Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Because of the development of new research techniques, knowledge of the brain acquired in the past 2 decades has exceeded the amount learned in all previous centuries. Numerous questions remain unanswered:
“What is consciousness?”
“What defines our personality?”
“How can we learn better?”
The central nervous system (CNS) is composed of the brain and spinal cord.
Integrates information received from the body
Coordinates and influences activity the body
As previously mentioned, the nervous system is broadly broken down into the CNS and the peripheral nervous system (PNS). Major anatomic differences are summarized in Table 3.1 .
| Embryonic Origin of Collections of Neural Cell Bodies | Neural Tube | Neural Crest Ectoderm |
|---|---|---|
| Myelin-forming glia cell | Oligodendrocyte (form parts of myelin for multiple axons) | Schwann cell (form 1 segment on 1 neuron) |
| Regenerative ability | Practically none | Limited: 1–4 mm/day, may grow back to wrong target or regenerate incompletely |
The differences in regeneration ability are particularly notable:
PNS
The axon is permitted to grow distally from site of injury
Portion distal to injury undergoes Wallerian (anterograde) degeneration with preservation of the distal endoneurial tube as a “pathway”
CNS
Damaged tissue forms a glial scar, restricting future regeneration
Reduced ability to recover from injury
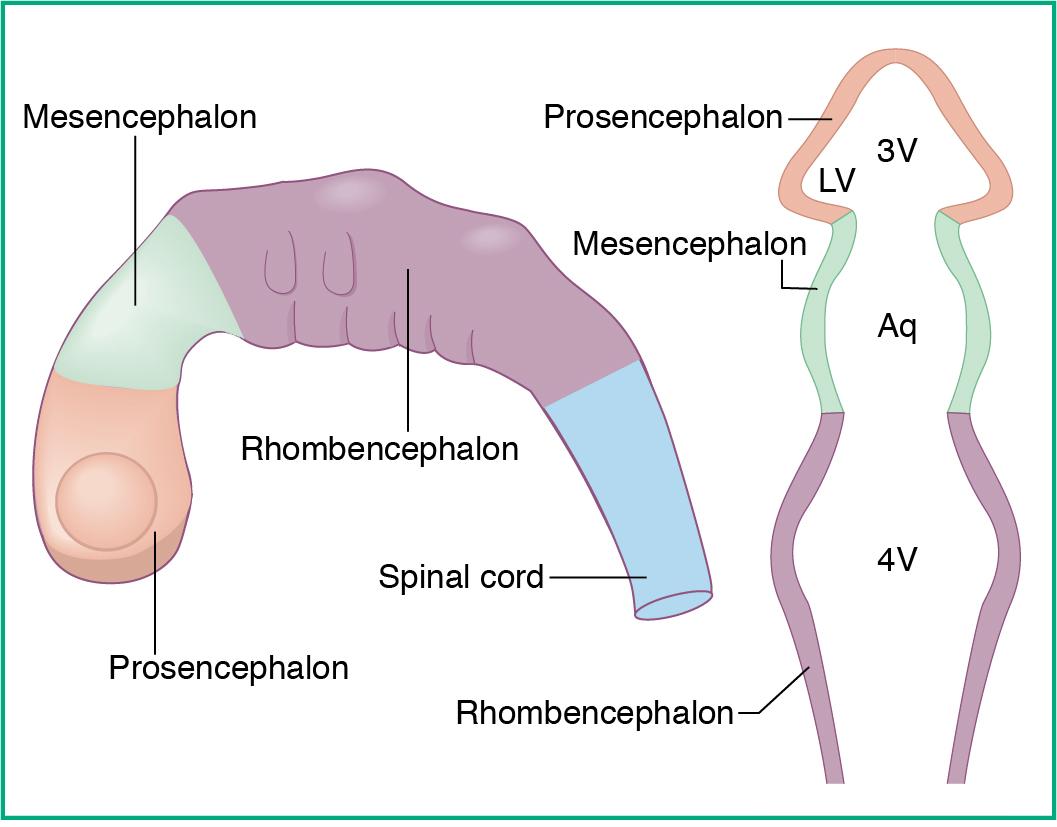
The brain ( Fig. 3.1 ) is the center of the nervous system. It can be divided into different sections based on developmental origins ( Fig. 3.2 ).
