Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
To maintain acid–base balance, the renal tubules secrete hydrogen ions into the lumen at a rate equal to the sum of extrarenal acid generation and bicarbonate filtration. In this chapter we review the cellular mechanisms responsible for transepithelial hydrogen secretion along the nephron and describe the regulators of these processes.
To maintain acid–base balance, the renal tubules secrete hydrogen ions into the lumen at a rate equal to the sum of extrarenal acid generation and bicarbonate filtration. In this chapter we review the cellular mechanisms responsible for transepithelial hydrogen secretion along the nephron and describe the regulators of these processes.
The fluid entering the proximal tubule is the glomerular ultrafiltrate, which is in Donnan equilibrium with plasma and has a bicarbonate concentration of approximately 25 mEq/L. The proximal convoluted tubule secretes H ions that acidify the luminal fluid leading to the reabsorption of approximately 14 apart from 75% of filtered bicarbonate and decreasing luminal bicarbonate concentration to 5–10 mEq/L and luminal pH from 7.4 to ~6.8. The proximal straight tubule has a smaller capacity for H secretion, but also contributes to bicarbonate reabsorption. With an acidic luminal fluid (relative to the peritubular space) and a relatively small transepithelial voltage [−2 (early proximal tubule) to +2 (late proximal tubule) mV], transepithelial H secretion requires active transport.
The proximal tubule cell voltage is between −50 and −70 mV relative to the peritubular fluid and cell pH of between 7.15 and 7.30. Using these values, it can be calculated that protons (hydrogen ions) can enter the cell across the basolateral membrane passively (or equivalently base can passively exit the cell across the basolateral membrane), while H ion secretion across the apical membrane from the cell to the luminal fluid is against the electrochemical gradient, and thus, requires active transport.
Net acidification of the tubular fluid may be mediated by apical membrane H secretion or HCO 3 absorption. These two processes, shown in Figure 55.1 , have been distinguished using the disequilibrium pH. The mechanisms shown in Figure 55.1 involve two reactions in series. The association/dissociation of H 2 CO 3 (carbonic acid) to or from H and HCO 3 (reaction 1) occurs instantaneously, while the association/dissociation of H 2 CO 3 to or from CO 2 and H 2 O (reaction 2) is very slow. Carbonic anhydrase catalyzes the latter reaction such that in its presence all constituents are at equilibrium. In the absence of luminal carbonic anhydrase, the second reaction will not be at equilibrium and the relative concentrations of the substrates can be used to determine the direction in which the reactions are occurring, and, thus, whether there is direct H secretion or direct HCO 3 absorption.
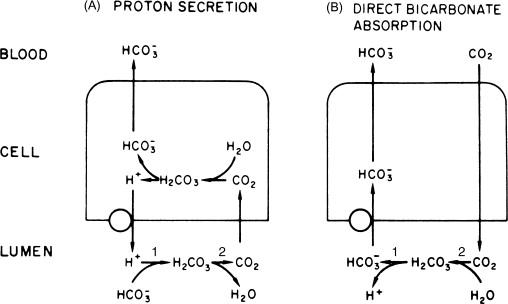
If carbonic anhydrase is present in the lumen, H 2 CO 3 will be in equilibrium with CO 2 and H 2 O, and H 2 CO 3 concentration will be that calculated from the concentration of CO 2 and the equilibrium constant for the second reaction. Equivalently, using the equilibrium constants for the above reactions, the concentrations of H, HCO 3 and CO 2 will be those predicted for equilibrium. But, if carbonic anhydrase is absent from the luminal fluid and H secretion is the mechanism by which the tubular fluid is acidified ( Figure 55.1a ), reactions 1 and 2 will move to the right, but the H 2 CO 3 concentration will be greater than that predicted for equilibrium with CO 2 , and H and HCO 3 concentrations, while in equilibrium with H 2 CO 3 , will also be greater than that predicted for equilibrium with CO 2 . Conversely, if luminal carbonic anhydrase is absent and HCO 3 absorption is the mechanism by which the tubular fluid is acidified ( Figure 55.1b ), luminal H 2 CO 3 , H, and HCO 3 concentrations will all be lower than that predicted for equilibrium with CO 2 .
