapid alternating movements are slow. Finally, her heel falls off her shin several times during the heel-shin slide examination.
Different Gait difficulties in Ataxia Patients. Turning difficulty in a patient with SCA1 ( Segment 1 ). Variable stride length and different walking directions in a patient with POLG -ataxia ( Segment 2 ). A slight wide-based gait in a patient with SCA6 ( Segment 3 ). A marked wide-based gait in a patient with SCA35 ( Segment 4 ).
Ataxia patients usually have truncal sways while standing still and sometimes when sitting without back support. To further identify subtle ataxia in the stance examination, patients are asked to stand with feet together, to stand on tandem stance, to stand on either foot, or to hop on either foot. Scanning speech is a classic speech associated with cerebellar ataxia, slow, with characteristic irregular force and unnecessary hesitation between some words. Words are usually broken into separate syllables.
In hand examination, three maneuvers are often used: nose-finger tests (the patient points repeatedly with his index finger from his nose to examiner’s index finger as precisely as possible), finger chase (the patient’s index finger follows examiner’s moving index finger as precisely as possible), and fast alternating movements (the patient performs repetitive alternation of pronation and supination of the hand). Patients with cerebellar ataxia often exhibit intention tremor, as increasing amplitude of oscillatory movements when voluntarily approaching a target, in the finger-nose test; over- or undershoot in the finger chase test; and slow and abnormal rhythm in the fast alternating movements. In the leg examination, ataxia patients are instructed to lift one leg, point with the heel to the opposite knee, and slide down along the shin to the ankle. Ataxia patients often have the heel falling off the shin during the slide. Functionally, one can consider this as the leg equivalent of the nose-finger test.
In patients with cerebellar ataxia, abnormal eye movements are common and sometimes can be diagnostic ( ![]() ). Neurologists should assess eyes in the fixation position, during smooth pursuit, and in saccadic movements. Certain eye movement abnormalities might be associated with specific types of ataxias: for example (1) square-wave jerks (saccadic intrusion in the fixed gaze) in Friedreich ataxia, (2) end-gaze nystagmus in many types of ataxia, (3) hypo- or hypermetric saccades, also in many types of ataxia, (4) breakdown of smooth pursuit (saccadic pursuit) in spinocerebellar ataxia type 3 (SCA3), (5) slow saccades in SCA2 (
). Neurologists should assess eyes in the fixation position, during smooth pursuit, and in saccadic movements. Certain eye movement abnormalities might be associated with specific types of ataxias: for example (1) square-wave jerks (saccadic intrusion in the fixed gaze) in Friedreich ataxia, (2) end-gaze nystagmus in many types of ataxia, (3) hypo- or hypermetric saccades, also in many types of ataxia, (4) breakdown of smooth pursuit (saccadic pursuit) in spinocerebellar ataxia type 3 (SCA3), (5) slow saccades in SCA2 ( ![]() ), (6) ophthalmoplegia/ophthalmoparesis in sensory axonal neuropathy with dysarthria and ophthalmoplegia (SANDO) syndrome with DNA polymerase gamma-1 ( POLG ) mutations, (7) ptosis in SANDO syndrome and mitochondrial ataxia, and (8) impaired vertical saccades in Niemann-Pick type C.
), (6) ophthalmoplegia/ophthalmoparesis in sensory axonal neuropathy with dysarthria and ophthalmoplegia (SANDO) syndrome with DNA polymerase gamma-1 ( POLG ) mutations, (7) ptosis in SANDO syndrome and mitochondrial ataxia, and (8) impaired vertical saccades in Niemann-Pick type C.
Various Eye Movement Abnormalities in Cerebellar Ataxia. End-gaze nystagmus in POLG -ataxia ( Segment 1 ). Slow saccades in SCA2 ( Segment 2 ). Hypermetric saccades in SCA1 ( Segment 3 ). Hypometric saccades in multiple system atrophy, cerebellar type ( Segment 4 ). Impaired vertical saccades with relatively preserved horizontal saccades in Niemann-Pick type C ( Segment 5 ).
A Case of SCA2. Dysarthria, slow saccades, dysmetria in the finger-chin test, impaired rapid alternating movements, overshoot in the finger-chase examination, hyporeflexia, and a wide-based gait with variable walking directions.