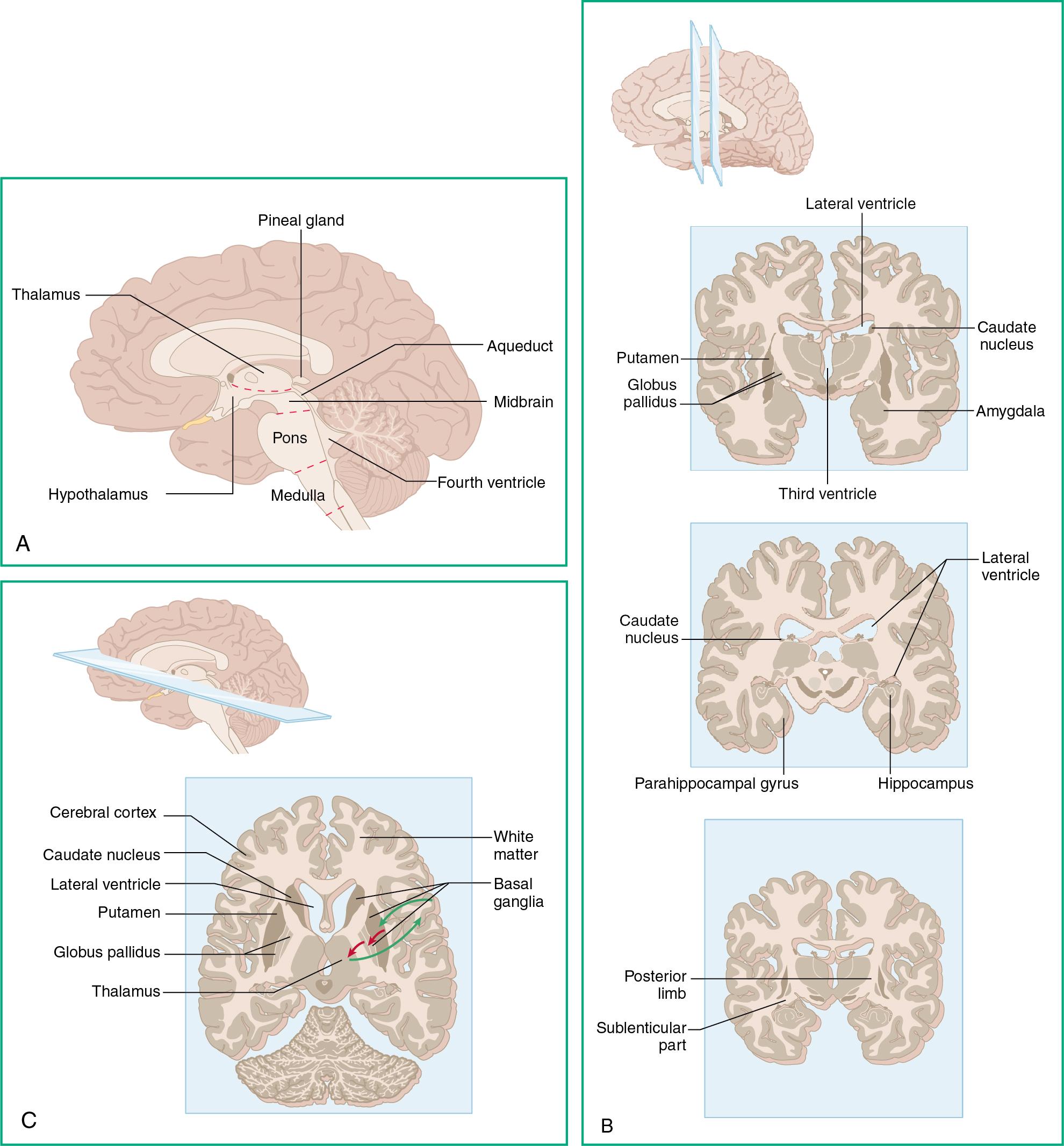
In addition to the skull, there are three layers that protect the brain called meninges ( Table 3.2 ).
Inflammation of these layers is called meningitis.
These membranes also form several potential and real spaces ( Table 3.3 ) which can become filled with pus, excess cerebrospinal fluid (CSF), or blood during pathologic states (see Clinical Correlation Box 3.1 ).
| Space | Location | Description | Clinical Correlate |
|---|---|---|---|
| Subarachnoid space | Between the pia and arachnoid and terminates at S2 vertebra | Where CSF is produced and contained | Subarachnoid hemorrhage causes the “worst headache of one’s life” |
| Subdural space | Between arachnoid and dura | Contains superior cerebral “bridging veins” from the brain | Ruptured veins cause subdural hematoma, a low-pressure hemorrhage that gradually causes headache and confusion. Appears crescent-shaped on CT scan |
| Epidural space | Between the dura and skull | Contains meningeal arteries, in the spinal cord it contains fatty areolar tissue, lymphatics, and venous plexuses | Trauma to the temporal region shears the middle meningeal artery and causes an epidural hematoma, a high-pressure hemorrhage that presents with a lucid interval followed by loss of consciousness. Appears lens-shaped on CT scan because of limitation by skull’s sutures |
| Layer | Location | Description |
|---|---|---|
| Pia mater | Covers brain and spinal cord surface | Delicate, highly vascular |
| Arachnoid | External to the pia mater, internal to the dura mater | Delicate, nonvascular, contains granulations that absorb cerebrospinal fluid |
| Dura mater | Exterior layer, tightly adherent to the skull | Dense, tough |
Meningitis typically affects the leptomeninges (pia and arachnoid) of the brain and spinal cord. The space between them is filled with cerebrospinal fluid (CSF), aiding in infection spread.
Causes of infectious meningitis include:
Bacteria
Viruses
Fungi
Tuberculosis (TB)
Parasites
Common clinical signs include:
Fever
Headache
Photophobia (are the lights switched off when you walk into the room?)
Nuchal rigidity (inability to flex the neck forward)
Kernig’s sign (while supine, inability to extend the knee while the hip is flexed)
Meningeal irritation caused by movement of the spinal cord
Brudzinski’s sign (passive flexion of the neck causes flexion of legs/thighs)
As earlier; movement of spinal cord by neck flexion “cancelled out” by hip flexion
To diagnose meningitis, one may perform a lumbar puncture to identify the responsible organism by culture and specific CSF characteristics.