Using slightly different approaches, three groups of investigators have examined this issue. All found that under baseline conditions the system is in equilibrium and measured in situ pH (H ion concentration) is equal to the predicted equilibrium pH. However, after systemic infusion of a carbonic anhydrase inhibitor luminal pH drops below that predicted for equilibrium (an acid disequilibrium pH), demonstrating secretion of H ( Figure 55.1a ).
These results provide two extremely important conclusions. First, they demonstrate that the mechanism(s) responsible for luminal fluid acidification involve H secretion rather than HCO 3 absorption across the proximal tubule apical membrane. This conclusion has been confirmed by numerous studies (see below) that have directly identified H rather than HCO 3 transport mechanisms on the apical membrane. The second conclusion of equal importance is that the luminal fluid is normally exposed to carbonic anhydrase. This luminal carbonic anhydrase allows secreted H ions to be buffered quickly, preventing an acid disequilibrium pH that would oppose continued H secretion. This latter point is discussed further below.
The discussion below first describes the mechanisms that mediate apical membrane H secretion and then discusses possible backleak mechanisms. The description of the H secretory mechanisms is oriented along lines of coupling to metabolism. The term primary active transport describes transport mechanisms directly coupled to the consumption of metabolic energy (ATP), such as the H-ATPase. Secondary active transport refers to a transporter that couples the transport of H to that of a second ion, for which a favorable driving force is established by another transporter that is directly coupled to energy consumption. An example of secondary active transport is Na/H exchange. Tertiary active transport refers to the presence of three transporters functioning in series, where the first transporter is directly coupled to energy utilization and establishes a favorable electrochemical gradient for molecular species A . A second transporter is a coupled transporter that uses the electrochemical gradient for species A to establish a favorable electrochemical gradient for species B , and the third transporter then uses the electrochemical gradient for species B to transport acid or base (species C ) against its electrochemical gradient. There is now good evidence that all of these mechanisms are present in the proximal tubule. The primary and secondary transport mechanisms play key roles in net acid secretion, while the tertiary transport mechanisms most likely contribute significantly to NaCl reabsorption, and play a minimal role in net acid secretion.
Five lines of evidence suggest the presence of an apical membrane H translocating ATPase. These include the demonstration of: (1) an H translocating ATPase in apical membrane vesicle preparations, (2) a role of an H-ATPase in the defense of cell pH against an acid load, (3) electrogenic H secretion in the microperfused proximal tubule, (4) apical membrane immunohistochemical labeling with antibodies against purified vacuolar H-ATPase subunits, and (5) a component of HCO 3 absorption that cannot be attributed to secondary or tertiary active H secretion.
Kinne-Saffrin and Kinne were the first to demonstrate the presence of HCO 3 -stimulated H-ATPase activity and ATP-dependent H translocation in brush border membrane vesicles. This H translocating ATPase is not of mitochondrial origin as it was insensitive to oligomycin.
In the rat proximal tubule microperfused in vivo symmetrically with Na-free solutions, Yoshitomi et al. found that cell pH decreases and then returns to control levels. Similarly, Alpern found that rat proximal tubules perfused symmetrically with Na-free solutions develop an alkaline cell pH in the steady state. Both of these observations are best explained by the presence of a Na-independent alkalinizing mechanism in these cells. Kurtz more specifically examined this phenomenon in the S3 proximal tubule perfused in vitro utilizing the fluorescent measurement of cell pH. Following an acute intracellular acid load (NH 3 /NH 4 + prepulse), cell pH is rapidly defended in the complete absence of Na. This cell alkalinization is inhibited by luminal dicyclohexylcarbodiimide (DCCD) and N-ethylmaleimide (NEM), two inhibitors of vacuolar H-ATPases. Nakhoul et al. demonstrated that S3 proximal tubules, acidified by removal of luminal and peritubular Na, returned their cell pH to values more alkaline than control cell pH. In these studies, the cell alkalinization was not inhibited by DCCD. All of these studies demonstrate the ability of the proximal tubule cell to transport protons out in response to cell acidification in the complete absence of extracellular Na. Based on our current knowledge of transporters capable of active H efflux from cells, the only Na-independent mechanism that has been identified is the H-ATPase. However, the lack of specificity of the inhibitors, DCCD and NEM, precludes a definitive interpretation from the above studies.