Many mimickers resemble cerebellar ataxia or present with overlapping symptoms ( Table 23.3 ). As such, neurological examinations should also assess the associated signs, such as tremor, dystonia, myoclonus, parkinsonism, sensory neuropathy, muscle weakness, and pyramidal signs. Note that sensory neuropathy could be a predominant feature in certain ataxia syndromes in the early stage of the disease, such as Friedreich ataxia and POLG -ataxia. Detailed physical examination sometimes can yield additional information for the diagnosis, such as telangiectasia for ataxia telangiectasia, splenomegaly for Niemann-Pick type C, scoliosis and pes cavus for Friedreich ataxia.
|
Serum and cerebrospinal fluid (CSF) biomarkers can be useful in diagnosing nutritional, immune-mediated, and autosomal recessive ataxia with metabolic dysfunction (certain forms of the latter). Serum levels of vitamin B 1 , B 12 , and E should be tested for deficiency. Vitamin B 1 deficiency causes Wernicke encephalopathy and can occur in a variety of clinical settings, such as cancer and malnutrition, besides alcoholism ( ). Vitamin E deficiency is relatively rare and can occur in two forms of recessive ataxia: ataxia with vitamin E deficiency and abetalipoproteinemia.
Serum autoantibodies can indicate specific immune-mediated causes of ataxia, such as ataxia associated with anti-glutamate acid decarboxylase (GAD) antibodies, anti-thyroperoxidase (TPO) antibodies (for steroid-responsive encephalopathy), paraneoplastic antibodies, anti-gliadin and anti-tissue transglutaminase antibodies (for gluten ataxia). Serum antibody levels are often diagnostic, especially when high levels of antibodies are present; occasionally, these autoantibodies can be only be detected in the CSF. Therefore, a lumbar puncture is warranted when immune-mediated ataxia is suspected, especially in cases with subacute onset of ataxia without marked cerebellar atrophy in the imaging studies. Infectious and inflammatory etiologies should also be examined in the CSF.
CSF analysis can provide additional information; for instance, a high protein 14-3-3 level can be seen in Creutzfeldt-Jakob disease (CJD), whereas a low CSF glucose level might point toward ataxia with glucose transporter type 1 deficiency.
Serum metabolic biomarkers sometimes can be helpful in identifying several forms of autosomal recessive ataxia. Ataxia telangectasia and ataxia with oculomotor apraxia type 2 both have elevated serum alpha fetoprotein levels, and cerebrotendinous xanthomatosis can have elevated blood cholestanol levels.
Brain magnetic resonance imaging (MRI) should be obtained in ataxia patients. This enables visualization of demyelinating, vascular, and structural causes for ataxia, such as multiple sclerosis, brain tumors, abscess, and ischemic or hemorrhagic strokes. Cerebellar cortical atrophy is the most common finding, and clinicians should assess the degree of the cerebellar atrophy in different cerebellar lobules and in vermis, paravermis, and hemisphere ( Fig. 23.2, A – C ). Prominent CSF space between cerebellar folia indicates the underlying degeneration. An enlarged fourth ventricle is often associated with cerebellar atrophy (see Fig. 23.2, D ). The cerebellum is divided into motor (predominantly anterior) and nonmotor (predominantly posterior) regions ( ). Therefore, clinicians should pay special attention to patients with prominent atrophy in the posterior lobules of the cerebellum and assess their cognitive dysfunction and emotional liability. In addition, speech and gait ataxia is associated with vermal atrophy, whereas appendicular ataxia is associated with paravermal atrophy. As mentioned above, certain forms of ataxia have predominant sensory neuropathy in the early stage (e.g., Friedreich ataxia, ataxia with vitamin E deficiency and POLG -ataxia); therefore, there might be no obvious cerebellar atrophy on the brain MRI.