| Opening Pressure | Cell Type | Protein | Sugar | |
|---|---|---|---|---|
| Bacterial | ↑ | ↑ polymorphonuclear leucocytes | ↑ | ↓ |
| Fungal/TB | ↑ | ↑ lymphocytes | ↑ | ↓ |
| Viral | Normal/↑ | ↑ lymphocytes | Normal/↑ | Normal |
The CSF serves the following roles for the brain:
Protection against trauma
Transportation of hormones
Removal of metabolic waste
CSF is produced by the ependymal cells of the choroid plexuses of the lateral, third, and fourth ventricles. The flow of CSF through the ventricles is as follows (see also Fig. 3.3 ):
Right and left lateral ventricles → interventricular foramen (of Monro) → third ventricle → cerebral aqueduct (of Sylvius) → fourth ventricle → lateral foramina (of Luschka) or medial foramen (of Magendie) → subarachnoid space → arachnoid villi → superior sagittal sinus (see Clinical Correlation Box 3.2 )
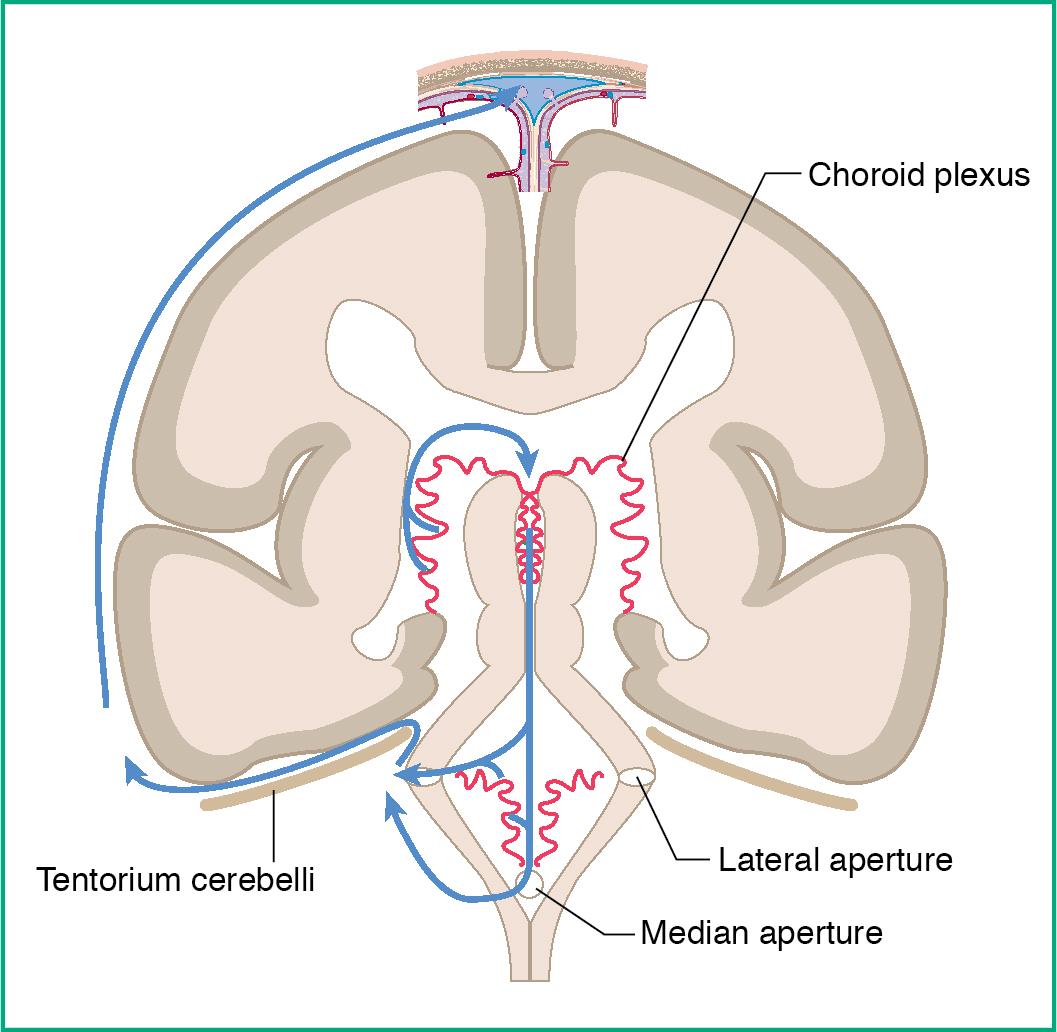
If there is an obstruction anywhere in the path of cerebrospinal fluid (CSF) flow, it can lead to hydrocephalus, which is a dilation of the cerebral ventricles (noncommunicating hydrocephalus) or subarachnoid space (communicating hydrocephalus) with CSF.
The blood-brain barrier is formed by tight junctions between choroid plexus cells, and the cerebral capillaries have few fenestrations. This has several implications:
Transport of substances (including immunologic cells and drugs) from the blood to the brain slow and limited.
Water-soluble, large molecular weight substances cannot cross this barrier, thus protecting the brain from many harmful substances.
Metabolic waste, such as carbon dioxide, however, is lipid-soluble and can easily leave the brain and go into the blood.
Some circulating peptides (e.g., insulin) and plasma proteins (e.g., prealbumin) can also cross the blood-brain barrier.
The blood supply to the brain arises from two main routes:
The internal carotid arteries give rise to the anterior and middle cerebral arteries (ACA, MCA)
The vertebral arteries unite to form the basilar artery, which then form the posterior cerebral arteries.
These three pairs of cerebral arteries connect to one another via communicating arteries at the base of the brain, forming the circle of Willis ( Fig. 3.4 ). The blood drains out through the jugular veins.