A third line of evidence suggesting the presence of an apical membrane H-ATPase is the demonstration of electrogenic H secretion. Fromter and Gessner first reported that when luminal and peritubular capillaries of rat proximal convoluted tubules are perfused at fast rates with bicarbonate Ringer's solutions (containing no organic components), transepithelial voltage is +1 mV in the early proximal tubule and +0.2 mV in the late proximal tubule. This lumen positive voltage is not affected by ouabain but is inhibited by acetazolamide, a carbonic anhydrase inhibitor. These results demonstrate electrogenic H secretion. While they are consistent with an apical membrane electrogenic H transporting mechanism, they are also consistent with a basolateral membrane electrogenic H/HCO 3 transport mechanism in series with any apical membrane conductance that need not mediate H transport.
Perhaps the best evidence for an apical membrane H-ATPase is the demonstration of the labeling of the apical membrane with antibodies against the purified vacuolar H-ATPase. High resolution immunogold labeling demonstrated that the H-ATPase is located at the neck of the apical invaginations between the clathrin-coated domains and the microvilli.
Lastly, as will be discussed below (see “Role of Apical Membrane H/HCO 3 Transport Mechanisms in Transepithelial H Secretion”), there is good evidence that a component of proximal tubule HCO 3 absorption exists that is not attributable to secondary or tertiary active transport mechanisms.
Based on all of the above, there is strong evidence for the existence of a proximal tubule apical membrane H translocating ATPase of the vacuolar type that contributes to luminal acidification. Its molecular composition was reviewed, this will be described in greater detail later in this Chapter under the “Distal Nephron,” and its role in proximal tubule function will be discussed below.
The apical membrane Na/H antiporter mediates most of NaHCO 3 absorption and all of transcellular NaCl absorption in the proximal tubule. Na/H antiporters represent a form of secondary active transport in that they are not directly coupled to metabolism, but rather use the low cell Na concentration established by the ouabain-sensitive basolateral membrane Na,K-ATPase to drive the ‘uphill’ extrusion of protons. Na/H antiporters are inhibited by amiloride and more potently by amiloride analogs with hydrophobic substitutions at the 5-amino nitrogen atom. Three pieces of evidence are consistent with a Na/H antiporter on the proximal tubule apical membrane.
Murer, Hopfer and Kinne were the first to demonstrate that apical membrane vesicles prepared from rat renal cortex possess Na/H antiporter activity. The antiporter acidifies the external medium in response to an inwardly directed Na gradient, and an outwardly directed H gradient drives Na uptake. The Na/H antiporter is coupled in a 1:1 manner such that transport is electroneutral. Similar results were reported by Warnock et al. and by Kinsella and Aronson.
Subsequent studies in vesicles demonstrated that the apical membrane Na/H antiporter displays saturation kinetics with respect to external Na with a K Na of approximately 5–15 mEq/l at pH 7.5. External protons compete with external Na for a single site such that increases in external H concentration decrease the apparent affinity for Na, the K H is 35 nM. The Na/H antiporter is inhibited by amiloride and lithium. Kinsella and Aronson showed that lithium and amiloride compete with Na at the external site and Ives et al. showed that the inhibition by lithium and amiloride involves both competitive and noncompetitive interactions. As will be discussed below, the Na/H antiporter is exquisitely sensitive to the H concentration on the inside (cytoplasmic side) of the vesicle.