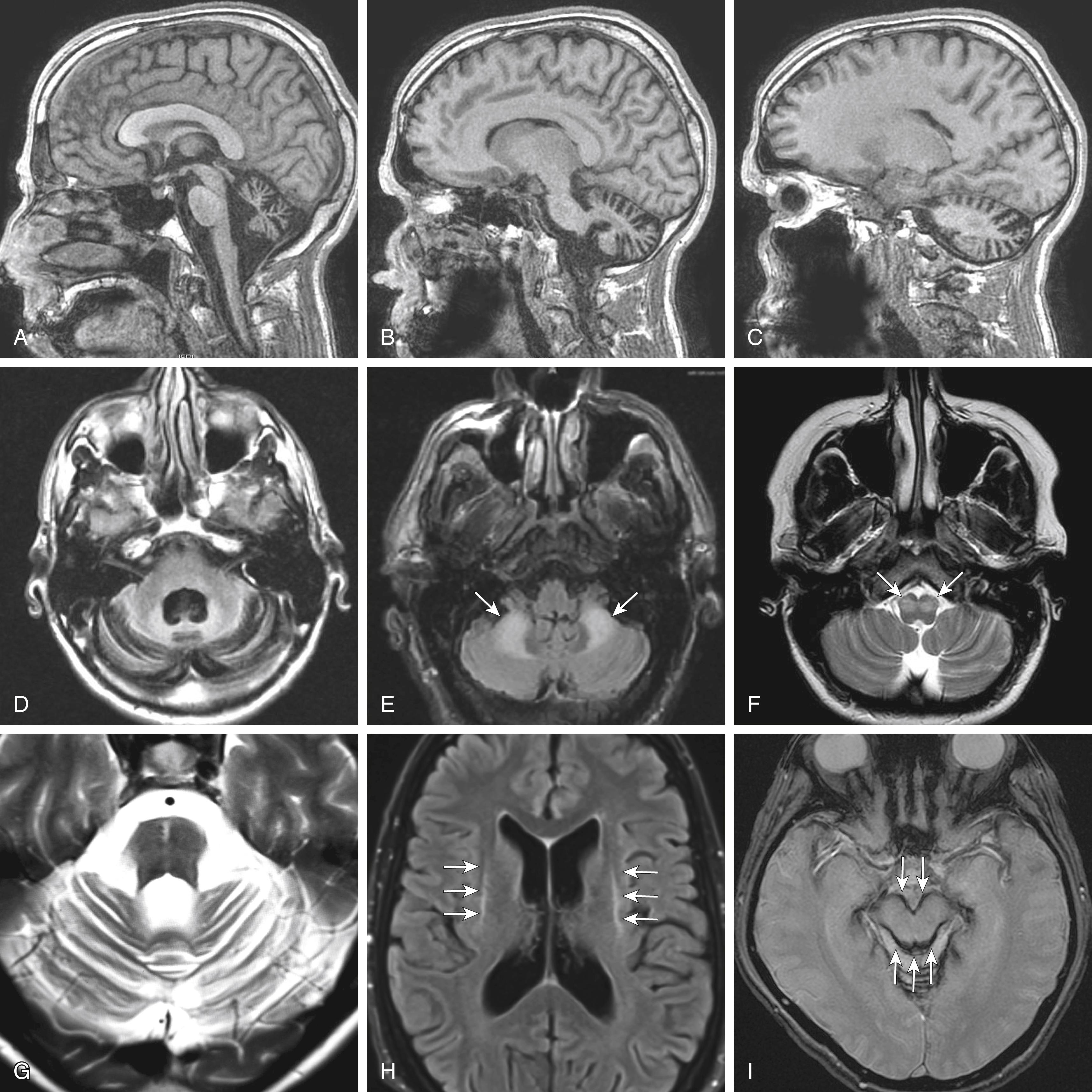
Clinicians should also look for specific changes associated with certain forms of cerebellar ataxia. Fragile X–associated tremor and ataxia syndrome has T2-hyperintensity in the bilateral middle cerebellar peduncles (see Fig. 23.2, E ). Wernicke encephalopathy has T2 hyperintensity in the mamillary bodies, periaqueductal gray, and paraventricular thalamus ( ). and adult-onset Alexander disease can have prominent subcortical white matter changes. POLG -ataxia, adult-onset Alexander disease, and ataxia with gluten sensitivity can have T2 hyperintensity in the bilateral inferior olivary nucleus (see Fig. 23.2, F ). Multiple system atrophy can have either a hot-cross-bun sign (a T2 hyperintense cross sign in the pons, associated with the cerebellar type, Fig. 23.2, G ) or linear T2 hyperintensity along the outer rim of the striatum (associated with the parkinsonism type, Fig. 23.2, H ). Superficial siderosis has hypointensity along the surface of the cerebellum and brainstem in the gradient echo sequence (GRE; see Fig. 23.2, I ). CJD has cortical ribboning on the diffusion-weighted imaging (DWI). While the presence of these features can help with the diagnosis, the absence of these features does not exclude these causes of cerebellar ataxia.
