Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
| Sign or symptom | Pathological basis |
|---|---|
| Headache | |
|
Raised intracranial pressure Constriction and dilatation of intracranial vessels (migraine) Irritation or inflammation of meninges |
|
Referred from paranasal sinuses, cervical or temporomandibular joints, teeth, ears, etc. Giant cell temporal arteritis |
| Reflexes | |
|
Upper motor neurone lesion Lower motor neurone lesion Compression of motor nerve roots Peripheral neuropathy |
| Neck stiffness | Irritation or inflammation of meninges Cervical spine osteoarthritis |
| Coma or impaired consciousness | Metabolic, e.g.:
Brainstem lesions, e.g.:
Cerebral hemisphere lesions, e.g.:
|
| Cognitive impairment and dementia | Loss of limbic or cortical neurones due to:
|
| Epileptic seizures | Paroxysmal neuronal discharges, either idiopathic or emanating from a focus of cortical disease or damage |
| Skeletal muscle deficit | |
|
Loss of motor stimulus from anterior horn cells or peripheral nerves Myopathy (e.g. muscular dystrophy, steroid-induced myopathy) |
|
Disease directly or indirectly affecting function of:
|
| Sensory impairment and/or paraesthesiae | Disease directly or indirectly affecting function of:
|
| Visual field defects or blindness | Disease involving the eyes, optic nerves and pathway or visual cortex:
|
| Tinnitus and/or deafness | Impaired transmission of sound through external meatus (e.g. wax) or through middle ear (e.g. otitis media), or disease affecting the organ of Corti or the auditory nerve |
The central nervous system (CNS) is the most complex system in the body, able to function both as a self-contained unit and the coordinating centre for activities of the peripheral nervous system (PNS), skeletal muscles and other organ systems.
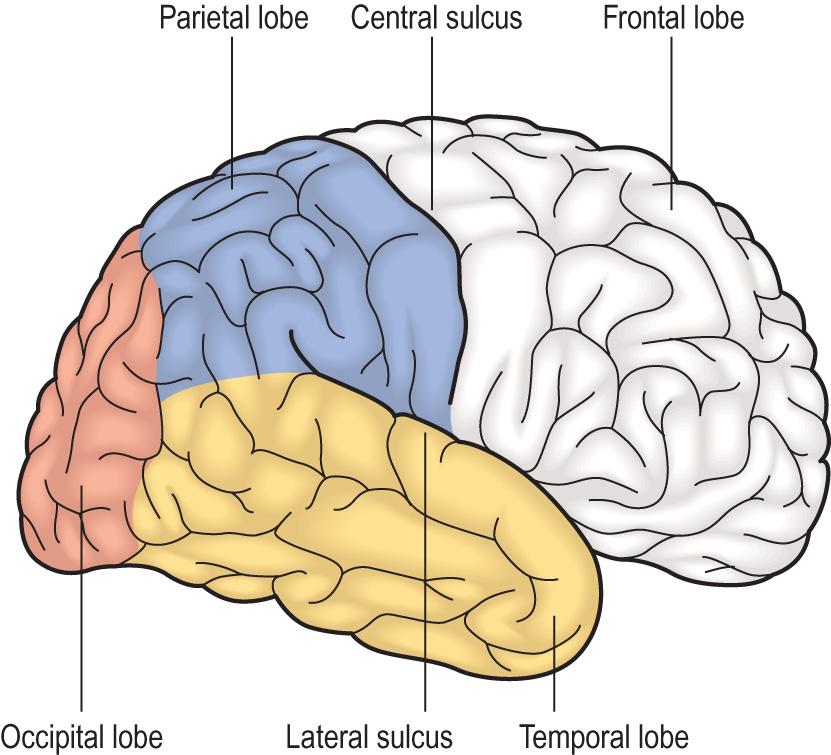
The CNS is composed of three principal structures: the brain, brainstem and spinal cord. The brain comprises two hemispheres covered by grey matter that are joined by a band of white matter known as the corpus callosum. The cerebral cortex is located on the outer surface of the hemispheres, and is composed of six layers of neurones. It is divided into four main regions: the frontal, temporal, parietal and occipital lobes. Each of these has distinct functions, which are summarised in Fig. 26.1 . The white matter beneath the cerebral cortex is composed of axons surrounded by myelin that connect the cortical neurones with neurones elsewhere. In the centre of the hemispheres are groups of grey matter nuclei (deep grey nuclei), the basal ganglia and the thalamus, whose principal functions are motor and sensory coordination, and the regulation of tone and posture. The cerebellum is connected by white matter bundles to the posterior surface of the brainstem. The cortex of the cerebellum lies on its outer surface, but its structure is different from that of the cerebral cortex. The cerebellum has a major role in movement coordination and the regulation of stance, posture and gait.
The brainstem contains many ascending and descending white matter fibre bundles that connect the spinal cord to the brain. It also contains many nuclei, including cranial nerves 3 to 12, the substantia nigra, the respiratory centre and the vomiting centre. The spinal cord is largely composed of ascending and descending white matter fibre bundles, for example, the lateral columns (descending motor fibres) and the posterior columns (ascending sensory fibres). The grey matter of the spinal cord is located centrally, and contains several groups of neurones, including the anterior horn cells that are lower motor neurones supplying all the skeletal muscle in the trunk and limbs. Motor nerve roots leave the anterior spinal cord to form peripheral motor nerves; sensory input from the skin, joints and organs enters the spinal cord by the posterior nerve roots, which pass into the ascending posterior columns.
Despite the structural and functional complexities of the CNS, the constituent cells can be divided into just five main groups:
neurones
glial cells
microglial cells
connective tissue cells
vascular cells.
Neurones are the structural and functional units of the CNS, generating electrical impulses that allow rapid cell–cell communication at specialised junctions known as synapses ( Fig. 26.2 ). Neurones are highly specialised postmitotic cells that cannot be replaced. They are subject to unique metabolic demands, for example, maintaining an axon that may be up to 1 m in length, which makes neurones particularly vulnerable to a wide range of insults, for example, hypoxia and hypoglycaemia.
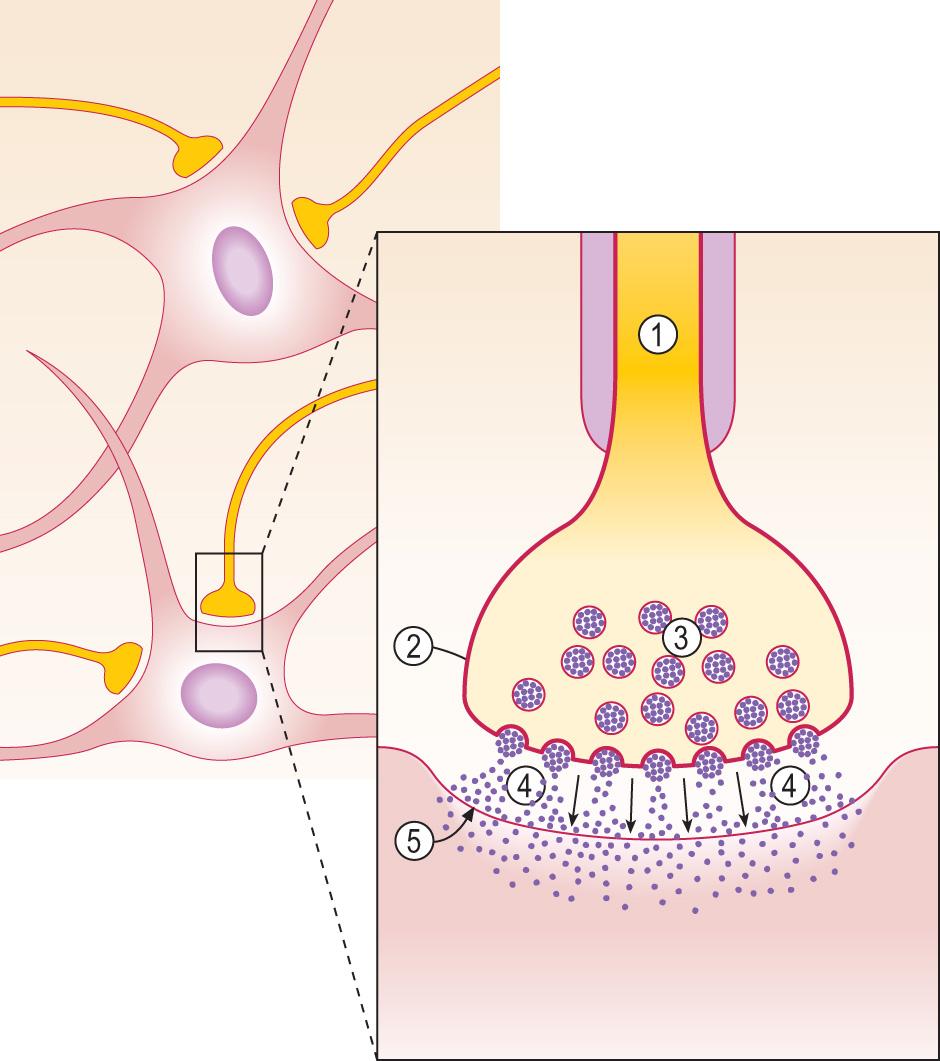
Neurones contain ion channels within the cell membrane that can be opened by either changing the voltage across the membrane, or by the binding of a chemical (neurotransmitter) to a receptor in or near the ion channel. In the resting state, the neuronal cell membrane is relatively impermeable to ions. Opening the ion channels allows an influx of sodium ions, which depolarises the membrane, forming an action potential that is transmitted rapidly down the axon by saltatory conduction. Transmission between neurones occurs at specialised junctions known as synapses (see Fig. 26.2 ). The most common excitatory neurotransmitter in the CNS is glutamate.
Neurones, or nerve cells, vary considerably in size and appearance within the CNS. All possess a cell body, axon and dendrites.
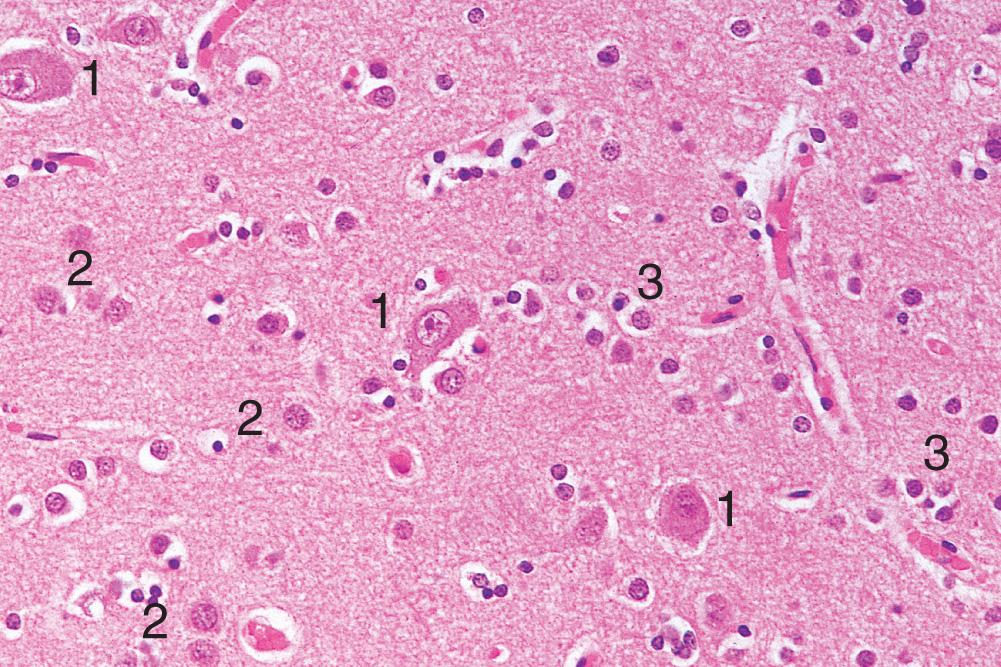
The cell body or perikaryon is easily seen by light microscopy ( Fig. 26.3 ). It contains neurofilaments, microtubules, lysosomes, mitochondria, complex stacks of rough endoplasmic reticulum, free ribosomes and a single nucleus with a prominent nucleolus.