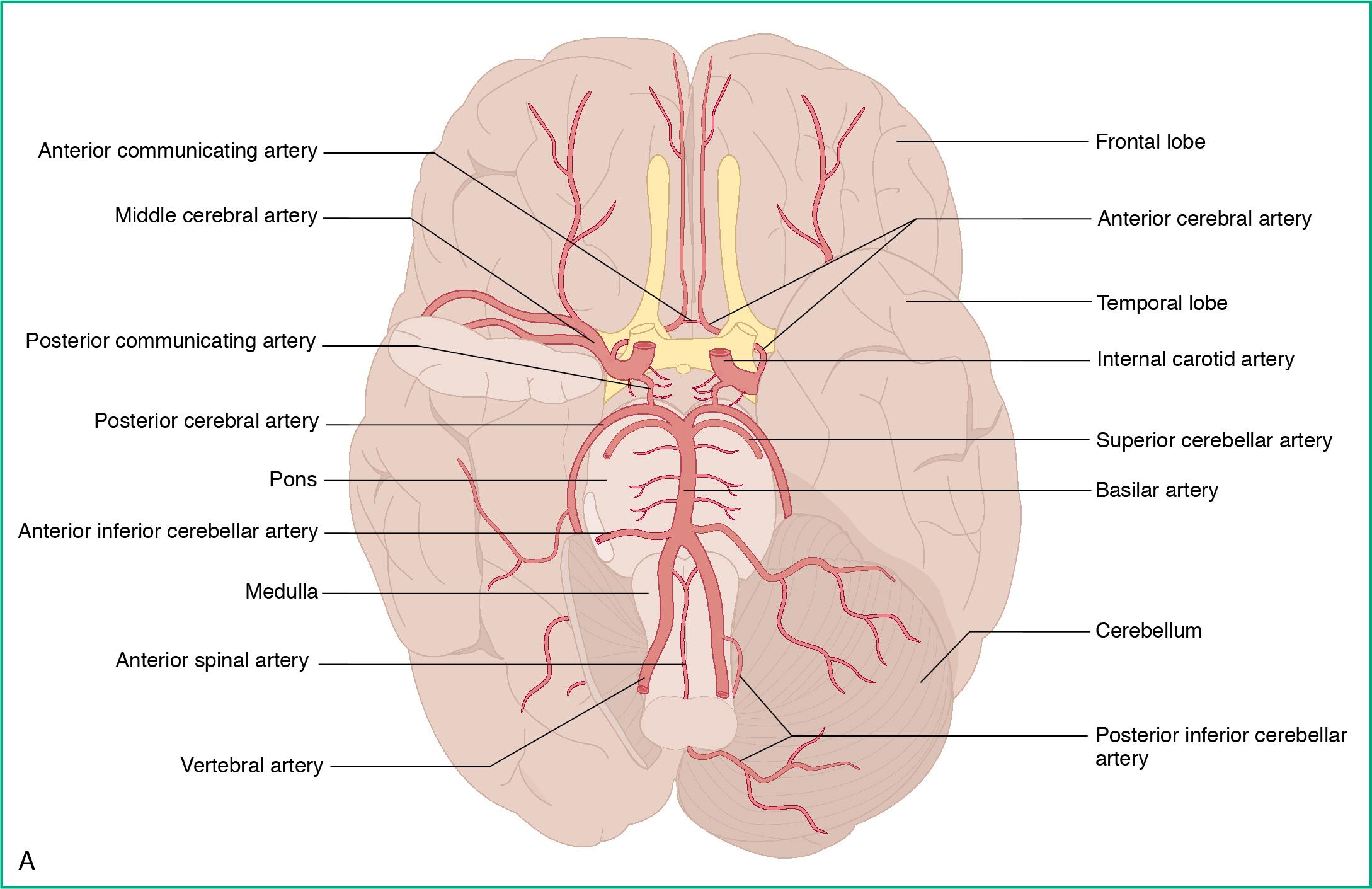
Blood supply to the brain must be tightly regulated ( Fig. 3.5 ). The brain, which is largely incompressible, is housed inside the rigid skull. The brain is comprised of the brain parenchyma and fluid. Because the volume of the brain is fixed, for one component to expand, the other must compensate if there is no change in pressure, as illustrated by the subsequent equation.
This is called the Monro-Kellie doctrine, where V represents the volume.
Intracranial volume remains constant in physiologic states.
V brain represents the amount of volume comprised of brain parenchymal cells, which is also largely constant in the developed brain.
V CSF is largely homeostatic provided there are no obstructions and under normal physiology.
V mass lesion can be added into the equation during pathologic states (e.g., tumor, hydrocephalus, hemorrhage).
Because intracranial volume must remain constant (assuming the skull is intact) another component of equation must decrease.
V blood is controlled by the body, through blood volume entering the brain.
As volume increases within the confined skull (assuming no brain herniation), intracranial pressure (ICP) must eventually increase. Mean arterial pressure (MAP) also decreases, causing a large decrease in cerebral perfusion pressure (CPP) according to:
CPP is necessary for life because the brain has a very high metabolic demand, but the brain is also very sensitive to overperfusion. The brain thus maintains proper cerebral blood flow (CBF) and CPP according to:
whereby the cerebral vascular resistance (CVR) controls the other two parameters (see Fast Fact Box 3.1 ).
Note that Eq. 3.3 is similar to Ohm’s law
where current (I) is represented as blood flow and voltage (V) is represented as a pressure gradient. R represents a resistance.