In addition to brain MRI, a dopamine transporter scan can be used to test the involvement of the dopamine system, which can be seen in multiple system atrophy.
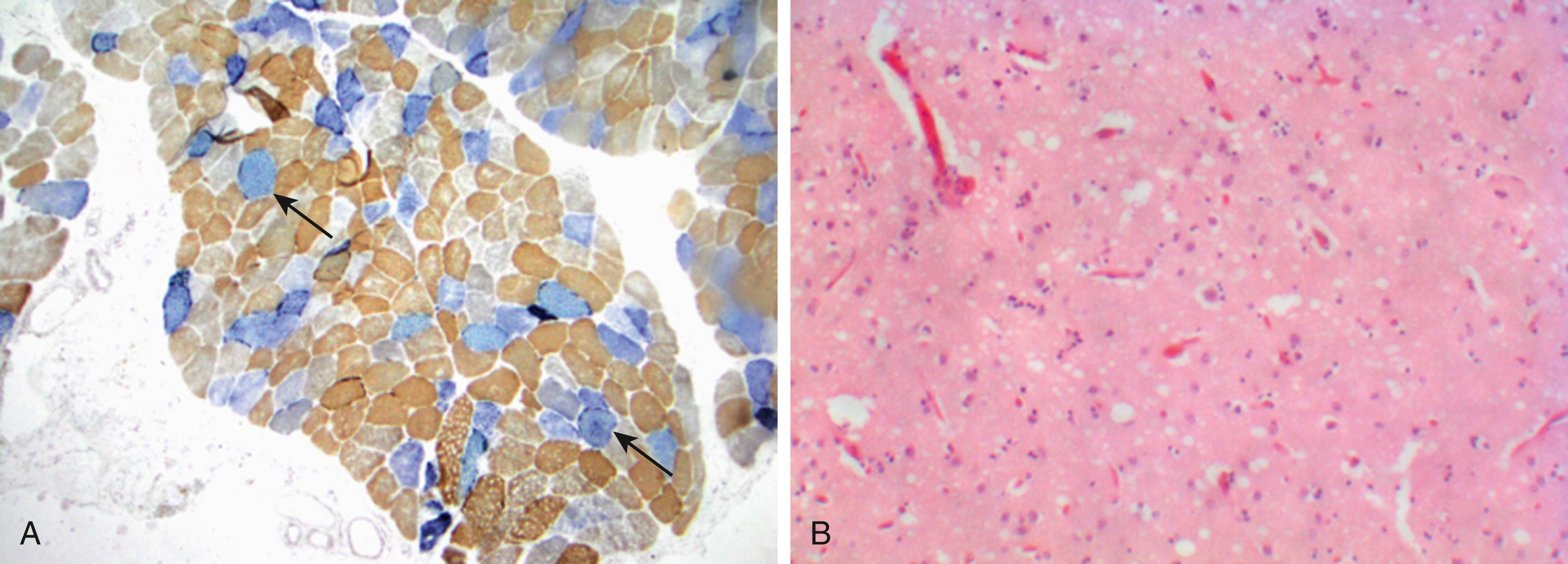
In addition to brain imaging studies, physiological measures can help to identify the involvement of additional systems. Autonomic nervous tests for orthostatic hypotension and/or urinary disturbance together with a sleep study to demonstrate rapid eye movement behavior disorder suggest the diagnosis of MSA. Electromyography and conduction studies can assess the associated motor-sensory neuropathy. In patients with ataxia and sensory neuropathy, the diagnosis of POLG -ataxia can be supported by muscle biopsy, demonstrating increased succinate dehydrogenase (SDH) expression as the result of mitochondrial proliferation ( Fig. 23.3, A ). In patients with CJD, an electroencephalogram may show typical periodic sharp-wave complexes, and brain biopsy may demonstrate spongiform changes (see Fig. 23.3, B ).