Axons and dendrites are the neuronal processes that convey electrical impulses from and towards the perikaryon, respectively. These processes vary enormously in size and complexity, and may be difficult to identify on routine microscopy.
Glia are specialised supporting cells of the CNS comprising four main groups:
astrocytes
oligodendrocytes
ependymal cells
choroid plexus cells.
Astrocytes are process-bearing cells that are poorly visualised by light microscopy (see Fig. 26.3 ) unless special staining techniques are used. They perform several important roles:
provision of a supportive framework for other cells in the CNS
control of the neuronal microenvironment by influencing local neurotransmitter and electrolyte concentrations
regulation of the blood–brain barrier by processes that are closely applied around capillary endothelium (see below)
proliferation and enlargement in response to neuronal injury.
Oligodendrocytes are the most numerous cells in the CNS. On microscopy, they are visible as darkly staining nuclei located around neurones and nerve fibres (see Fig. 26.3 ). The most important function of oligodendrocytes is the synthesis and maintenance of myelin in the CNS, a lipid-rich protein that insulates myelin to facilitate rapid electrical conduction.
Ependymal cells form the single-cell lining of the ventricular system and the central canal of the spinal cord. They are short columnar cells that bear cilia that may help regulate the flow of cerebrospinal fluid (CSF).
Choroid plexus cells form a cuboidal epithelial covering over the ventricular choroid plexus, where CSF is secreted.
Microglia belong to the macrophage/monocyte system of phagocytic cells. They are normally quiescent, and inconspicuous on light microscopy, but can enlarge and proliferate in reaction to CNS injury, for example, in inflammatory and demyelinating disorders.
Connective tissue in the CNS is confined to two main structural groups: the meninges and perivascular fibroblasts.
The meninges contain fibroblast-like cells in the pia, arachnoid and dura mater, and arachnoidal cap cells, which form the main site of CSF absorption.
The larger blood vessels in the CNS are similar in structure to those elsewhere; however, CNS capillaries differ from most other capillaries in several respects.
They are nonfenestrated, with tight junctions between adjacent cells.
Relatively few microvilli are present on the luminal surface of the endothelial cells, with only occasional pinocytotic vesicles present in the cytoplasm.
The endothelial cell basement membrane is intimately surrounded by a network of astrocyte processes.
These special structural features are important constituents of the blood–brain barrier, a functional unit that controls the entry and exit of many substances — including proteins, ions, nonlipid-soluble compounds and drugs — to and from the CNS.
Axonal damage results in central chromatolysis in neuronal perikarya, with anterograde degeneration of the damaged axon
Axonal regeneration does not occur to a significant extent in the CNS
Axonal degeneration results in breakdown of the myelin sheath around damaged fibres
Hypertrophy and hyperplasia of astrocytes results in gliosis around areas of tissue damage
Microglia and recruited blood monocytes form a population of phagocytic cells
Neurones can undergo various reactive changes to cell injury:
central chromatolysis
anterograde degeneration
synaptic degeneration.
Central chromatolysis is a reaction that usually occurs in response to axonal damage ( Fig. 26.4 ). This reaction is accompanied by increased RNA and protein synthesis, suggesting a regenerative response.
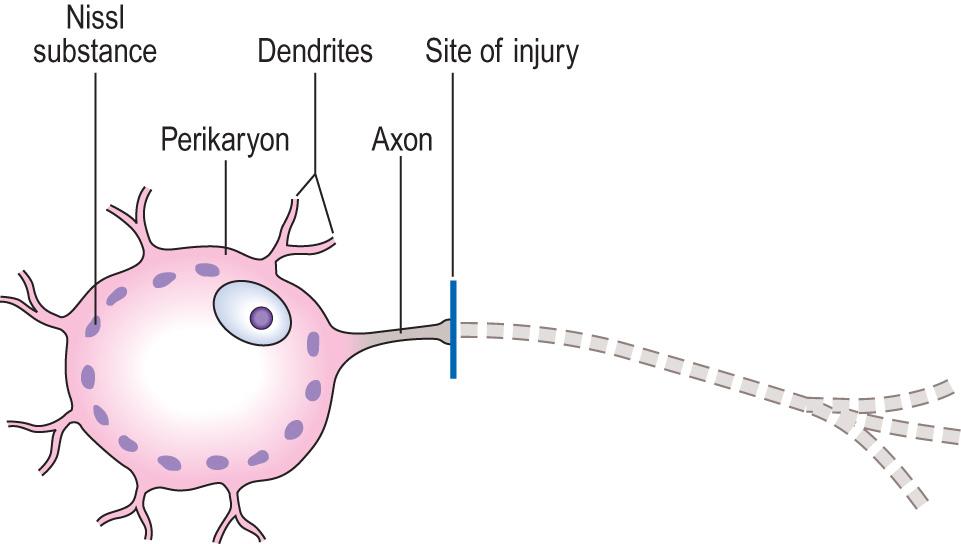
Anterograde degeneration occurs as a result of axonal transection, and is usually accompanied by central chromatolysis (see Fig. 26.4 ). Degeneration of the distal part of the axon will occur following its separation from the intact perikaryon, for example, by transection. The myelin sheath surrounding the axon also fragments after axonal degeneration is established. Axonal and myelin debris is then phagocytosed by macrophages, which often remain around the site of injury for several months.
Synaptic degeneration occurs in many neurodegenerative disorders, for example, Alzheimer disease. Trans-synaptic neuronal atrophy may occur following the loss of afferent connections, for example, in neurones of the lateral geniculate body following damage to the optic nerve.
Astrocytes undergo hyperplasia and hypertrophy following almost all forms of CNS damage, in a response known as ‘reactive gliosis’. Gliotic tissue is translucent and firm, often forming a limiting barrier around areas of tissue damage, for example, at the edge of a cerebral infarct.
Microglia and recruited blood monocytes are involved in the response to CNS damage, acting as phagocytes. When myelin is damaged, microglia ingest the breakdown products and become distended with droplets of lipid.
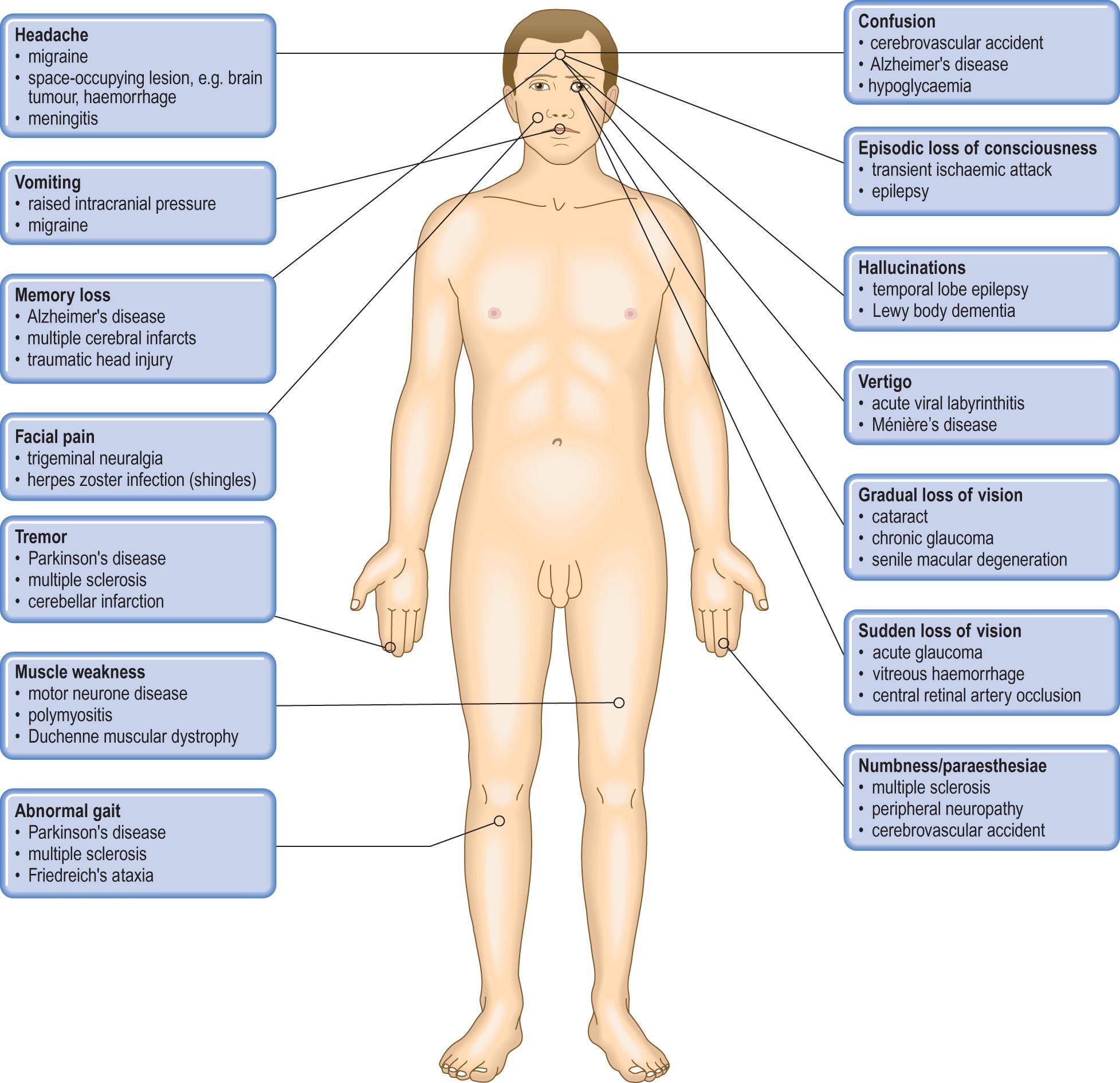
Diseases of the CNS impair the highly complex integration that is necessary for normal neurological function. The resulting clinical abnormalities can often indicate the anatomical site of the lesion in the CNS, which can be investigated by imaging the CNS by magnetic resonance imaging (MRI) scanning. The pathological basis box ( pp. 671-672 ) gives an introduction to some common clinical abnormalities and their pathological basis in the CNS.
Brain swelling may be diffuse or focal
Diffuse brain swelling is usually due to vasodilatation or oedema
Focal brain swelling may be due to inflammatory, traumatic, vascular or neoplastic lesions
Result in intracranial shift and herniation once a critical stage of mass expansion occurs
Produce characteristic signs and symptoms due to raised intracranial pressure and intracranial shift or herniation, and can result in ‘brain death’
Intracranial space-occupying lesions (SOLs) arise from a variety of causes, but share a common feature: expansion in volume of the intracranial contents, resulting in raised intracranial pressure. Brain swelling may be either diffuse or focal.
Diffuse brain swelling denotes a generalised increase in the volume of the brain, which usually results from vasodilatation or oedema.
Failure of the normal vasomotor control system results in vasodilatation, with an increased cerebral blood volume that results in brain swelling. This occurs particularly in response to hypercapnia and hypoxia, but may also occur following severe head injury.
Oedema in the brain is defined as an abnormal accumulation of fluid in the cerebral parenchyma that produces an increase in cerebral volume. Cerebral oedema can be classified into three main types:
vasogenic: due to increased cerebral vascular permeability (blood–brain barrier dysfunction)
cytotoxic: due to cellular injury involving endothelial cells, glia and neurones
interstitial: due to damage to the ventricular lining in hydrocephalus ( pp. 680-681 ).
In many instances, cerebral oedema occurs due to a combination of mechanisms; for example, both vasogenic and cytotoxic mechanisms are involved in ischaemia. Cerebral oedema frequently surrounds focal lesions in the brain, thereby exaggerating their mass effects.