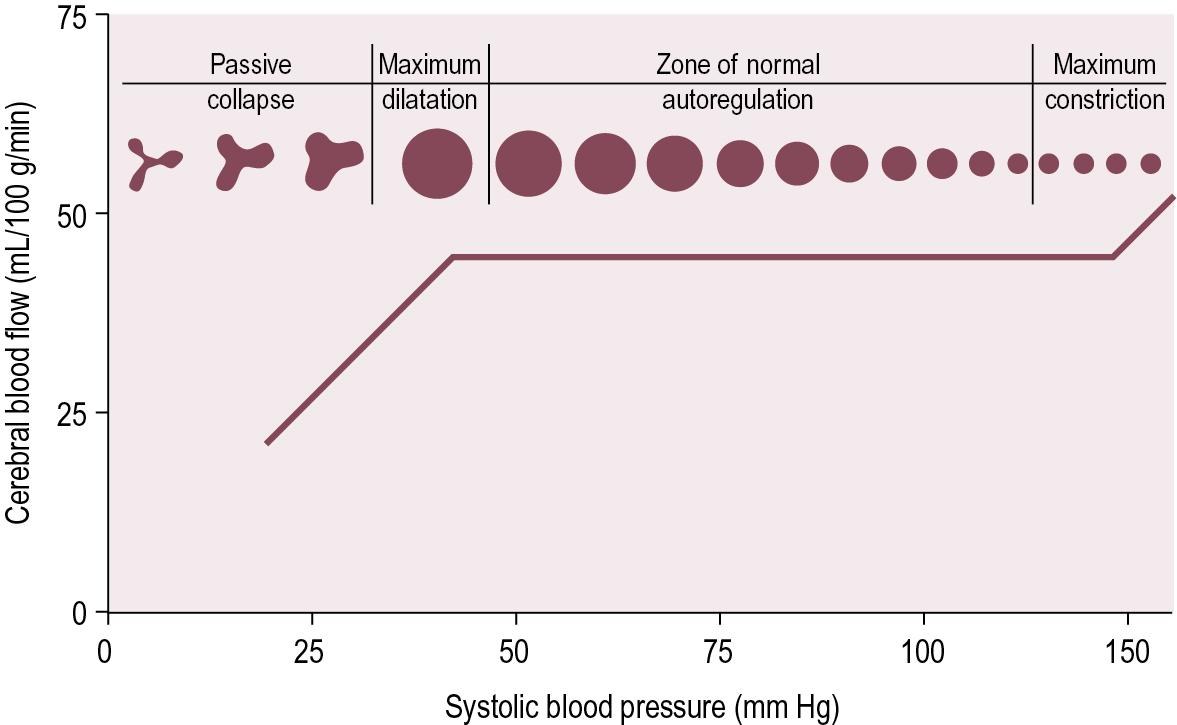
To vary the CVR, the cerebral small arteries and arterioles constrict and dilate, changing the CBF so that it remains at approximately 50 mL/100 g /min (see Fig. 3.3 ).
CBF is maintained with a MAP between 50 to 125 mm Hg
Blood flow decreases dramatically at MAP less than 50 mm Hg
Blood flow increases dramatically at MAP more than 125 mm Hg
The change in vessel size through cerebral autoregulation is thought to potentially work through three different mechanisms:
Myogenic regulation: Changes in transmural blood pressure can be detected by vascular smooth muscle in arterioles through mechanical sensors.
Neurogenic regulation: Resistance arterioles receive sympathetic innervation from brainstem blood pressure (BP) control centers for vasoconstriction, and para-sympathetic innervation that releases nitric oxide and causes vasodilation.
Metabolic regulation: Metabolic demand is balanced with blood flow, such that Increased metabolic activity (inc. PCO 2 ) leads to vasodilation while excessive oxygen concentrations lead to vasoconstriction to protect against oxygen toxicity (see Clinical Correlation Box 3.3 ).
Local vasodilation increases blood flow to keep up with metabolic demand, and also to carry away CO 2 -rich blood from the brain. This is the basis for measuring the use of different brain regions under functional magnetic resonance imaging (fMRI).
The cerebrum controls all voluntary actions in the body and is composed of:
Two cerebral hemispheres (left and right)
Four primary lobes of cerebral cortex
Frontal
Parietal
Temporal
Occipital
Several subcortical structures
Thalamus
Hypothalamus
Basal ganglia
Hippocampus
The cerebral cortex is composed of six layers that are largely segregated by their connections with other cortical and subcortical regions of the brain:
Layers I (molecular layer, contains few neurons), II (external granular layer), and III (external pyramidal layer) are the primary input for corticocortical afferents from the same cerebral hemisphere.
Layer III is the primary output layer of corticocortical efferents to both ipsilateral and contralateral cortices.
Layer IV (internal granular layer) is the input for specific ipsilateral thalamic and cortical afferents.
Layer V (internal pyramidal layer) contains the efferents to subcortical structures (i.e., basal ganglia), the brain stem, and spinal cord.
Layer VI (multiform layer) contains excitatory and inhibitory efferents to the thalamus.