Before one starts searching for genetic causes of cerebellar ataxia in a patient, it is important to identify acquired causes of ataxias, as some of them are potentially treatable or partially reversible, in contrast to genetic cerebellar ataxias. Common acquired causes of ataxias include metabolic (nutrition, toxins), vascular insults (ischemic stroke, bleed), neoplasms, infections, and autoimmune reactions ( Table 23.4 ).
| Entity | Diagnostic Process |
|---|---|
| Vascular Disease | History of strokes, imaging |
| Hypoxic encephalopathy | History of hypoxic episodes |
| Demyelinating disease | Remitting and relapsing episodes, imaging, CSF analysis |
| Tumors in the posterior fossa | Imaging |
| Cranio-vertebral junction anomalies | Imaging |
| Toxic Disorders Alcohol Chemotherapy (5-fluorouracil, ara-C, methotrexate) Metals (mercury, bismuth, lithium, lead) Solvents (toluene) Anticonvulsants (phenytoin) |
History |
| Infectious/Inflammatory Disease Acute cerebellar ataxia of childhood, acute cerebellitis Post-infectious Bickerstaff encephalitis Human immunodeficiency virus (HIV) Creutzfeldt-Jakob disease (CJD) Whipple disease |
History, imaging, serology, CSF analysis History, imaging, CSF analysis Imaging, CSF analysis Serology CSF 14-3-3, imaging, electroencephalogram, biopsy Small intestine biopsy |
| Autoimmune: Paraneoplastic Gluten sensitivity Anti-GAD ataxia Anti-GluRδ2 ataxia |
Serology Anti-Hu, anti-Yo, anti-Ri, others Anti-gliadin, anti-tissue transglutaminase Anti-GAD Anti-GluRδ2 |
| Superficial siderosis | Imaging |
| Nutritional: Vitamin B 1 deficiency Vitamin B 12 deficiency Vitamin E deficiency |
Blood Vitamin B 1 level Vitamin B 12 level Vitamin E level |
Thiamine deficiency can lead to Wernicke encephalopathy, which may present with altered mental status, ophthalmoplegia, and ataxia ( ). While thiamine deficiency is frequently associated with chronic alcohol use, there is evidence that thiamine deficiency can cause cerebellar dysfunction independent of alcohol toxicity ( ). Proposed mechanisms of thiamine-deficiency-induced ataxia include tissue edema, altered blood-brain barrier integrity, impaired energy metabolism, reduced thiamine-utilizing enzymes in cerebellum and subsequent loss of amino acids, lactic acidosis, excitotoxicity, mitochondrial uncoupling, oxidative stress, reactive microglia, apoptosis, and microvascular damage ( ).
Acquired vitamin E (α-tocopherol) deficiency can occur in patients with insufficient intake or poor absorption. Vitamin E deficiency can also be hereditary, stemming from mutations in the gene for α-tocopherol transfer protein in ataxia with vitamin E deficiency or mutations in the MTTP gene in abetalipoproteinemia ( ). Patients typically present with progressive cerebellar ataxia with limb and gait changes, titubation of the head, and evidence of peripheral neuropathy. Ataxia with vitamin E deficiency and abetalipoproteinemia are further discussed under genetic ataxias (see below).
Paraneoplastic cerebellar degenerations (PCDs) are the most frequently encountered paraneoplastic neurological syndrome (around 24.3%; ). Patients with PCDs usually present with a subacute-onset cerebellar syndrome over several months ( ). Neurological symptoms may precede the diagnosis of the neoplasm ( ). Brain MRI typically demonstrates cerebellar atrophy, although it may be normal at an early stage ( ). Onconeural antibodies associated with PCDs include anti-Yo, anti-Hu, anti-Ma, anti-Ri, anti-VGCC, anti-CV2/CRMP5, anti-Tr/DNER, and anti-mGluR1 ( ). The most common cancers associated with PCDs are small-cell lung cancer, ovarian tumor, breast cancer, and Hodgkin lymphoma ( ). The role of onconeural antibodies is still unclear as most of the targets are intracellular antigens, which limits antibody accessibility ( ). The mainstream treatment is to identify and treat underlying cancer. However, the response to either immunotherapy or to the tumor itself has been poor ( ).