Expanding lesions of many types result in focal brain swelling, for example, cerebral abscesses, haematomas and intrinsic neoplasms. Expanding extrinsic lesions, for example, subdural haematomas and meningiomas, also exert a mass effect within the cranial cavity and act as SOLs.
The consequences of intracranial SOLs may be:
raised intracranial pressure
intracranial shift and herniation
epilepsy
hydrocephalus
systemic effects.
Raised intracranial pressure is an invariable consequence of expanding intracranial SOLs, as there is very little space within the rigid cranium to accommodate an enlarging mass. Initially, a phase of spatial compensation occurs by reductions in the CSF space and the blood volume within the skull. Pressure atrophy of the brain may occur around slow-growing extrinsic lesions, for example, meningiomas (see Fig. 26.36 ).
Any further increase in the volume of the intracranial contents will cause an abrupt increase in intracranial pressure. Characteristic signs and symptoms of raised intracranial pressure and their likely causes include:
headache: due to compression of pain and stretch receptors around intracranial vessels and within the dura mater, usually worse on arising
nausea and vomiting: due to pressure on vomiting centres in the brainstem
visual disturbances: due to papilloedema, when axonal flow is impeded by raised pressure around the optic nerve
impairment of consciousness, ranging from drowsiness to deep coma, related to the level of increased intracranial pressure
‘brain death’ when the intracranial pressure exceeds the cerebral arterial perfusion pressure.
Intracranial shift and herniation are the most important consequences of SOLs. They usually occur following a critical increase in intracranial pressure, which may be precipitated by withdrawing CSF at lumbar puncture. Lumbar puncture is contraindicated in any patient with raised intracranial pressure and a suspected intracranial SOL to prevent this potential complication.
Lateral shift of the midline structures is an early complication of supratentorial SOLs. Patients with acute lateral displacement of the brain have a depressed level of consciousness even in the absence of an intracranial herniation. The clinical features are summarised in Table 26.1 .
| Site of herniation | Effect | Clinical consequence |
|---|---|---|
| Transtentorial | Ipsilateral 3rd cranial nerve compression | Ipsilateral fixed dilated pupil |
| Ipsilateral 6th cranial nerve compression | Horizontal diplopia, convergent squint | |
| Posterior cerebral artery compression | Occipital infarction Cortical blindness |
|
| Cerebral peduncle compression | Upper motor neurone signs | |
| Brainstem compression and haemorrhage | Coma | |
| Cardiorespiratory failure | ||
| Death | ||
| Foramen magnum | Brainstem compression and haemorrhage | Coma |
| Cardiorespiratory failure | ||
| Death | ||
| Acute obstruction of CSF pathway | Coma | |
| Cardiorespiratory failure | ||
| Death |
Herniations occur at several characteristic sites within the cranial cavity, depending on the location of the SOL ( Fig. 26.5 ). Axial displacement is frequently fatal because of secondary haemorrhage into the brainstem ( Fig. 26.6 ).
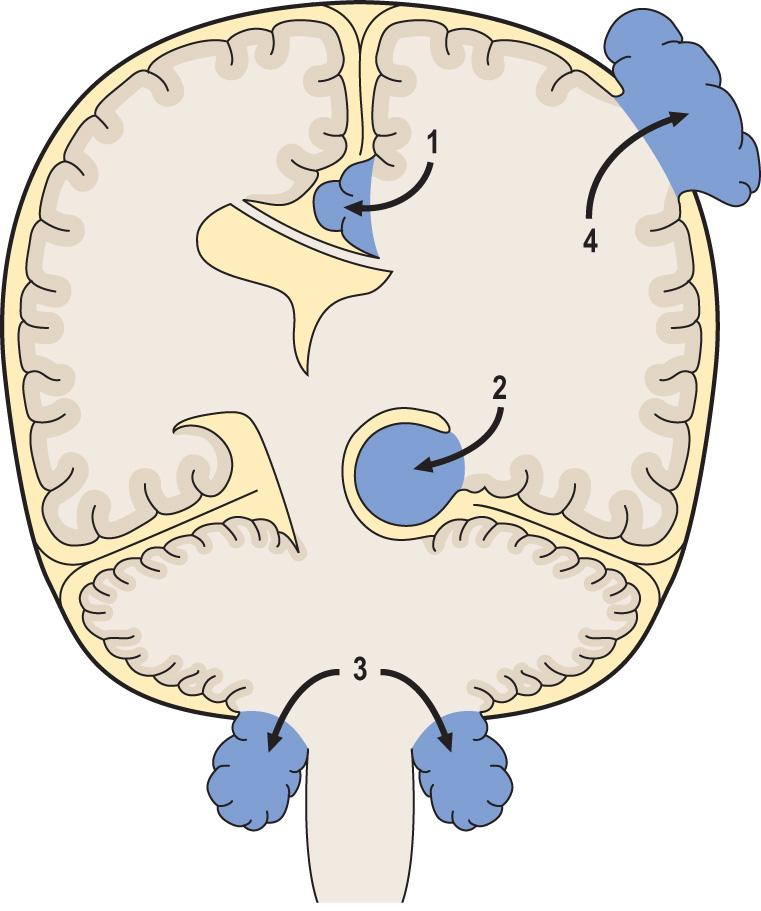
Epileptic seizures may be focal or generalised ( p. 694 ), and are particularly common in patients with raised intracranial pressure due to cerebral abscesses and neoplasms.
Hydrocephalus is a common complication of SOLs in the posterior fossa that compress the cerebral aqueduct and fourth ventricle ( p. 703 ).
The systemic effects of raised intracranial pressure are of major clinical importance and may result in a life-threatening deterioration. These are thought to result from autonomic imbalance and overactivity as a result of hypothalamic compression, resulting in:
hypertension and bradycardia
pulmonary oedema
gastrointestinal and urinary tract ulceration and haemorrhage
acute pancreatitis.
Results from missile or nonmissile injuries: the latter are more common
CNS damage occurs as primary damage (immediate) or secondary damage (after the injury)
Primary damage includes focal lesions (contusions and lacerations) and diffuse axonal injury
Secondary damage includes intracranial haematomas, oedema, intracranial herniation, infarction and infection
Important complications include epilepsy, persistent vegetative state and dementia
In the UK, 200 to 300 per 100, 000 population present to hospital each year with head injuries, most of which result from road traffic accidents and falls. Head injuries are classified according to their aetiology: missile and nonmissile (blunt) injuries. The latter are more common.
Missile injuries to the brain are caused by bullets or other small objects propelled through the air. Three main types of injury are recognised.
Depressed injuries, in which the missile causes a depressed skull fracture with contusions, but does not enter the brain.
Penetrating injuries occur when the missile enters the cranial cavity, but does not exit. Focal brain damage may be accompanied by infection.
Perforating injuries, occur when a missile enters and exits from the cranial cavity, leaving a large exit wound. Brain damage around the missile tract is severe, with extensive haemorrhage. The risk of infection and epilepsy in survivors is high.
Nonmissile injuries to the brain range from relatively minor injuries with spontaneous improvement (as in concussion), to severe injuries that are rapidly fatal. These injuries occur most commonly in road traffic accidents (55%) and falls (35%), when rotational forces acting on the brain are accompanied by impact-related forces that often result in a skull fracture. However, around 20% of fatal head injuries occur without a fracture. The types of brain damage occurring in nonmissile injuries may be classified as either primary or secondary.
Primary brain damage occurs at the time of injury in two main forms: focal damage and diffuse axonal injury.
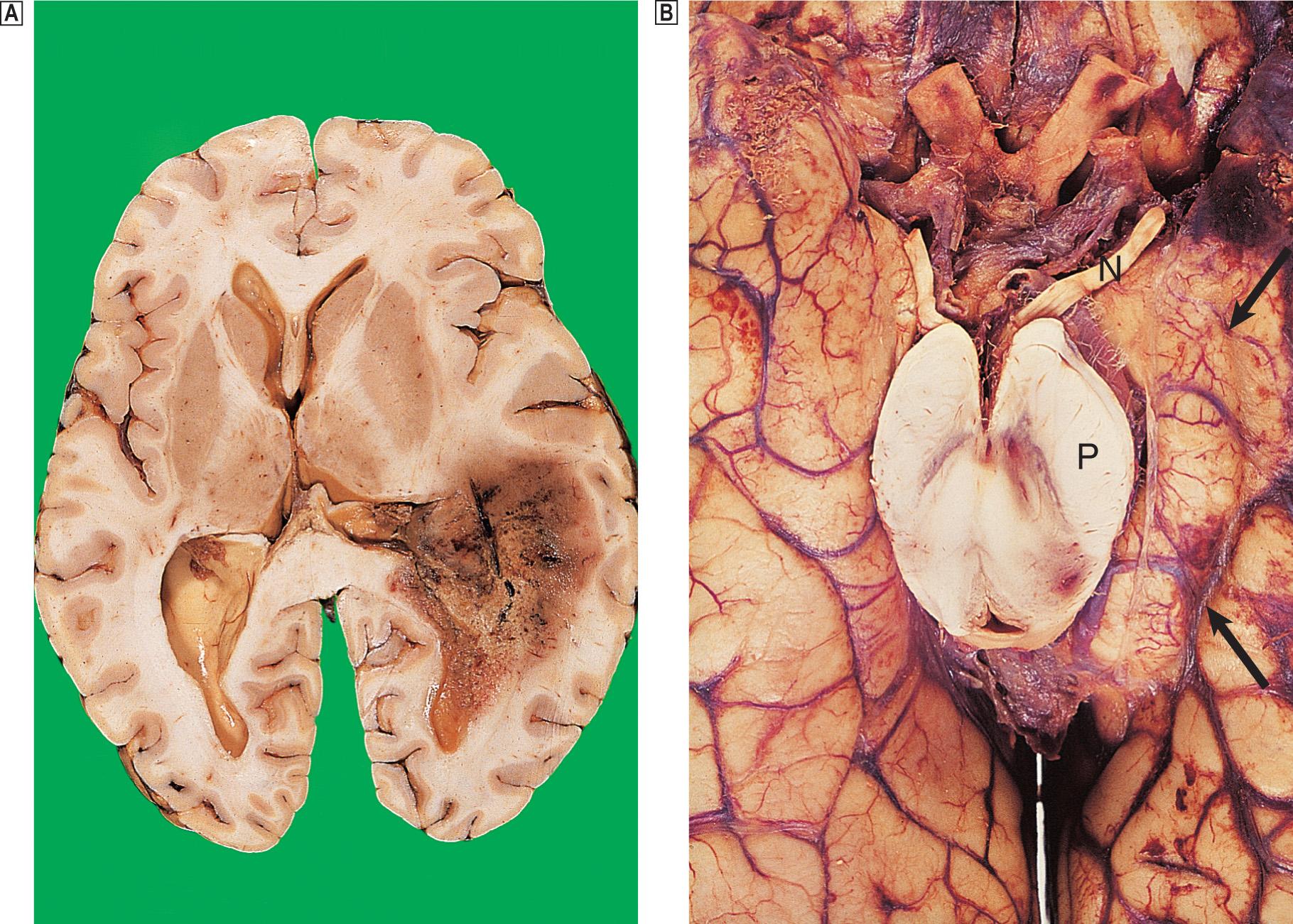
The most common form of focal brain injury is contusions, which usually occur at an impact site, particularly if a skull fracture is present. Contusions are asymmetrical and may be more severe on the side opposite the impact — the ‘contrecoup’ lesion ( Fig. 26.7 ). Following injury, the brain moves within the skull and comes into contact with adjacent bone, resulting in local injury. Large contusions may be associated with intracerebral haemorrhage or cortical lacerations. Healed contusions are represented by wedge-shaped areas of gliosis that are yellow-brown due to the presence of haemosiderin. Severe forms of focal damage include tears of cranial nerves or the brainstem.