An apical membrane Na/H antiporter has also been demonstrated in the intact tubule by measuring the effect of luminal Na on cell pH. Boron and Boulpaep measured cell pH using a pH-sensitive microelectrode in the in vitro perfused salamander proximal tubule and found that following an acid load cell pH recovery is accelerated in the presence of luminal Na, an effect that is blocked by luminal amiloride. Sasaki et al. measured cell pH in the in vitro perfused rabbit proximal straight tubule using a pH sensitive microelectrode and showed that luminal Na removal and readdition causes cells to acidify and realkalinize, respectively. Alpern and Chambers measured cell pH in the in vivo perfused rat proximal convoluted tubule microfluorimetrically and found similarly that luminal Na removal causes cell acidification and Na readdition causes cell alkalinization. In the studies of Sasaki et al. and Alpern and Chambers, the cell pH change was blocked by amiloride and by amiloride analogs.
The last line of evidence supporting the existence of an apical membrane Na/H antiporter is the demonstration of the dependence of HCO 3 absorption on Na transport. In the in vivo microperfused rat proximal convoluted tubule and the in vitro microperfused rabbit proximal convoluted tubule and proximal straight tubule, HCO 3 absorption is almost entirely inhibited by removal of Na from luminal and peritubular fluids or inhibition of the Na,K-ATPase. As will be discussed below, these results suggest a role for the Na/H antiporter in HCO 3 absorption, but are complicated by other possible interpretations. A more convincing result is inhibition of HCO 3 absorption by addition of amiloride to the luminal fluid.
In summary, the above studies provide strong evidence for an apical membrane amiloride-sensitive, electroneutral Na/H antiporter in the proximal tubule.
Sardet et al. was the first to clone a complementary DNA (cDNA) for a human amiloride-sensitive Na/H antiporter. Based on the sequence, the Na/H antiporter is predicted to be a 99 kDa protein with an amino terminal domain, 10–12 transmembrane spanning regions, and a carboxy terminal hydrophilic region representing a large cytoplasmic tail. Three putative N-linked glycosylation sites (aa 75, 370, and 410) have been identified and the C-terminus possesses several potential phosphorylation sites. The isoform encoded by this cDNA is now referred to as NHE1.
Subsequent studies from multiple labs have identified at least 8 other isoforms, with 3 isoforms (NHEs 1, 3, and 8) expressed in the proximal tubule. NHE1 is ubiquitously expressed with few exceptions, and is probably the most important isoform for cell pH and volume defense, cell growth, migration, adhesion, and other ‘housekeeping’ functions. In the proximal tubule cell, NHE1 is expressed on the basolateral membrane. Haggarty et al. demonstrated that the apical membrane Na/H antiporter of LLC-PK 1 cells (proximal tubule cell line) has an IC 50 of 13 μM for EIPA (ethylisopropylamiloride) (classified as amiloride-resistant), whereas the basolateral membrane Na/H antiporter has an IC 50 of 44 nM (amiloride sensitive). The inhibitor sensitivity of the LLC-PK 1 apical membrane transporter is similar to that found in apical membrane vesicles prepared from renal cortex, while the inhibitor sensitivity of the basolateral membrane Na/H antiporter is similar to that of the “housekeeping” isoform.
Substantial evidence suggests that NHE3 is the apical membrane Na/H antiporter of the proximal tubule. (1) Immunohistochemical studies have localized NHE3 to the apical membrane of the proximal tubule and thick ascending limb ( Figure 55.2 ). (2) NHE3 mRNA is highly expressed in kidney cortex. (3) NHE3 is inhibited by amiloride, amiloride analogs, and HOE694, with sensitivities similar to that of the apical membrane Na/H antiporter. (4) In cultured cells and in intact animals, regulation of NHE3 protein and mRNA abundances by glucocorticoids, thyroid hormone, and acidosis parallels regulation of the proximal tubule Na/H antiporter. (5) In cultured cells regulation of NHE3 activity (defined as EIPA resistant activity) parallels regulation of the proximal tubule apical membrane Na/H antiporter in that both are stimulated by angiotensin II and endothelin, and both are inhibited by parathyroid hormone and protein kinase A. (6) Lastly, mice in which the NHE3 gene has been knocked out develop a mild metabolic acidosis and proximal tubule HCO 3 absorption is inhibited by 61%.