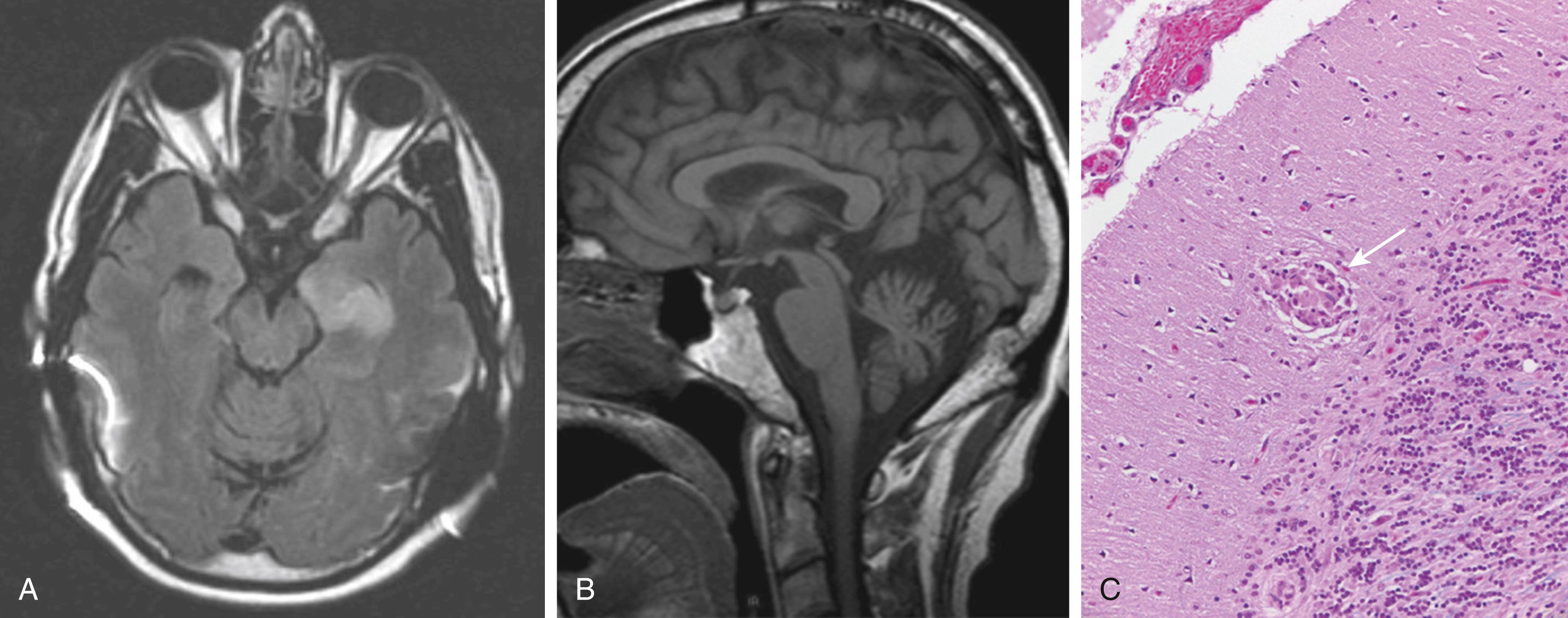
Sarcoidosis is a chronic inflammatory disease with formation of non-caseating granulomas. About 3%–10% of patients with sarcoid have involvement of the central nervous system (CNS; ). Cerebellar symptoms arise when the cerebellum or its in- or outflow tracts are affected by neurosarcoid. The brain MRI typically shows hyperintensity changes on fluid-attenuated inversion recovery (FLAIR) images ( Fig. 23.4, A ). In chronic cases, cerebellar atrophy may be seen (see Fig. 23.4, B ). Confirmation of diagnosis requires biopsy demonstrating non-caseating granulomas (see Fig. 23.4, C ).
Gluten-sensitive ataxia was initially categorized as part of the extra-intestinal manifestation of celiac disease (gluten-sensitive enteropathy), but increasing evidence suggests that these and gluten-sensitivity with skin involvement (dermatitis herpetiformis) may all belong to the spectrum of “gluten sensitivity” ( ). Patients with gluten-sensitive ataxia manifest with progressive ataxia, with limb, truncal, and ocular involvement that worsens slowly over the years ( ). Thirteen percent of the patients have gastrointestinal symptoms, suggesting an overlapping syndrome with gluten-sensitive enteropathy. Similar to celiac disease, adopting a gluten-free diet may improve the symptoms of gluten-sensitive ataxia ( ). In addition to anti-gliadin antibodies found in patients with gluten sensitivity, antibodies against transglutaminase 6 (anti-TG6) are recently found to be associated with gluten-sensitive ataxia ( ). Injection of antibodies with reactivity to TG2 and TG6 to mice can cause ataxia, suggesting that anti-TG6 may have a pathogenic role ( ).