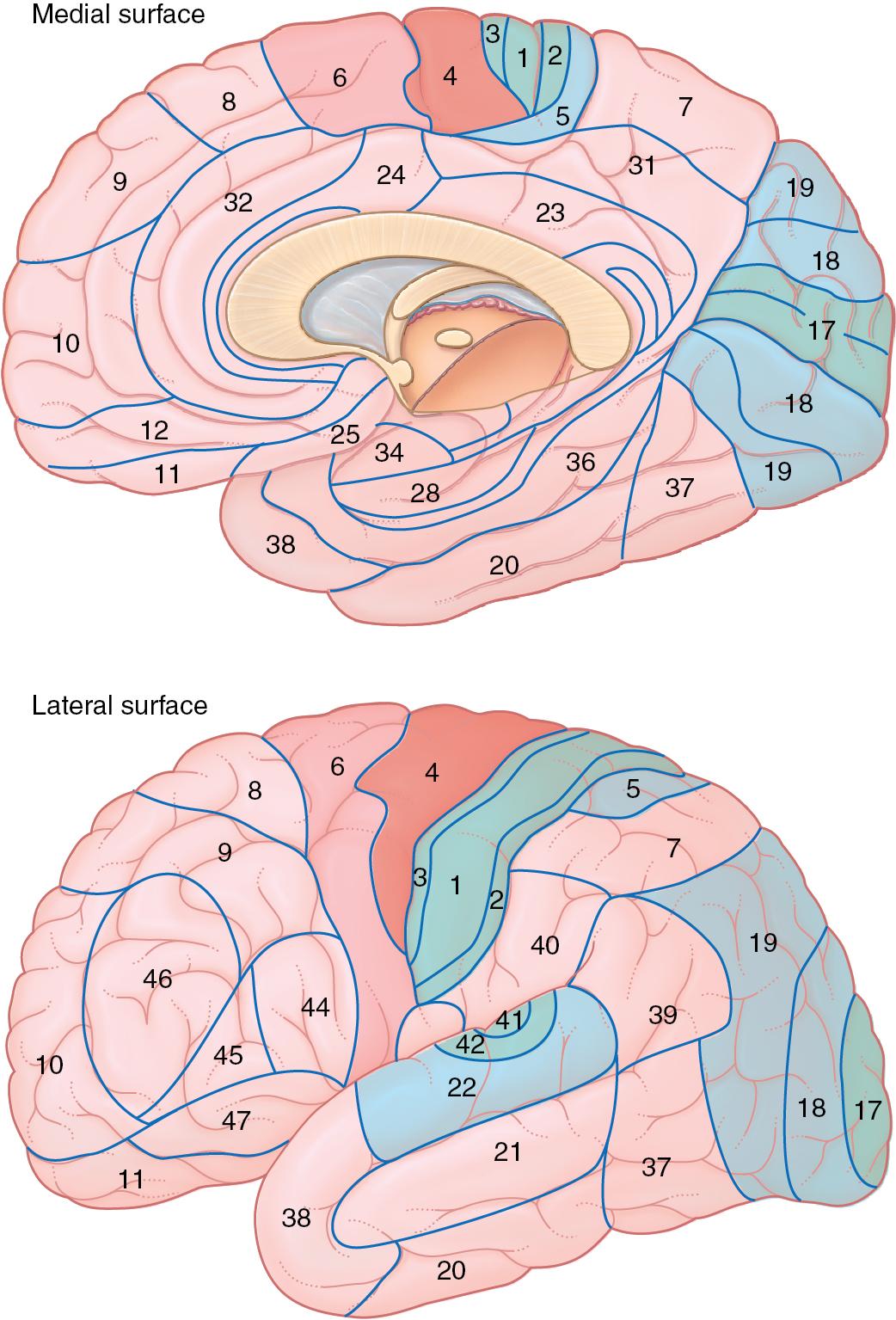
Columnar layers of the cerebral cortex form characteristic cortical columns that share similar functions or pay attention to the same portion of a particular topographic map. Based on the differences in cortical column organization across the cortex, German anatomist Korbinian Brodmann defined numerous cortical areas that were anatomically similar ( Fig. 3.6 ).
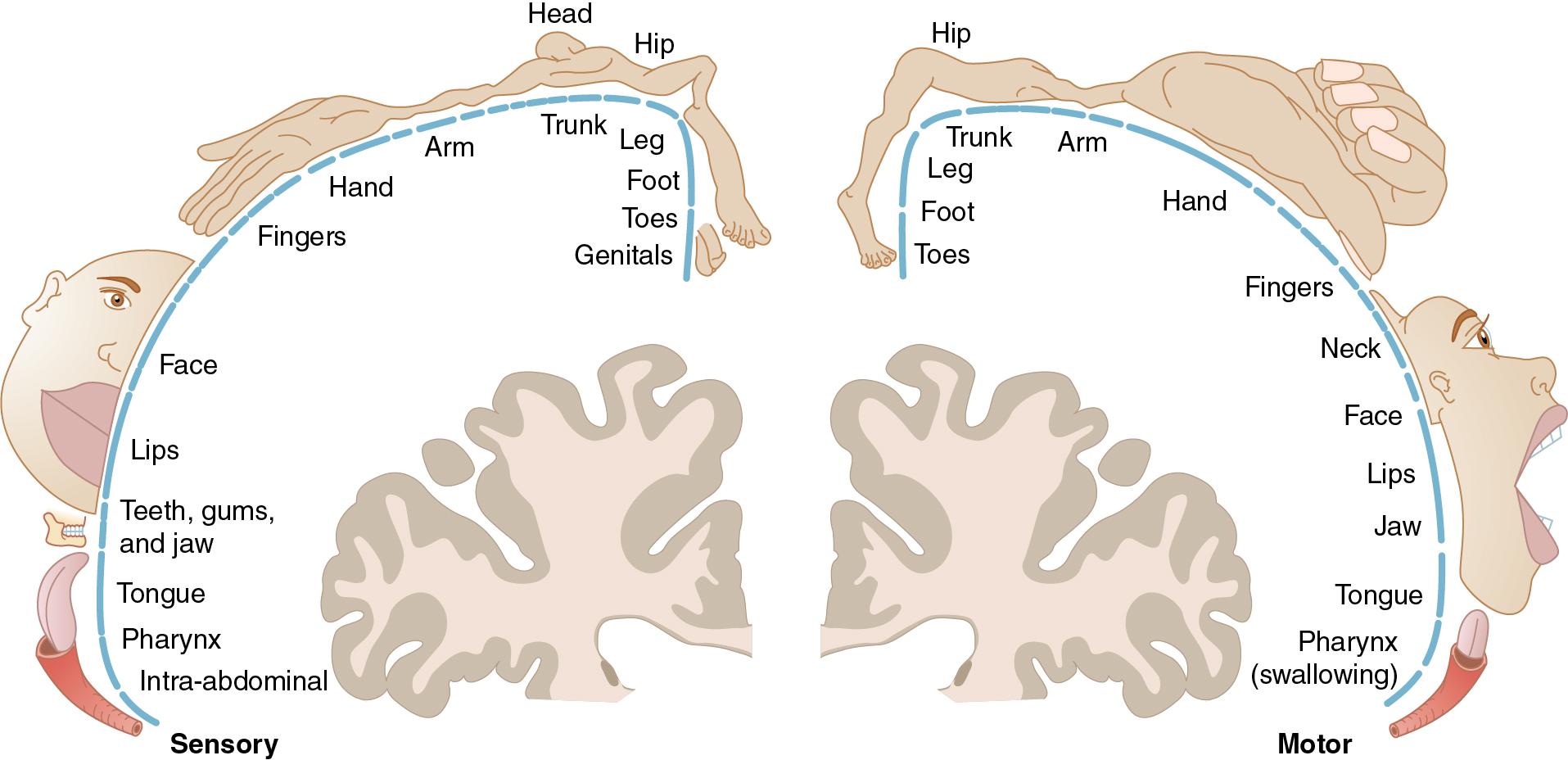
Different topographic maps can largely be separated into sensory, motor, and association areas. These may be visually represented as homunculi ( Fig. 3.7 ).
Sensory
Receive and process information from primary sense modalities from contralateral body.
Controlled by:
Visual cortex (vision)
Auditory cortex (hearing)
Somatosensory cortex (touch)
Piriform cortex (olfaction)
Motor
Executes voluntary motor movements to contralateral body
Controlled by:
Primary motor cortex
Supplemental motor area and premotor cortex assist in selecting voluntary movements
Association
Integrate input from various cortical regions into single cortical region.
Aids in accurate perception of environment.
Relate information to past experiences to inform future actions.
Language processing and production
For written word, information must travel from primary visual cortex → visual association cortex → angular gyrus → Wernicke’s speech area (text/language understanding)
For vocalization, information must travel from arcuate fasciculus → Broca’s speech area (motor speech patterns) → primary motor cortex (see Clinical Correlation Box 3.4 ).
The anterior association area, or prefrontal cortex, is essential for planning, executive decision-making, and appropriate social behavior. Famously, an American railroad worker named Phineas Gage reportedly underwent major personality changes (including a tendency toward inappropriate, uninhibited social behavior) after surviving a work accident in which an iron rod was driven completely through his head, destroying most of his left frontal lobe.
Finally, although the right and left cerebral cortex are connected by the corpus callosum (a large tract of corticocortical fibers running across the interhemispheric fissure), there is still functional lateralization of tasks to specific hemispheres.