In NHE3 null mice, about 60% of the remaining HCO 3 absorption is inhibited by bafilomycin, a vacuolar H-ATPase inhibitor, demonstrating a significant role for the H pump in mediating NHE3-independent HCO 3 absorption. The mechanism of H secretion mediation of the remaining 40% of HCO 3 absorption remains controversial. A small EIPA-sensitive component of HCO 3 absorption was observed by some, but not all labs, in mice null for both NHE2 and NHE3, suggesting expression of another Na/H antiporter isoform. In support of expression of another NHE isoform, Aronson's group demonstrated expression of an invertebrate NHE isoform (NHE8) in the proximal tubule and localized expression to the apical membrane by in situ hybridization, immunoblotting, and immunofluorescence microscopy. Apical membrane NHE8 is highly expressed in neonates, likely accounting for much of the NHE activity when apical membrane NHE3 protein expression is scant at this age. In both neonatal mice and rats, acid feeding-induced increases in NHE activity are prevented by a dose of EIPA that inhibits NHE8, but not NHE3 activity, and both NHE3 and NHE8 apical membrane protein abundances are increased. With maturation apical membrane expression switches from predominately NHE8 to NHE3 expression, but total cortical NHE8 expression is higher than in the neonatal proximal tubule, suggesting that NHE8 may serve predominantely as an intracellular organelle exchanger in adults. Regulation of this maturational switch appears to be under endocrine control, with both thyroid hormone and glucorticoids being responsible. However, studies in senile rats provide support for NHE8 contributing to apical membrane Na/H exchange even in the adult by the finding that age-related decreases in proximal acidification occur without changes in NHE3 or H-ATPase expression, but with decreased abundance of both NHE8 mRNA and protein.
Molecular biological studies have provided some characterization of NHE3. The protein has 12 transmembrane domains and a large cytoplasmic C-terminal domain. Transmembrane domain IV is implicated in ion transport, with amiloride inhibition mediated by transmembrane domain IX, and regulation of transporter activity mediated by the C-terminal tail. In the C-terminal tail are binding domains or phosphorylation sites for NHERF [required for cAMP inhibition of NHE3 through EPAC (exchange protein directly activated by cAMP)-dependent and PKA-dependent mechanisms), CHP [(calcineurin homologous protein ) involved in ezrin-dependent increases in NHE3 abundance and constitutive function], casein kinase 2 (involved in exocytic and delivery of newly synthesized protein to the membrane surface), PKA phosphorylation sites (which when phosphorylated by PKA do not alter NHE3 activity). In addition there is evidence for novel phosphorylation sites that when dephosphorylated by a calyculin A-sensitive phosphatase (likely PP1) stimulate NHE3 activity. It is likely that these sites are functionally significant, but their effect masked by the net contribution of other known phosphorylation sites.
Aronson was the first to propose that active H extrusion could be mediated by tertiary active transport. Siebens and Boron first demonstrated such a process in the Ambystoma proximal tubule perfused in vitro . This segment, which contains a basolateral membrane Na,K-ATPase, possesses an apical membrane Na/lactate cotransporter and a basolateral membrane lactate/hydroxyl exchanger (or equivalently an H/lactate cotransporter). These transporters working in series could effect the active extrusion of protons, but the proton extrusion would be across the basolateral membrane.
Nakhoul and Boron found evidence for such a system in the S3 segment of the rabbit proximal tubule. This segment, which contains a basolateral membrane Na,K-ATPase, possesses an apical membrane Na/acetate cotransporter, and pathways for acetic acid movement (equivalent to H/acetate cotransport or acetate/OH countertransport) on the apical and basolateral membranes. The acetic acid pathways may merely represent nonionic diffusion across the lipid bilayer rather than a specific transporter. With this model, the Na,K-ATPase establishes a low cell Na concentration that provides a driving force for the Na/acetate cotransporter, mediating acetate uptake across the apical membrane. The high cell acetate concentration can then drive protons (equivalently as acetic acid nonionic diffusion, H/acetate cotransport, or acetate/OH countertransport) out across the apical or basolateral membrane.