Antibodies against GAD, the rate-limiting enzyme for γ-aminobutyric acid (GABA) synthesis, are associated with neurological conditions including stiff person syndrome, limbic encephalitis, epilepsy, and cerebellar ataxia ( ). Most of the patients with cerebellar ataxia with anti-GAD antibodies present with an insidious-onset gait and limb ataxia, dysarthria, and nystagmus. Peripheral neuropathy or rigidity in the leg may also be seen ( ). It is more commonly seen in women (80%–90%; ). MRI may show cerebellar atrophy ( ). The pathogenic role of anti-GAD antibody is still unclear, although rats infused with anti-GAD antibodies seem to have more irregular gait ( ). In patients with subacute-onset anti-GAD ataxia treated with intravenous immunoglobulin (IVIG) with corticosteroid or other immunosuppressors, 35% showed improvement ( ).
Steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT) is also called Hashimoto encephalopathy. It is an encephalopathy associated with antithyroid antibodies (e.g., antithyroperoxidase and antithyroglobulin antibodies; ). The role of antithyroid antibodies is still unclear, and there is still debate whether its presence is coincidental. Another antibody associated with SREAT is anti-NAE, an antibody against the N-terminus of α-enolase, and its pathogenic role is again undetermined ( ). Patients may present with a wide range of neuropsychiatric symptoms, including altered mental status, psychosis, cognitive impairment, seizure, cerebellar ataxia, and other involuntary movements ( ).
Acute cerebellitis is an inflammatory process involving the cerebellum, affecting children more than adults. Clinical presentation includes ataxia, nausea, vomiting, headache, dysarthria, fever, nystagmus, vertigo, and altered mental status. The onset of symptoms is typically preceded by a viral infection or recent vaccination ( ). The delayed onset of ataxia after a prodromal infection and the specific targeting of cerebellum suggest acute cerebellitis is more likely an immune-mediated inflammatory disorder rather than the result of a direct infection to the cerebellum. The infectious agent may be detected by polymerase chain reaction (PCR) or by elevated serum immunoglobulin M (IgM) titer against an organism, but in most cases, the etiology was never identified. Organisms associated with acute cerebellitis include varicella zoster virus, Epstein-Barr virus, mumps, influenza, herpes simplex virus 7, cytomegalovirus, Coxsackie virus, enterovirus, and Mycoplasma pneumoniae ( ). The prognosis is usually self-limiting, but severe cases may sustain permanent neurological deficits.
Bickerstaff encephalitis is characterized by progressive ophthalmoplegia, ataxia, and altered consciousness or hyperreflexia ( ). Bickerstaff encephalitis is similar to Fisher syndrome, which consists of ophthalmoplegia, ataxia, and areflexia ( ). Both are associated with elevated serum anti-GQ1b antibodies ( ), and now the two are considered entities that are in the same spectrum. Patients with Bickerstaff encephalitis may have hyperintensity on the T2-weighted images, involving brainstem, cerebellum, thalamus, or subcortical white matter ( ). The imaging findings of cerebellar and brainstem involvement as well as the few autopsy cases ( ) suggest that the ataxia in Bickerstaff encephalitis is more likely to be cerebellar. In contrast, the ataxia in Fisher syndrome is more likely the result of proprioception impairment: namely, sensory ataxia. However, patients with Fisher syndrome may also have abnormalities on MRI, making the debate unsettled ( ).
As the name indicates, chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) is a chronic inflammatory process of the pontine region that responds to steroids, and pathological findings showed predominant perivascular lymphocytic inflammation ( ). Patients present with subacute-onset gait ataxia and diplopia along with dysarthria, tingling of the face, dizziness, nystagmus, and paraparesis ( ). MRI shows small punctate of gadolinium enhancement ( ). Corticosteroid is the treatment of choice, but maintenance immunosuppressant is required. Most patients experienced improvement with treatment, and in a study 10 out of 23 patients had complete resolution of MRI findings ( ).