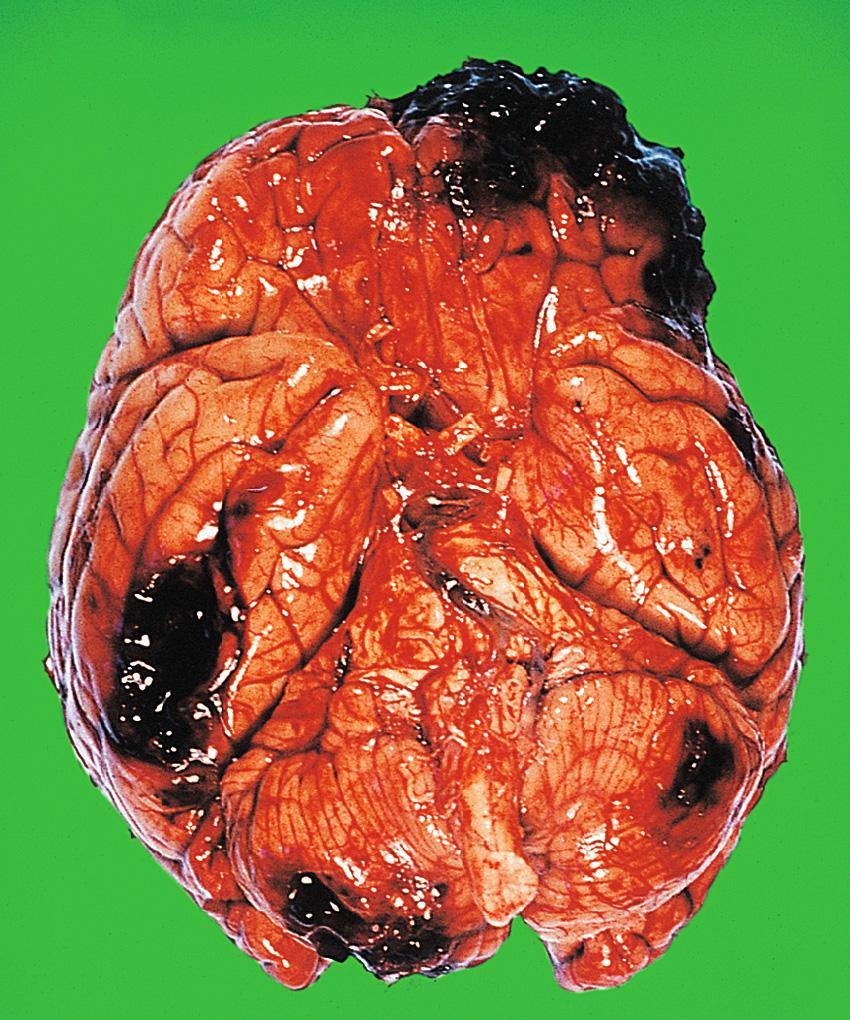
This common form of brain damage occurs as a result of shearing and tensile strains produced by rotational movements of the brain within the skull. It often occurs in the absence of a skull fracture and cerebral contusions. It is most common in road traffic accidents, being seen less often in falls or assaults. Two main components exist.
Small haemorrhagic lesions in the white matter of the corpus callosum and dorsolateral brainstem.
Diffuse damage to axons, which can only be detected microscopically in the form of axonal beads and swellings ( Fig. 26.8 ) in the white matter, called diffuse traumatic axonal injury. Damaged axons eventually degenerate, resulting in a loss of fibres in the white matter.
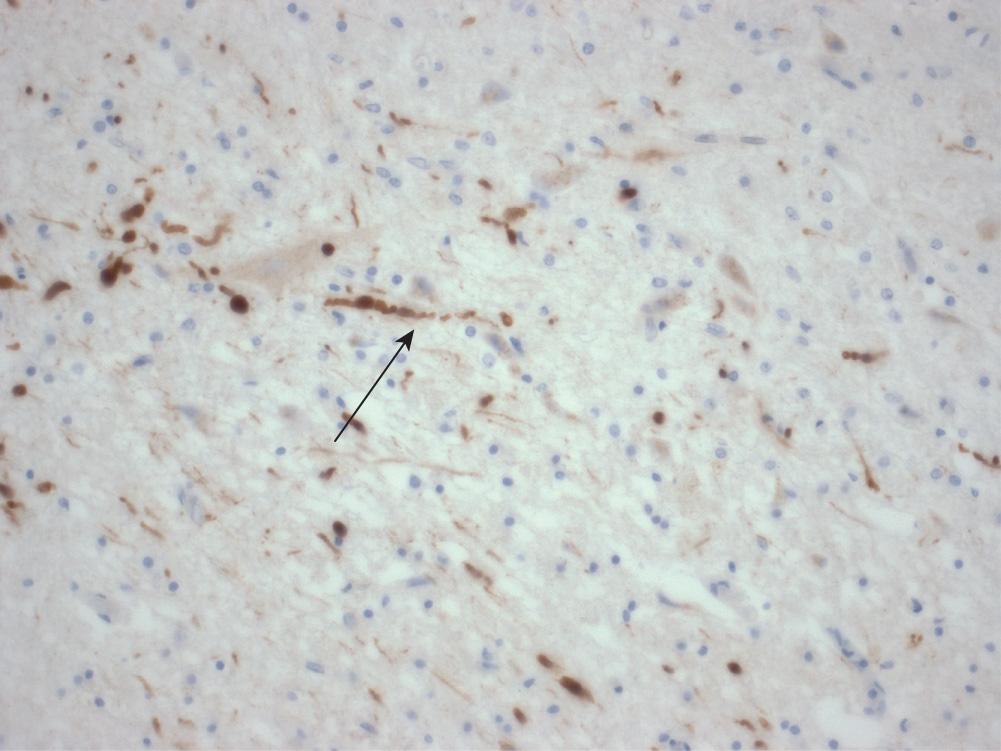
Secondary brain damage results from complications developing after the moment of injury. These complications often dominate the clinical picture, and are frequently responsible for death.
Intracranial haemorrhage. The mechanisms and clinical manifestations of traumatic intracranial haemorrhage are summarised in Table 26.2 .
| Lesion | Mechanism | Clinical manifestations |
|---|---|---|
| Extradural haematoma | Skull fracture with arterial rupture, e.g. middle meningeal artery | Lucid interval followed by a rapid increase in intracranial pressure |
| Subdural haematoma | Rupture of venous sinuses or small bridging veins due to torsion forces | Acute presentation with a rapid increase in intracranial pressure |
| Chronic presentation with personality change, memory loss and confusion, particularly in the elderly | ||
| Subarachnoid haemorrhage | Arterial rupture | Meningeal irritation with a rapid increase in intracranial pressure |
| Intracerebral haemorrhage | Cortical contusions Rupture of small intrinsic vessels with intracerebral haematoma ‘Burst lobe’ with intracerebral haematoma contusions and subdural haematoma |
May cause seizures Increased intracranial pressure with focal deficits; usually fatal Profound coma, usually rapidly fatal |
Traumatic damage to extracerebral arteries. Although uncommon, these are important because some cases can be treated surgically, for example, dissection of the internal carotid artery.
Intracranial herniation. See above.
Ischaemic brain damage. Ischaemic brain damage often results from severe systemic hypotension following blood loss, and raised intracranial pressure. Rarer causes include fat emboli and trauma to extracerebral vessels.
Meningitis. This is particularly common in patients with an open skull fracture.
Most patients with minor head injuries make a satisfactory recovery. However, only 20% of survivors of severe head injuries make a good recovery, while 10% remain severely disabled. Important causes of persisting debility are:
posttraumatic epilepsy, which is the most common delayed complication of nonmissile head injury
persistent vegetative state, in which patients remain severely neurologically impaired due to severe diffuse axonal injury and hypoxic brain damage
posttraumatic dementia, due to severe neuronal loss and axonal damage.
Spinal cord injuries are the major cause of paraplegia and tetraplegia. Over 80% occur in road traffic accidents; most of the patients are males under 40 years of age. Two main groups occur.
Perforating injuries can cause extensive disruption and haemorrhage, but penetrating injuries may result in incomplete cord transection, which may result in the Brown–Séquard syndrome (flaccid paralysis and loss of position and vibration sense on the affected side, with contralateral loss of pain and temperature sense — see Fig. 26.21 ).
Closed injuries account for most spinal injuries and are usually associated with a fracture/dislocation of the spinal column. Damage to the cord depends on the extent of the bony injuries and can be considered in two main stages:
primary damage: contusions, nerve fibre transection, haemorrhagic necrosis
secondary damage: extradural haematoma, infarction, infection, oedema.
Late effects of cord damage include:
ascending and descending anterograde degeneration of damaged nerve fibres
posttraumatic syringomyelia
systemic effects of paraplegia: urinary tract and chest infections, pressure sores and muscle wasting.
The outcome of cord injuries depends mainly on the site and severity of the cord damage. Patients with incomplete lesions in the cauda equina have an almost normal life expectancy, while patients with high cervical lesions have much higher morbidity and mortality.
The principal causes of spinal cord and nerve root compression are:
intervertebral disc prolapse
neoplasms (e.g. metastatic carcinoma, myeloma, schwannoma)
skeletal disorders (e.g. spondylosis, rheumatoid arthritis, Paget disease)
infection (e.g. tuberculosis, abscess)
trauma.
The most common causes of subacute or chronic nerve root and cord compression are intervertebral disc prolapse and spondylosis.
Intervertebral disc prolapse ( Ch. 25 ) occurs in two main ways:
disc rupture following strenuous exercise or sudden exertion in young adults
disc herniation following minimal stress due to degenerative disc disease and spondylosis (see below) in older patients.
In both instances, a tear in the annulus fibrosus allows the soft nucleus pulposus to herniate laterally, causing nerve root compression. Central herniation is less common, but can cause direct cord damage. Disc prolapse occurs most commonly at the C5/C6 and L5/S1 levels; nerve root compression in the latter results in sciatica.
Spondylosis due to osteoarthritis ( Ch. 25 ) of the vertebral column affects around 70% of adults over 40 years of age, and is usually accompanied by degenerative disc disease. Bony outgrowths, known as osteophytes, develop on the upper and lower margins of the vertebral bodies. These may encroach upon the spinal canal or intervertebral foramina to produce nerve root pain that is exacerbated by movement.
A group of disorders resulting in excess CSF within the intracranial cavity
Two main groups: primary hydrocephalus, usually accompanied by increased intracranial pressure; and secondary hydrocephalus, compensatory to loss of cerebral tissue
Primary hydrocephalus usually results from obstruction of the CSF pathway
Produces irreversible brain damage unless the raised intracranial pressure is relieved by surgical drainage
CSF is secreted by the choroid plexus epithelium by processes that carefully regulate its biochemical composition. In adults, the total volume of CSF is around 140 mL; this volume is renewed several times daily ( Fig. 26.9 ). CSF resorption occurs primarily at the arachnoid villi. Hydrocephalus is the term used to denote an excess quantity of CSF within the cranial cavity and is classified as follows:
primary hydrocephalus
secondary or compensatory hydrocephalus.