Nakhoul et al. showed that this system participates in the defense of cell pH against an acid load. The role of this system on transepithelial H secretion or transepithelial acetate transport depends on whether the “acetic acid” exits across the apical or basolateral membrane. The contribution of tertiary active transport systems to transepithelial H secretion was addressed by Geibel et al. who demonstrated that in tubules incubated in the absence of CO 2 /HCO 3 , acetate stimulates transepithelial H secretion, but in tubules perfused in the presence of CO 2 /HCO 3 , acetate addition inhibits rates of transepithelial H secretion. These data suggest that the acetate tertiary active system does not contribute significantly to transepithelial H secretion under physiologic conditions.
The previous sections have discussed mechanisms responsible for active H extrusion. The proximal tubule also possesses a number of pathways that lead to the net backleak of acid from the lumen into the cell across the apical membrane. These transporters are chloride-dependent and exchange chloride (moving from lumen to cell) for another anion, such as OH, HCO 3 , formate, or oxalate (moving from cell to lumen, equivalent to a backleak of acid). Evidence for such transport on the apical membrane has come from several studies.
Lucci and Warnock were the first to suggest the presence of apical membrane anion exchange. In in vivo microperfused rat proximal tubule, NaCl absorption is inhibited by luminal addition of disulfonic stilbenes or high doses of furosemide (10 −3 M), agents that inhibit the red cell Cl/HCO 3 exchanger. Baum also found in the in vitro perfused rabbit proximal convoluted tubule that luminal addition of disulfonic stilbenes inhibits Cl absorption.
Using apical membrane vesicles isolated from rabbit renal cortex, Warnock and Yee demonstrated that a pH gradient (inside pH greater than outside pH) drives Cl uptake into vesicles. Shiuan and Weinstein found similar results. Both groups found that pH gradients drive Cl uptake even in the presence of a voltage clamp, suggesting direct chemical coupling of Cl and base transport. Burnham et al. found similar results measuring the effect of Cl gradients on proton transport measured by acridine orange. Chen et al. demonstrated Cl/OH exchange using a Cl-sensitive fluorescent dye to measure Cl transport. On the other hand, Seifter et al. showed that when apical membrane vesicles are voltage clamped, pH gradients do not drive Cl uptake. Other investigators have also been unable to demonstrate Cl driven H transport using acridine orange. The reason for this discrepancy is not clear, but is most likely due to the low level of Cl/OH exchanger activity. Even those investigators who demonstrated Cl/OH exchanger activity found that it is far less than Na/H antiporter activity. Cl/OH exchange in vesicles is inhibited by disulfonic stilbenes.
Karnisky and Aronson showed that formate gradients are able to drive the countertransport of Cl, and Cl gradients are able to drive the countertransport of formate in rabbit renal apical membrane vesicles. The rate of this transporter is far greater than that found by other investigators for Cl/OH exchange. Similar evidence was found for a Cl/oxalate exchanger. Based on this they proposed that the physiologically relevant Cl/base exchangers were Cl/formate and Cl/oxalate exchangers.
Measuring cell pH microfluorimetrically in the in vivo perfused rat proximal convoluted tubule, Alpern showed that if basolateral cell pH defense mechanisms are inhibited, changes in luminal Cl concentration affect cell pH in a manner consistent with Cl/base exchange. If 1 mM formate is added to luminal and peritubular solutions, the effect of Cl removal and addition increases 3-fold and all effects of luminal Cl substitution are blocked by addition of DIDS to the luminal fluid. Similar results were found by Baum in the in vitro perfused rabbit proximal convoluted tubule. Thus, these studies demonstrate Cl/base exchange, and confirm the vesicle results, namely Cl/OH and Cl/formate exchange with Cl/formate exchange activity being greater than Cl/OH exchange activity.