Left: Often dominant for speech, writing, language, and algebraic calculation.
Right: Often dominant for construction and nonverbal ideation, and perceives both sides of visual space (rather than only contralateral side) (see Clinical Correlation Box 3.5 ).
Left-sided neglect, or inability to see the left visual space, may occur with damage of right hemisphere.
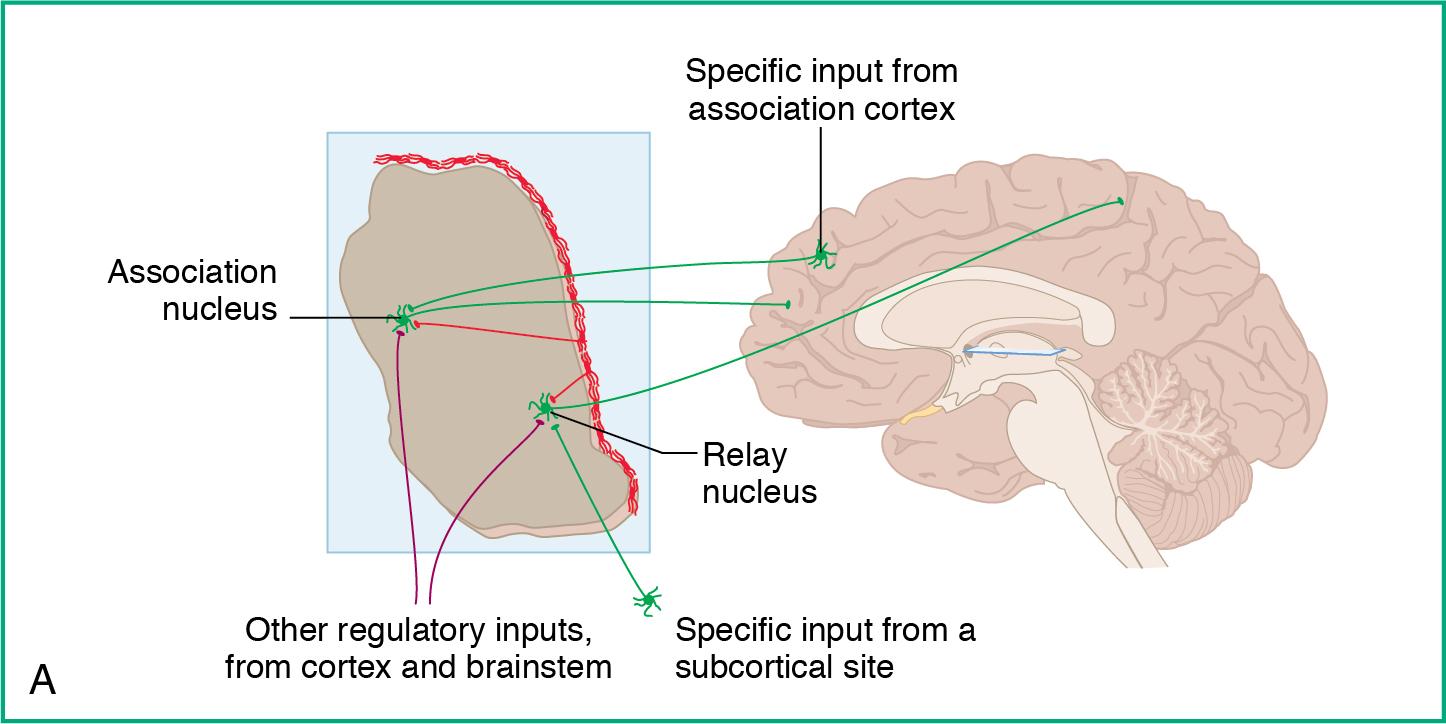
The thalamus ( Fig. 3.8 ) serves several essential functions that are spatially divided:
Main “relay station” for sensory and motor information between the cerebral cortex (except for olfaction)
Ventral anterior (VA) nuclei carry motor signals from cortex to arms.
Also carries motor signals from basal ganglia
Ventral lateral nuclei carry motor signals from cortex to legs.
Also carries motor signals from cerebellum
Ventral posteromedial nuclei carry sensory signals to cortex from arms.
Ventral posterolateral nuclei carry sensory signals to cortex from legs.
Additional key roles in:
Emotion (anterior nucleus)
Memory (dorsomedial nucleus)
Vision (lateral geniculate body)
Hearing (medial geniculate body)
Integration of sensory and motor systems (pulvinar nucleus)
Given its varied roles as a central signal integrator in the cerebrum, we will discuss the specific thalamic circuits throughout the chapter.
As the name implies, the hypothalamus sits just beneath the thalamus ( Fig. 3.9 ). The hypothalamus also serves varied functions in the CNS:
Regulation of the autonomic nervous system
Appetite
Circadian rhythm
Hormone synthesis and regulation
Emotion

As with the thalamus, the hypothalamus is divided into several nuclei to serve these functions:
Anterior nucleus: thermal cooling, parasympathetic tone
Posterior nucleus: thermal heating, sympathetic tone
Lateral hypothalamus: stimulates hunger
Ventromedial nucleus: stimulates satiety
Suprachiasmatic nucleus (SCN): direct retinal input to regulate circadian rhythm
Mammillary body: memory (see Fast Facts Box 3.2 . Also Trauma Box 3.1 , Pharmacology Box 3.1 , Development Box 3.1 and Genetic Box 3.1 .)