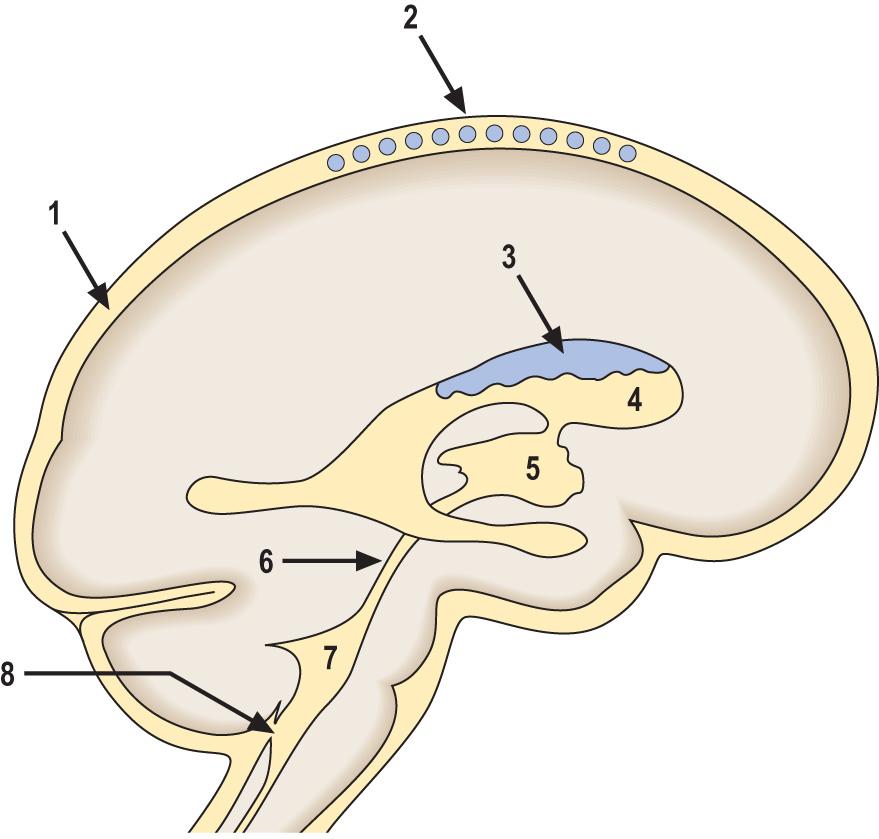
Primary hydrocephalus includes any disorder in which the accumulation of CSF is usually accompanied by an increase in intracranial pressure. It can be due to:
obstruction to CSF flow (noncommunicating hydrocephalus)
impaired CSF absorption at the arachnoid villi (rare)
excess CSF production by choroid plexus neoplasms (very rare).
Obstructive hydrocephalus is by far the most common form; it may be either congenital or acquired.
Congenital hydrocephalus occurs in around 1 per 1000 births. Most cases are diagnosed antenatally by ultrasonography. Congenital malformations, for example, Arnold–Chiari malformation (see Fig. 26.23 ), are the principal causes of congenital hydrocephalus. A few cases in males are due to an X-linked disorder that results in aqueduct stenosis. Aqueduct stenosis is more commonly due to acquired disorders, for example, viral infections that affect both sexes.
Acquired hydrocephalus can result from any lesion that obstructs the CSF pathway (see Fig. 26.9 ). Expanding lesions in the posterior fossa are prone to cause hydrocephalus, as the fourth ventricle and aqueduct are small and easily obstructed. Some lesions may cause intermittent obstruction, for example, colloid cysts of the third ventricle, which may block the foramen of Monro. Obstructive hydrocephalus commonly results from the organisation of blood clot or inflammatory exudate in the CSF pathway following an episode of haemorrhage or meningitis ( Fig. 26.10 ). Intermittent pressure hydrocephalus is thought to result from defective CSF absorption at the arachnoid villi.
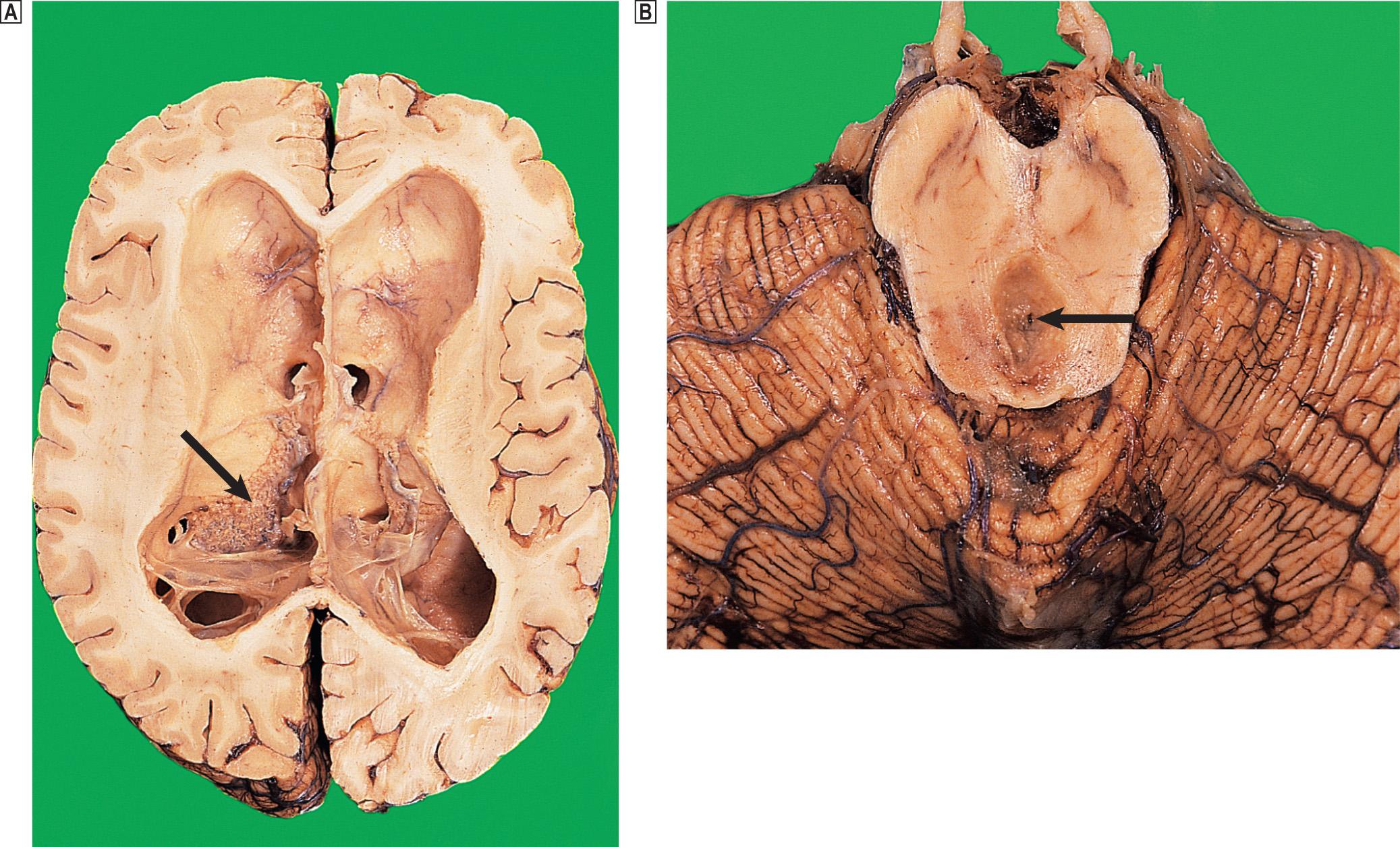
In secondary or compensatory hydrocephalus, an increase in CSF volume follows a loss of brain tissue, for example, cerebral infarction or atrophy, so that overall, there is no increase in intracranial pressure (see Fig. 26.25 ).
Primary hydrocephalus can be relieved by the insertion of a ventricular shunt with a one-way valve system to drain CSF into the peritoneum. Untreated patients may suffer irreversible brain damage due to raised intracranial pressure (see Fig. 26.10 ). Shunts often need to be replaced in growing children and are prone to become infected with low-virulence bacteria, for example, Staphylococcus epidermidis , resulting in blockage and raised intracranial pressure.
Syringomyelia is an uncommon condition in which a cavity (syrinx) expands within the central spinal cord, sometimes up into the brainstem (syringobulbia). Syringomyelia occurs most frequently following spinal cord injury, or an intrinsic neoplasm of the cord, and is most common in the cervical region. However, extensive cavities involving almost the entire length of the cord have been described. Modern radiological techniques are of great value in delineating the extent of the lesion (see Fig. 26.23 ).
Clinical manifestations of syringomyelia include:
lower motor neurone lesions (muscle weakness and atrophy) in upper limbs due to compression of anterior horn cells
dissociated anaesthesia (loss of pain and temperature sensation) due to damage to nerve fibres crossing the cord in the lateral spinothalamic tracts — see Fig. 26.21 .
Surgery can sometimes alleviate symptoms by draining the fluid in the expanding syrinx.
A common cause of morbidity in the elderly in the UK
CNS damage occurs in cerebrovascular disease as a result of ischaemia or haemorrhage
Neurones are the cells most vulnerable to ischaemia; they become irreversibly damaged after 5 to 7 minutes
Important risk factors are atheroma, heart disease, hypertension and diabetes mellitus
Often presents clinically as a stroke or transient ischaemic attack
Most strokes are due to cerebral infarction; the remainder are due to intracerebral and subarachnoid haemorrhages
Cerebrovascular disease is the third most common cause of death in the UK (after heart disease and cancer) and is a major cause of morbidity, particularly in the middle-aged and elderly. The ultimate effect of cerebrovascular disease is to reduce the blood supply to the CNS, resulting in local tissue damage.
Ischaemic damage to the CNS occurs when there is insufficient blood flow to fulfil metabolic requirements. Tissue damage may also occur:
when the blood supply is normal, but oxygen is carried at a reduced tension (hypoxia) or is absent (anoxia)
in rare circumstances when cellular respiratory enzyme function is impaired, for example, in cyanide poisoning.
The cells most vulnerable to ischaemia are the neurones, which depend almost exclusively on the oxidative metabolism of glucose for energy. Experimental evidence suggests that the earliest stages of ischaemic neuronal damage are reversible; the final stages, however, result in neuronal death.
The neurones most vulnerable to ischaemia are those in the third, fifth and sixth layers of the cortex, in the CA1 sector of the hippocampus and the Purkinje cells in the cerebellum. In infants, certain brainstem nuclei are also vulnerable. The basis of this selective vulnerability is unknown. Ischaemic neuronal death is characterised by activation of glutamate receptors, causing uncontrolled entry of calcium into the cell. Complete cessation of the circulation, for example, following myocardial infarction, results in global cerebral ischaemia. In less severe cases, a critical reduction of cerebral blood flow may result in boundary zone infarcts between the territories supplied by each of the main cerebral arteries.
The term stroke denotes a sudden event with disturbance of CNS function due to vascular disease which persists for more than 24 hours or causes death within that time. The annual incidence of stroke is 3 to 5 per 1000 of the general population worldwide, but it is much more common in the elderly. Transient ischaemic attacks are CNS disturbance due to vascular disease lasting for less than 24 hours. Transient ischaemic attack is a major risk factor for stroke; most attacks are due to circulatory changes in the CNS occurring as the result of disease in the heart or extracranial arteries.
The clinical features of stroke depend on the localisation and nature of the lesion ( Table 26.3 ). Recurrent or multiple strokes often occur in patients with recognised risk factors, particularly heart disease, hypertension and diabetes mellitus.
| Cause | % | Clinical presentation | 30-day mortality (%) | Pathogenesis | Predisposing factors |
|---|---|---|---|---|---|
| Cerebral infarction | 82 | Slowly evolving signs and symptoms | 15–45 | Cerebral hypoperfusion Embolism Thrombosis |
Heart disease (e.g. infective endocarditis, endocardial thrombus) Hypertension Atheroma Diabetes mellitus |
| Intracerebral haemorrhage | 15 | Sudden onset of stroke with raised intracranial pressure | 80 | Rupture of arteriole or microaneurysm | Hypertension Vascular malformation |
| Subarachnoid haemorrhage | 3 | Sudden headache with meningism | 45 | Rupture of saccular aneurysm on circle of Willis | Hypertension Polycystic renal disease Coarctation of the aorta |
Most infarcts occur within the internal carotid territory, particularly in the distribution of the middle cerebral artery. Infarction of the corticospinal pathway in the region of the internal capsule is a common event, resulting in contralateral hemiparesis. Although many infarcts produce clinical symptoms, small infarcts may not result in any apparent neurological disturbance. Multiple cerebral infarcts may also result in dementia ( p. 698 ).