Further support for these anion exchangers is derived from volume flux measurements. The addition of formate or Cl to the luminal fluid increases the proximal tubule volume and Cl absorption. This increase is inhibited by DIDS, an anion exchange inhibitor, suggesting that NaCl absorption is mediated by parallel Na/H and Cl/base exchangers with the H and base recycling. Support for this model comes from the observation that amiloride inhibits Cl absorption in the absence of added formate or oxalate, and that EIPA inhibits the formate-induced stimulation of Cl absorption. The demonstration that oxalate-dependent Cl transport is not EIPA-sensitive, but is sulfate-dependent, while formate-dependent Cl transport is sulfate-independent, but EIPA-sensitive has lead to a proximal tubule model in which formate-stimulated NaCl absorption is dependent on Na/H exchange, while oxylate-stimulated NaCl absorption is dependent on Na-sulfate cotransport in parallel with sulfate-oxalate exchange. In the case of formate and OH, stimulation of anion exchange not only increases the rate of NaCl absorption, but inhibits the rate of luminal acidification.
The quantitative role of Cl/base exchangers in proximal tubule/base exchangers in proximal tubule luminal acidification is unclear, but likely to be small. Three studies have been unable to demonstrate an effect of luminal and peritubular Cl removal on net HCO 3 absorption, while one lab found that luminal DIDS stimulates net HCO 3 absorption. Thus, in summary, the Cl/formate, Cl/OH, and Cl/oxalate exchangers appear to function in parallel with Na-dependent transport mechanism to mediate net NaCl absorption from the lumen to cell. In circumstances where Na/H exchange is coupled to “base” secretion, net H secretion is reduced. The role of the Cl/base exchangers in proximal tubule function is discussed in more detail in Chapter 33 : “Sodium and Chloride Transport: Proximal Nephron.”
An H leak pathway in the apical membrane, especially in the late proximal tubule, would provide an additional backleak pathway for secreted acid. Reenstra et al. showed that brush border membranes possess a significant H/OH permeability. Ives measured internal buffer capacity of vesicles and was able to calculate an H permeability that is not inhibited by amiloride or DCCD and, thus, is not due to proton movement across the Na/H antiporter or the H-ATPase.
Preisig and Alpern measured apical membrane proton permeability by examining the rate of change of cell pH in response to a rapid luminal pH change. Apical membrane Na- and Cl-coupled transporters were inhibited by perfusing tubules in the absence of luminal and peritubular Na and Cl and rates of change in cell pH were converted to fluxes by measuring buffer capacity. These studies demonstrated an apical membrane proton permeability of 0.52 cm/sec, a value that is in close agreement with that calculated by Ives from vesicle studies when corrected for the surface area of the brush border membrane.
As shown in Figure 55.3 , the proximal tubule apical membrane possesses an H-ATPase, a Na/H antiporter and tertiary active transport mechanisms all capable of effecting active acid extrusion. In addition, possible apical membrane net proton backleak pathways include the Cl/HCO 3 , Cl/OH, and Cl/formate exchangers as well as proton diffusion across the membrane. As discussed above, the Cl/base exchangers and tertiary active transport mechanisms probably play a very small, if any, role in proximal tubule acidification, and thus, won’t be discussed further.

Using the apical membrane H permeability (see above), Preisig and Alpern calculated that a passive H leak from lumen to cell would be of minor quantitative importance in the early proximal tubule where the luminal pH is close to 7.4 (and cell pH ~7.3). However, in the late proximal tubule with a luminal pH of ~6.8, 1/3 of secreted protons could leak back across the apical membrane.
The role of the Na/H antiporter in mediating transepithelial HCO 3 absorption has been studied in a number of ways. In in vivo and in vitro perfused proximal tubules, either complete Na removal from luminal and peritubular fluids or inhibition of the Na,K-ATPase by peritubular ouabain or peritubular K removal inhibits most of HCO 3 absorption. While these studies were initially interpreted as demonstrating that all apical membrane H secretion is Na-coupled and utilizes the lumen-to-cell Na gradient established by the Na,K-ATPase, they are equally consistent with a Na-coupled, electrogenic mechanism mediating HCO 3 efflux on the basolateral membrane working in series with a Na-independent H secretory mechanism on the apical membrane, which was demonstrated to be the situation (see below). However, inhibition of the Na,K-ATPase leads to cell depolarization, which inhibits the electrogenic Na/HCO 3 cotransporter on the basolateral membrane, as shown in studies in which inhibition of the Na,K-ATPase in the bullfrog proximal tubule causes a cell alkalinization. This observation is consistent with inhibition of base exit, and not consistent with inhibition of apical membrane Na-independent H secretion, as that would have resulted in cell acidification. Thus, while the effect of both Na removal and Na,K-ATPase inhibition on HCO 3 absorption are consistent with an important role of the Na/H antiporter in HCO 3 absorption, neither can quantify the magnitude of this contribution.