Orexin is a neuropeptide primarily released by the lateral hypo-thalamic area. When triggered by signals from the suprachiasmatic nucleus, it drives arousal through stimulation of monoaminergic and cholinergic neurons in the brainstem. It is also triggered by ghrelin, a hormone signaling decreased energy stores, and thus increases hunger. Thus hunger indirectly promotes wakefulness, whereas satiety promotes sleep—explaining the phenomenon of “food coma”!
One dramatic aspect of narcolepsy is the “sleep attack," when an affected patient suddenly and unexpectedly falls asleep in the middle of whatever he or she as doing. A related phenomenon is cataplexy, where patients have the abrupt loss of muscle tone in response to strong emotions (laughter, anger, fear, surprise). As you can imagine, these events, leading to the unanticipated loss of consciousness, can be highly dangerous if a patient falls or is operating a motor vehicle or heavy equipment, making catalepsy a major risk for traumatic injury.
Narcolepsy is often treated with stimulants, such as modafinil and armodafinil. These drugs selectively inhibit the reuptake of dopamine and thereby indirectly increase the release of histamine and hypocretins/orexins from hypothalamic neurons. Tangentially related is the observation that first generation anti-histamines often cause somnolence in adults, but may cause hyperactivity in children, indicating that endogenous histamine is important in neural networks involved in sleep wake cycling.
Narcolepsy can affect patients of any age, but typically manifests initially in adolescents. Pathologically, there is a loss of hypothalamic neurons that produce hypocretins (also termed orexins) that regulate normal circadian sleep-wake cycles.
Narcolepsy is a disease of sleep dysregulation in which affected patients have excessive daytime somnolence, suffer from “sleep attacks” in which they cannot resist the urge to fall asleep, have difficulty sleeping at night and may have vivid dreams, hypnagogic hallucinations, and motor paralysis around transition to sleep. The gene HLA-DQB1*06:02, which encodes peptide presenting proteins on immune cells, is tightly associated to narcolepsy, but how this MHC class II immune system gene is mechanistically related is unclear.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here