The following mechanisms may be responsible for cerebral infarction:
arterial thrombosis occurring as a complication of atheroma in the intracranial or extracranial arteries supplying the CNS
embolic arterial occlusion occurring as a complication of atheroma in extracranial vessels, for example, around the carotid artery bifurcation, or mural thrombus in the heart following myocardial infarction; fat and emboli may also result in cerebral infarction following major trauma
head injury may result in cerebral ischaemia, vascular occlusion or rupture, all of which may cause cerebral infarction
subarachnoid haemorrhage following rupture of a saccular aneurysm may be accompanied by vascular spasm, resulting in cerebral infarction
generalised arterial disease, for example, vasculitis, may affect both intracranial and extracranial vessels and result in cerebral infarction
critical reduction of cerebral blood flow, for example, following cardiac arrest
critical reduction in arterial oxygenation, for example, profound hypoxia following respiratory arrest
intraventricular haemorrhage in neonates is often accompanied by infarction in the adjacent white matter (periventricular leucomalacia)
venous thrombosis as a complication of local sepsis or drugs, for example, oral contraceptives.
Around 24 hours after infarction, the affected tissue becomes softened and swollen, with loss of definition between grey and white matter. There may be oedema around the infarct, potentiating the local mass effect. Within 4 days, the infarcted tissue undergoes colliquative (liquifactive) necrosis. Histology shows infiltration by macrophages containing the lipid products of myelin breakdown. Reactive astrocytes and proliferating capillaries are often present at the edge of the infarct. Eventually, all the dead tissue is phagocytosed, leaving a cystic cavity with a gliotic wall ( Fig. 26.11 ). Some infarcts are haemorrhagic, due to reflow of blood through anastomotic channels. Anterograde degeneration of axons occurs distal to the site of infarction, for example, in the ipsilateral cerebral peduncle in infarcts involving the internal capsule.
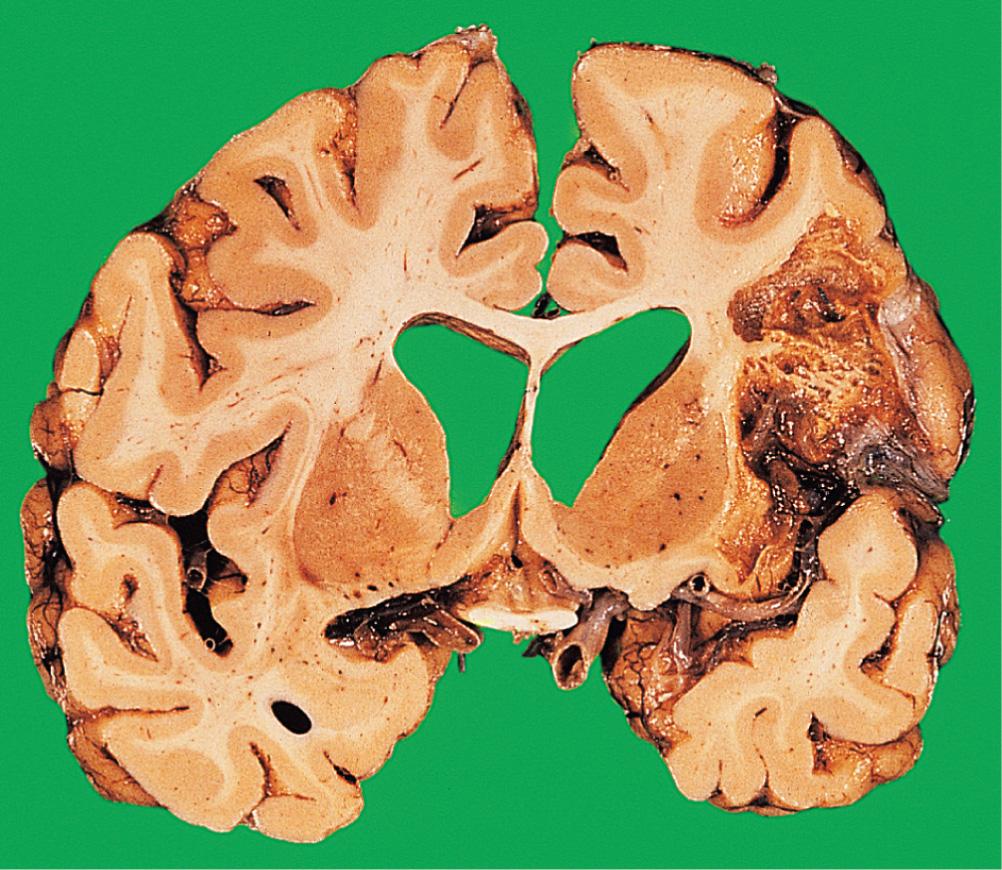
Venous infarction is a consequence of venous thrombosis, and results in haemorrhagic infarction of the cerebral cortex and subcortical white matter. It usually occurs secondary to other disease processes, for example, local sepsis, dehydration or drugs (e.g. oral contraceptives).
Intracerebral and subarachnoid haemorrhage together account for around 18% of strokes. Extradural and subdural haemorrhages usually occur following trauma and are considered in Table 26.3 .
Intracerebral haemorrhage occurs most frequently in the basal ganglia (80% of cases), the brainstem, cerebellum and cerebral cortex. Most occur in hypertensive adults over 50 years of age. The haematoma acts as an SOL, causing a rapid increase in intracranial pressure and intracranial herniation ( Fig. 26.12 ). In survivors, resorption of the haematoma eventually occurs, and a cyst with a gliotic wall is formed. The mortality from spontaneous intracerebral haemorrhage is greater than 80%, and many survivors suffer severe neurological deficits.
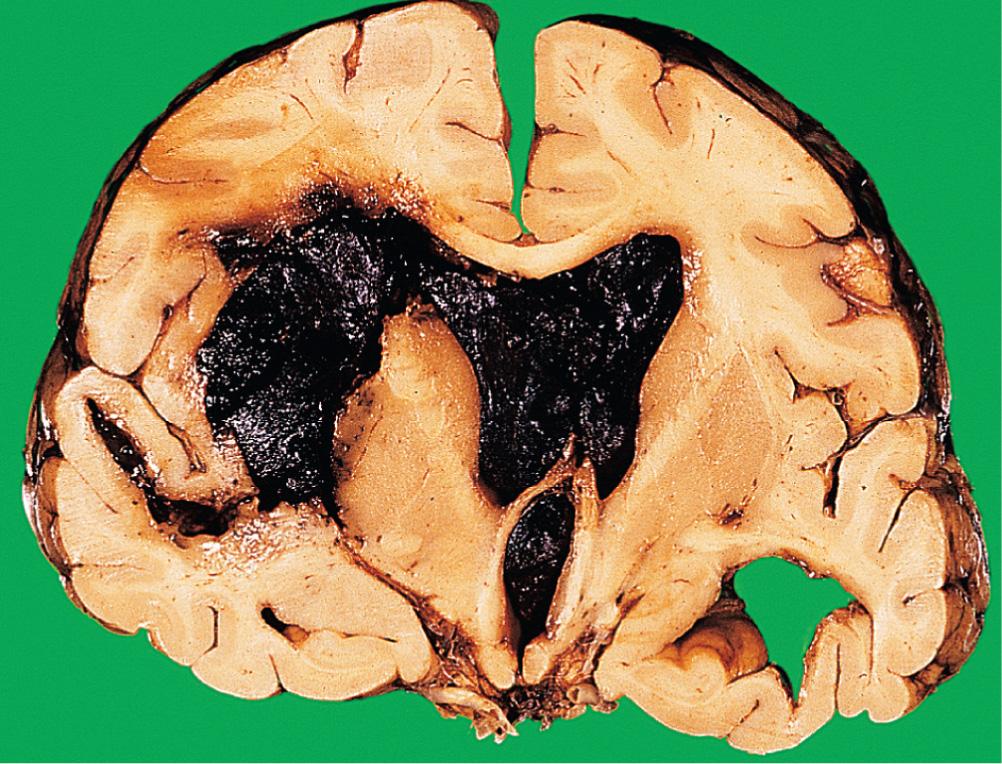
Most intracerebral haemorrhages occur following rupture of the lenticulostriate branch of the middle cerebral artery. Recent studies have found that the ruptured vessels are arterioles, which show replacement of smooth muscle by lipids and fibrous tissue (lipohyalinosis), predisposing to rupture. Intracerebral haemorrhage in children and younger adults may occur as a consequence of trauma, or rupture of an arteriovenous malformation. In older adults, haemorrhage into the lobes of the brain may be due to amyloid deposition in the vessel walls (amyloid angiopathy), which is associated with Alzheimer disease ( pp. 697-698 ).
Subarachnoid haemorrhage usually occurs following spontaneous rupture of a saccular aneurysm on the circle of Willis. Other causes include trauma, hypertensive haemorrhage, vasculitis, tumours and coagulation disorders.
Saccular aneurysms occur in 1% to 2% of the general population, but are more common in the elderly. Most patients with ruptured saccular aneurysm are between 40 and 60 years of age; males are affected twice as often as females. The role of hypertension in the pathogenesis of saccular aneurysms is uncertain, but it appears that hypertensive patients are more likely to have multiple aneurysms than are normotensive patients. Local vascular abnormalities, for example, atheroma, are also important. Their pathogenesis is thought to relate to defects in the smooth muscle of the tunica media at the site of an arterial bifurcation, where local haemodynamic factors act to produce a slowly enlarging aneurysm.
Saccular aneurysms are usually sited at proximal branching points on the anterior portion of the circle of Willis, particularly on the internal carotid, anterior communicating and middle cerebral arteries. Most are less than 10 mm in diameter, but some may be partly filled by thrombus, which can obscure their true size on radiological studies ( Fig. 26.13 ).
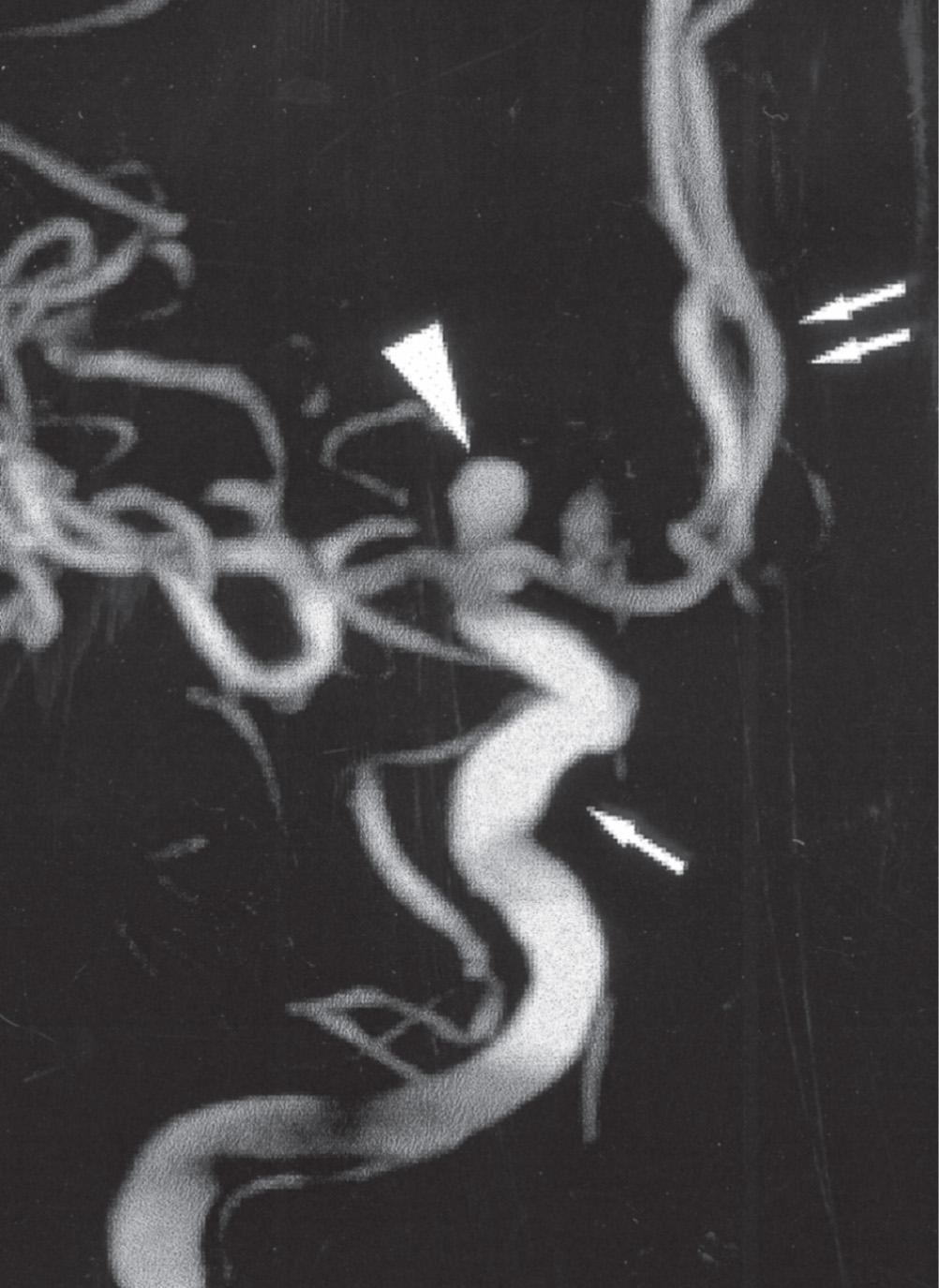
Subarachnoid haemorrhage often presents with a characteristic clinical history of sudden onset of severe headache. Blood accumulates in the basal cisterns and around the brainstem following rupture of a saccular aneurysm. Subarachnoid haemorrhage may be instantly fatal in as many as 15% of cases, with some patients dying later due to rebleed at the site of rupture, or arterial spasm.