CJD can present with mainly cerebellar ataxia before developing other cognitive symptoms ( ). CJD is a form of rapidly progressive dementia caused by misfolded prion protein, PrP, encoded by the gene PRNP ( ). Polymorphism of the PRNP gene affects the clinical phenotype ( ). In patients expressing a 21 kDa PrP with homozygous methionine at codon 129 (MM1), 33% presented with cerebellar ataxia at symptoms onset, whereas in patients carrying heterozygous methionine/valine at codon 129 (MV1), 75% presented with cerebellar ataxia at onset ( ). Diagnostic tools include MRI DWI sequence showing cortical ribbon sign and double hockey stick sign, elevated CSF 14-3-3 protein, CSF RT-QuIC and protein misfolding cyclic amplification (PMCA) test, electroencephalogram with periodic sharp wave complex, and spongiform pathology on brain biopsy (see Fig. 23.3, B ).
Whipple disease is a chronic multisystemic infectious disease caused by Tropheryma whipplei . The incidence is about 1 per 1,000,000 ( ). It predominantly affects the gastrointestinal tract, leading to abdominal pain, diarrhea, and weight loss. Involvement of the CNS can occur in 6%–43% of the patients ( ). Among the patients with CNS Whipple disease, 11%–45% have cerebellar ataxia. Other neurological symptoms include cognitive impairment, seizure, psychiatric symptoms, supranuclear gaze palsy, cranial nerve involvement, upper motor neuron signs, and myoclonus. The pathognomonic symptoms for CNS Whipple disease, oculomasticatory myorhythmia, and oculo-facial-skeletal myorhythmia, occur in about 20% of patients ( ). PCR can detect T. whipplei in CSF in 92% of patients with CNS Whipple disease ( ).
Infection caused by Listeria monocytogenes typically presents as self-limited gastroenteritis. However, L. monocytogenes can also cause meningitis, encephalitis, and brain abscess owing to its CNS tropism ( ). Interestingly, Listeria encephalitis tends to involve just the brainstem: hence the term “rhombencephalitis”. Clinical symptoms may include cranial nerve palsy (single or multiple), altered mental status, and cerebellar ataxia. Arrhythmia or respiratory compromise may occur, depending on the extent of the brainstem involvement. Although less frequent, basal ganglia, thalami, cerebral cortex, and spinal cord may also be affected ( ).
In patients with human immunodeficiency virus (HIV) infection, neurological symptoms may arise from direct toxicity of HIV to the nervous system, opportunistic infection, side effects of antiretroviral agents, and increased risk of developing CNS lymphoma ( ). Cerebellar ataxia can be the result of lymphoma or a localized opportunistic infection in the posterior fossa (e.g., progressive multifocal leukoencephalopathy [PML] or toxoplasmosis). PML is caused by the death of the oligodendrocytes and loss of myelination as the result of JC virus reactivation in immunocompromised patients. Initiation of antiretroviral agents may unmask or worsen PML because of immune reconstitution inflammatory syndrome (PML-IRIS; ). A study reviewed literature between 1998 and 2016, finding that 28% of patients with PML-IRIS have cerebellar ataxia ( ). In rare cases, HIV patients may develop a pure cerebellar syndrome not associated with cognitive impairment, opportunistic infection, or CNS lymphoma ( ).
Cerebellar symptoms can occur in both acute and chronic alcohol intoxication, in addition to other neurological symptoms including cognitive impairment, seizure, slurred speech, and peripheral neuropathy. Ethanol has a direct effect on ion channels such as receptors for N -methyl- d -aspartate (NMDA), GABA, and glycine as well as neuronal nicotinic receptors and potassium channels ( ). Ethanol can cause secondary thiamine deficiency from malnutrition and direct impairment of thiamine metabolism ( ), leading to Wernicke encephalopathy, characterized by the triad of altered mental status, ophthalmoplegia, and ataxia ( ).
Multiple anticonvulsants can cause ataxia, including phenytoin, valproic acid, carbamazepine, oxcarbazepine, lamotrigine, zonisamide, lacosamide, vigabatrin, and gabapentin ( ). The majority of cases are reversible with discontinuation or reduction of the offending medication. Chronic phenytoin use can cause cerebellar atrophy, but cerebellar symptoms may not always be present, and most seemed to correlate with supratherapeutic serum level of phenytoin ( ). Valproic acid can itself cause ataxia, but more often it acts through lowering the metabolism of other medications. Benzodiazepine-related ataxia occurs more often in children, and symptoms are usually milder and reversible ( ).
Several chemotherapy agents can cause cerebellar ataxia among other neurological symptoms, including 5-fluorouracil (5-FU), capecitabine (prodrug of 5-FU), cytarabine, and methotrexate ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here