Arterial spasm following rupture causes cerebral ischaemia, which is often accompanied by brain swelling due to oedema. One-third of survivors are permanently disabled as a consequence of ischaemic brain damage.
Hydrocephalus can occur acutely following rupture as blood accumulates in the basal cisterns, or at a later stage due to organisation and fibrosis of old haemorrhage in the subarachnoid space or arachnoid granulations.
As well as being a major risk factor for stroke, systemic hypertension causes many other changes in the CNS that result in neurological dysfunction:
alteration in autoregulation of cerebral blood flow (e.g. a sudden drop in systemic blood pressure to normal levels may result in hypoperfusion)
vascular changes, especially atheroma
aneurysms, including atheromatous aneurysms of the basilar artery and saccular aneurysms on the circle of Willis
dementia due to multiple cerebral infarcts.
Spinal cord infarction is most often due to trauma or compression, but may result from cord ischaemia following myocardial infarction or aortic dissection. In such cases, the infarct occurs in the midthoracic region of the cord in the distribution of the anterior spinal artery, where the arterial blood supply is relatively poor. Infarction results in paraplegia with dissociated sensory loss, as the posterior columns are spared (see Fig. 26.21 ).
Intracranial haemorrhage in neonates has a markedly different pathology from intracranial haemorrhage in adults, for example, rupture of superficial veins following traumatic delivery. Haemorrhage from the subependymal germinal matrix can result in infarction of the adjacent white matter, and is a major cause of death in premature neonates.
Three main types of vascular malformation occur in the CNS:
arteriovenous malformations
cavernous angioma
capillary telangiectasis.
Arteriovenous malformations are clinically the most important; these usually consist of an irregular plexus of dilated thick-walled vessels in the superficial grey matter of the cerebral hemispheres or spinal cord. All cerebral vascular malformations may be clinically silent, but are also associated with epilepsy ( p. 694 ), or may rupture to result in a subarachnoid or intracerebral haemorrhage.
Follow direct spread of infection, or by haematogenous spread
Meningitis is the most common form of bacterial infection; it occurs most frequently in children and the elderly
CSF in bacterial meningitis contains many neutrophil polymorphs and bacteria; the fluid has high protein and low glucose concentrations
Complications of bacterial meningitis include hydrocephalus, cerebral thrombophlebitis and cerebral abscess
Cerebral abscesses are encapsulated foci of suppuration which act as SOLs
Tuberculous infections occur as subacute meningitis or, rarely, as intracerebral tuberculomas
The CNS is normally sterile, but once bacteria gain access, spread of infection can occur rapidly. Bacteria gain access to the CNS by three main routes:
direct spread from an adjacent focus of infection, for example, the paranasal sinuses or middle ear, or from outside the body, for example, head injury with open skull fracture
blood-borne spread as a consequence of septicaemia, or as septic emboli from infections elsewhere, for example, bacterial endocarditis and bronchiectasis
iatrogenic infection, following introduction of organisms into the CSF at lumbar puncture. Low-grade meningitis may occur in up to 20% of patients with a ventriculoperitoneal shunt ( p. 680 ), usually due to skin commensal organisms.
The clinical term ‘meningitis’ usually refers to inflammation in the subarachnoid space involving the arachnoid and pia mater, for example, leptomeningitis. However, inflammation of the meninges may involve predominantly the dura mater (pachymeningitis).
Pachymeningitis is usually a consequence of direct spread of infection from the bones of the skull following otitis media or mastoiditis, and is a well-recognised complication of skull fracture. Common bacterial pathogens include Gram-negative bacilli from the middle ear, alpha- or beta-haemolytic streptococci from paranasal sinuses, or mixed organisms from skull fractures. An epidural or subdural abscess may then occur.
Epidural abscess . This is the result of suppuration between the dura mater and the skull or vertebral column. Epidural abscesses can act as SOLs, and require treatment by surgical drainage and antibiotics.
Subdural abscess . Subdural abscess is an uncommon lesion, as pus can readily spread in the subdural space to form a subdural empyema. Involvement of subdural vessels may result in cerebral cortical thrombophlebitis with infarction. Spontaneous resolution is rare, so surgical drainage and antibiotic therapy is usually required.
Leptomeningitis (‘meningitis’) frequently results from blood-borne spread of infection, particularly in children, but many cases arise from direct spread of infection from the skull bones. The most important organisms are:
in neonates: Escherichia coli, Streptococcus agalactiae, Listeria monocytogenes
2 to 18 years: Neisseria meningitidis (meningococcus)
over 30 years: Streptococcus pneumoniae.
Tuberculosis and syphilis are considered separately on page 687 .
Following successful vaccination programmes, bacterial meningitis due to Haemophilus influenzae is now rare. Vaccines are now also available for serogroups A and C of N. meningitidis , and for S. pneumoniae . Meningococcal meningitis can occur as isolated cases or as an epidemic outbreak in small communities. The serogroup B meningococcus is the most common cause of bacterial meningitis, and is spread in droplets from asymptomatic nasal carriers; the carriage rate in small communities may reach over 25%. The organism reaches the CNS by haematogenous spread; the onset of the symptoms of meningitis may follow symptoms of upper respiratory tract infection. A petechial rash may herald the onset of disseminated intravascular coagulation (Waterhouse–Friderichsen syndrome), which is often fatal. Vigorous antibiotic therapy is essential: incomplete or inappropriate therapy can be fatal or may result in chronic meningitis.
Examination of the CSF following lumbar puncture is essential; the CSF changes in the CNS infections are summarised in Table 26.4 . The CSF in bacterial meningitis usually contains many organisms, although these are sometimes detected only on culture. In fatal cases, pus is present in the cerebral sulci and around the base of the brain, extending down around the spinal cord ( Fig. 26.14 ).
| Cells | Protein (g/L) | Glucose (mmol/L) | Appearance | |
|---|---|---|---|---|
| Normal | 0–4 lymphocytes/mm 3 | 0.15–0.40 | 2.7–4.0 | Clear and colourless |
| Bacterial meningitis | ↑↑ polymorphs | ↑ | ↓ or absent | Opaque and turbid |
| Tuberculous meningitis | ↑ polymorphs initially, then lymphocytes | ↑ | ↓ or absent | Clear or opalescent |
| Viral meningitis | ↑ polymorphs initially, then ↑↑ lymphocytes | ↑ | Normal | Clear and colourless |
| Viral encephalitis | ↑ polymorphs initially, then lymphocytes | ↑ | Normal | Clear and colourless |
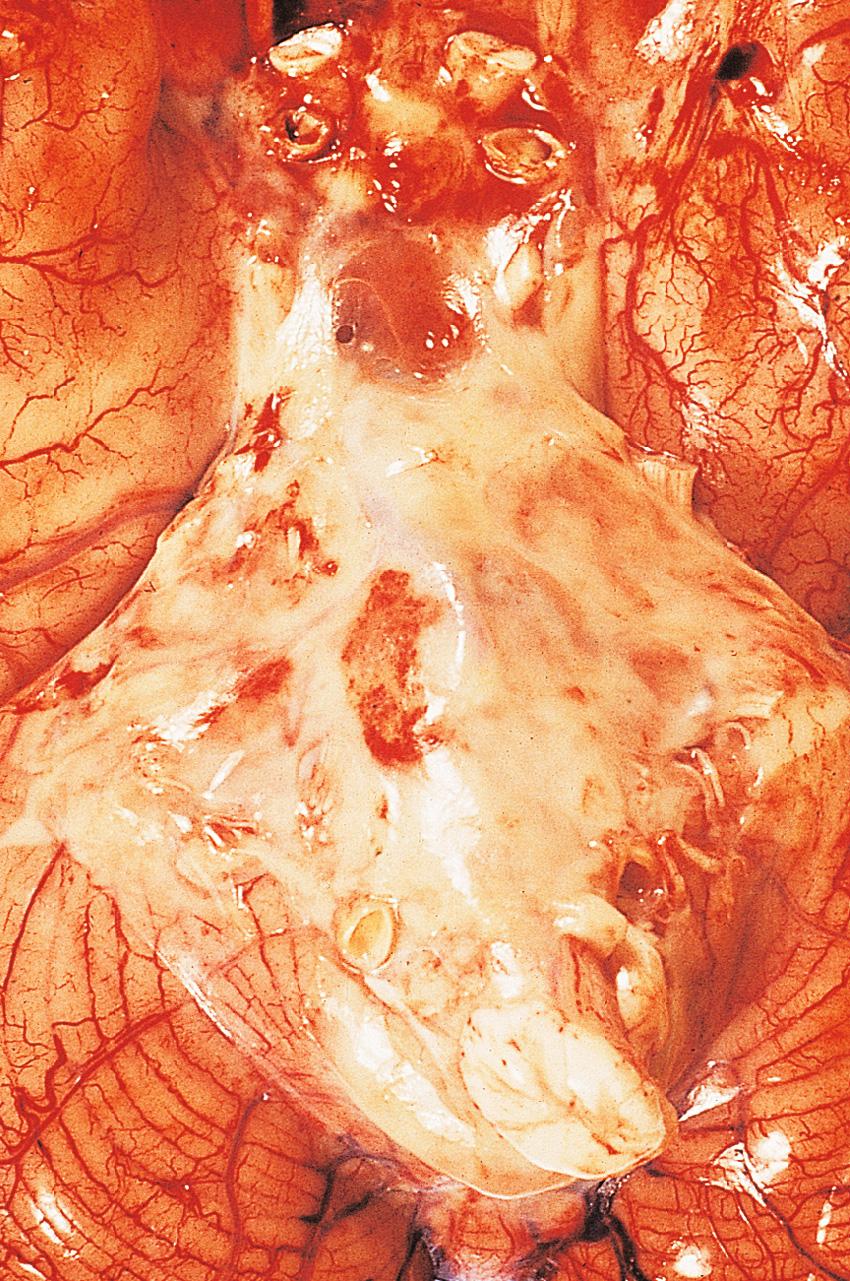
The meningeal and superficial cortical blood vessels are congested, often with small foci of perivascular haemorrhage. The CSF is usually turbid, even in the ventricles, which often show signs of acute inflammation with fibrin deposition. Common complications of bacterial meningitis are:
cerebral infarction
obstructive hydrocephalus
cerebral abscess
subdural empyema
epilepsy.
A cerebral abscess usually develops from an acute suppurative encephalitis in three ways.
Direct spread of infection, usually Gram-negative bacilli, from the paranasal sinuses or middle ear.
Septic sinus thrombosis due to spread of infection from the mastoid cavities or middle ear via the sigmoid sinus.
Haematogenous spread, for example, in patients with infective endocarditis (particularly in congenital heart disease) or bronchiectasis. Haematogenous abscesses are most often found in the parietal lobes, and are often multiple.
Abscess formation occurs when suppuration is accompanied by local tissue destruction ( Fig. 26.15 ). A pyogenic membrane is formed and the abscess develops a capsule composed of granulation tissue, surrounded by reactive gliosis. The adjacent brain is markedly oedematous, containing perivascular collections of lymphocytes and plasma cells. Cerebral abscesses frequently enlarge and become multiloculate.
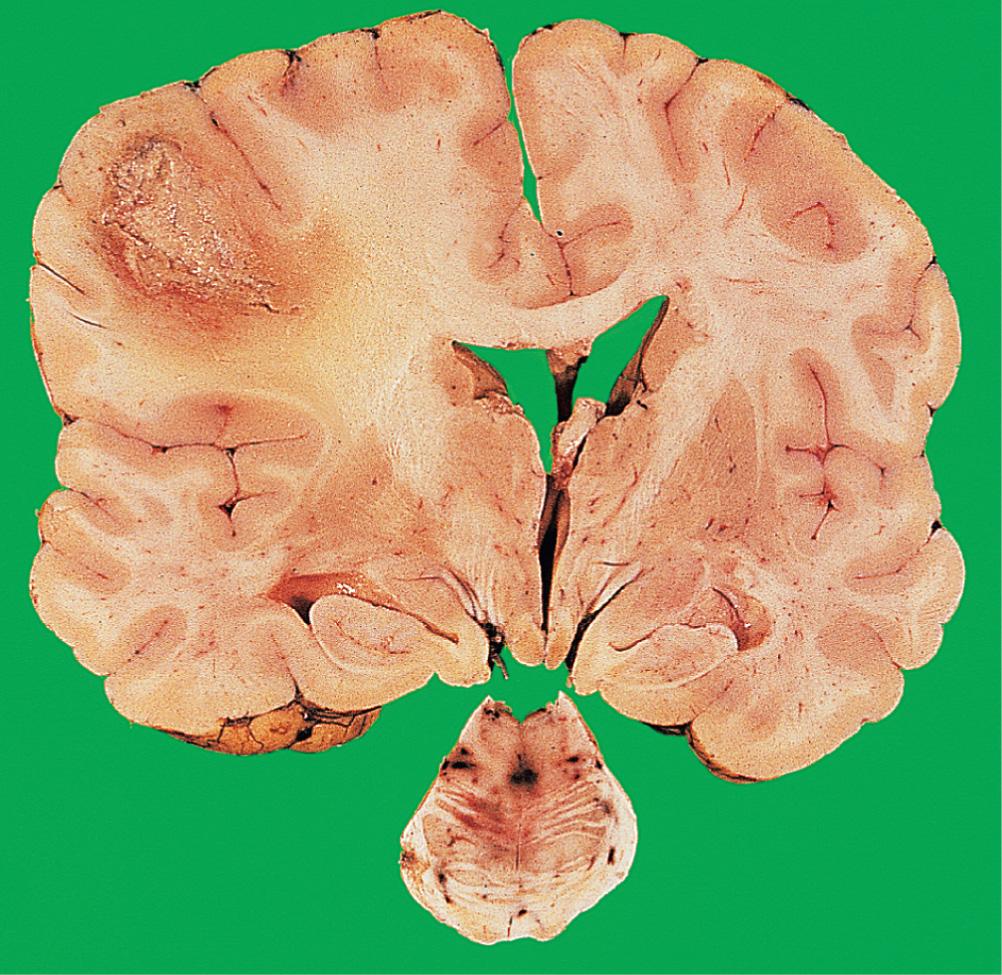
The clinical presentation is similar to that of acute bacterial meningitis, but focal neurological signs, epilepsy and fever are more common. Abscesses act as SOLs; a lumbar puncture should not be performed as an initial investigation on a patient with a suspected cerebral abscess as this may precipitate intracranial herniation. Antibiotic therapy is useful in the treatment of abscesses in an early stage, but surgical aspiration or excision is usually necessary once a capsule has formed. Complications of cerebral abscesses include:
meningitis
intracranial herniation
focal neurological deficit
epilepsy.
Tuberculous infection of the CNS is always secondary to infection elsewhere in the body, usually in the lungs. CNS involvement takes two main forms: tuberculous meningitis and tuberculomas.
Tuberculous meningitis usually results from haematogenous spread from a primary or secondary complex in the lungs. Rarely, it can result from direct spread of infection from a vertebral body to the meninges. The resulting meningitis is characterised by a thick gelatinous exudate, which is most marked around the basal cisterns and within cerebral sulci. The CSF findings are summarised in Table 26.4 . On microscopy, meningeal involvement consists of granulomas with central caseation in which giant cells may be present.
Patients usually present with signs and symptoms of a subacute meningitis, occasionally accompanied by isolated cranial nerve palsies. However, the clinical features may be entirely nonspecific. This disorder is frequently fatal and requires intensive antituberculous chemotherapy.
Tuberculomas are uncommon in the UK, but are still encountered in patients originating from some other countries (particularly in Asia). These consist of focal areas of granulomatous inflammation with caseation, surrounded by a dense fibrous capsule. Tuberculomas usually present with symptoms of raised intracranial pressure; features of meningitis rarely occur. Antituberculous therapy and surgical excision are usually required.
Syphilis is now rare in the UK. After the initial infection, Treponema pallidum gains access to the CNS by haematogenous spread. CNS involvement includes:
clinically silent meningitis during primary and secondary stages
meningeal thickening in the tertiary stage, causing cranial nerve palsies
gummas (focal inflammatory lesions) causing cerebral or spinal compression
tabes dorsalis due to degeneration of dorsal spinal columns.
Infections spread to the CNS by the haematogenous route, or by retrograde neural transport
Viral meningitis is a common, self-limiting illness with characteristic CSF changes
Encephalitis is less frequent, but may result in death or severe disability
Reactivation of a latent viral infection (e.g. herpes zoster) may damage the CNS
CNS involvement in HIV infection is common and often accompanied by other viral, bacterial or parasitic infections
Acute disseminated encephalomyelitis, a demyelinating disorder, may result from a virus-induced immune reaction
Viruses can spread to the CNS by the following routes:
haematogenous spread as part of a systemic infection with viraemia, usually causing meningitis or encephalitis
neural spread along peripheral sensory nerves by retrograde axonal transport.
Certain viruses exhibit neurotropism — a tendency to spread specifically to the CNS from the initial site of infection, for example, poliovirus from the gut. Viruses can cause neurological dysfunction either as a result of viral multiplication within cells of the CNS, or very rarely as a result of an immunological response to a viral infection.
Although acute in onset, viral meningitis is usually clinically less severe than bacterial meningitis. In most instances, the viruses reach the CNS by haematogenous spread. Common organisms are:
echovirus 7, 11, 24, 33
Coxsackie B1 to 5
Coxsackie A9
mumps virus
other enteroviruses.
Characteristic changes are present in the CSF (see Table 26.4 ), and serology or polymerase chain reaction (PCR) techniques are often used to confirm the diagnosis.
Viral meningitis is characterised by infiltration of the leptomeninges by mononuclear cells (lymphocytes, plasma cells and macrophages), along with perivascular lymphocytic cuffing of blood vessels in the meninges and superficial cortex.
Infection of the brain is a well-recognised complication of several common viral illnesses, for example, measles and mumps. Most cases are mild, self-limiting conditions, but others, for example, rabies and herpes simplex type I infections, result in extensive tissue destruction and are often fatal. Herpes simplex encephalitis is the most common variety of acute viral encephalitis in the UK. Despite these differences in severity, all viral infections of the brain and spinal cord produce similar pathological changes in the CNS:
mononuclear cell infiltration by lymphocytes, plasma cells and macrophages; this is often noticeable as perivascular cuffing which usually extends into the parenchyma ( Fig. 26.16 )
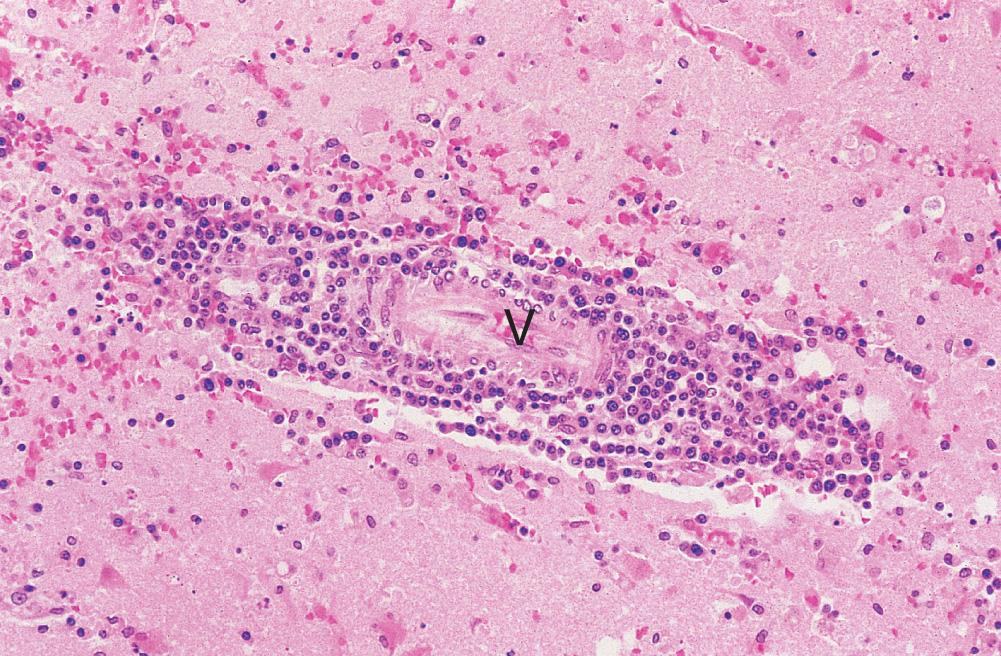
reactive hypertrophy and hyperplasia of astrocytes and microglia
cell lysis (cytolytic viral infection) and phagocytosis of cell debris by macrophages; when neurones are involved, for example, in poliovirus infection, this process is known as neuronophagia
viral inclusions, which can be present in infected neurones, for example, Negri bodies in rabies, or glia.
Herpes zoster results from reactivation of latent varicella-zoster virus within sensory ganglia in the CNS, the infection having been established following chickenpox in childhood. Reactivation (resulting in shingles) usually occurs during periods of intercurrent illness or immunosuppression, particularly in the elderly. Acute inflammation of the sensory ganglion (usually a thoracic dorsal root ganglion or the trigeminal ganglion) is accompanied by pain and hyperalgesia along the nerve distribution, followed by erythema and vesicle formation. Involvement of the ophthalmic division of the trigeminal nerve may result in blindness as a consequence of corneal ulceration and scarring.
Progressive multifocal leucoencephalopathy results from CNS infection by the JC papovavirus. Most cases occur in immunosuppressed patients, for example, in AIDS. The virus produces a cytolytic infection of oligodendrocytes, resulting in demyelination of the white matter. The disease is uniformly fatal.
This uncommon disease usually affects children aged 7 to 10 years and is characterised by a progressive neurological deficit with dementia and myoclonus leading to death. Subacute sclerosing panencephalitis is caused by the measles virus, which is usually acquired before the age of 1 year. Large numbers of measles viral inclusion bodies are present within neurones, and high titres of measles antibody can be detected in the CSF. The pathogenesis of this disorder is not fully understood.
The fetal CNS can be damaged during the first trimester of pregnancy following maternal infection with cytomegalovirus or rubella virus; the latter is becoming less common following immunisation in schoolgirls. Both viruses cause a necrotising encephalomyelitis resulting in developmental damage and microcephaly.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here