The best approach toward quantifying the contribution of the Na/H antiporter has been to examine the effect of inhibiting the apical membrane Na/H antiporter on HCO 3 absorption. Howlin et al. and Chan and Giebisch demonstrated that luminal amiloride significantly inhibits the rate of HCO 3 absorption in the in vivo microperfused rat proximal convoluted tubule. However, neither group found the magnitude of inhibition to be as large as expected if the Na/H antiporter mediates all of the H secretion involved in HCO 3 absorption.
To further examine this issue, Preisig et al. looked at the effect of high concentrations of luminal amiloride and t-butyl amiloride (a more potent analog) on transepithelial HCO 3 absorption. In the in vivo microperfused tubule, these investigators found that both agents inhibit only 45% of HCO 3 absorption. While one interpretation of this observation is that the Na/H antiporter mediates only a fraction of transepithelial HCO 3 absorption, other interpretations are that the rat Na/H antiporter is insensitive to these agents or that the inhibitor concentration is not consistent along the perfused segment. Preisig et al. then showed that when corrections are made for the measured luminal inhibitor concentration profile (amiloride and its analogs are highly lipophic and diffuse out of the lumen along the length of the perfused segment), and for the luminal [HCO 3 ] profile, Na/H antiporter activity, assayed as the rate of change in cell pH in response to luminal Na removal or addition, is inhibited by amiloride and t-butyl amiloride as predicted by vesicle studies. Thus, these studies concluded that an amiloride-sensitive Na/H antiporter mediates approximately 65% of transepithelial HCO 3 absorption, with the remaining 35% mediated by an amiloride-insensitive, Na-independent mechanism. This conclusion is supported by the observation that in NHE3 null mice, proximal tubule HCO 3 absorption is inhibited by 61%.
The studies by Preisig et al. were performed in the absence of organic anions in the perfused lumen making it very unlikely that tertiary active transport mechanisms are responsible for the remaining 39% of HCO 3 absorption. Thus, the most likely explanation is that the H-ATPase mediates the remaining 35% of transepithelial HCO 3 absorption. Bank et al. have more directly demonstrated a role of the H-ATPase in HCO 3 absorption by showing that luminal DCCD, an inhibitor of vacuolar H-ATPases, inhibits 21% of proximal tubule HCO 3 absorption.
Because cell pH is above electrochemical equilibrium, basolateral membrane base efflux can occur passively.
Studies have uniformly demonstrated that the key basolateral membrane base efflux mechanism is an electrogenic Na/3HCO 3 cotransporter. Evidence for this rests on results that have demonstrated: (1) effects of this transporter on cell pH and cell voltage, (2) the presence of this transporter in basolateral membrane vesicles, and (3) the importance of this transporter in transepithelial HCO 3 absorption.
In intact tubules, initial studies examined the electrogenicity of basolateral membrane H/HCO 3 movement. It was reasoned that if cell pH and HCO 3 concentration are above electrochemical equilibrium, a simple basolateral membrane H/OH/HCO 3 conductance could mediate base efflux and transepithelial H secretion. Burckhardt et al. provided evidence for a large basolateral membrane H/HCO 3 conductance in the in vivo perfused rat proximal convoluted tubule. When HCO 3 concentration is rapidly decreased from 30 to 3 mEq/L, cells rapidly depolarize and then slowly repolarize. A subsequent increase in peritubular HCO 3 concentration leads to a rapid hyperpolarization followed by a slow depolarization. This basolateral membrane conductance is inhibited by acetazolamide and peritubular SITS. While initial studies had difficulty demonstrating such a HCO 3 conductance in the in vitro perfused rabbit proximal convoluted and straight tubules, improvements in the perfusion technique that permit sufficiently rapid fluid changes, led to confirmation of Burckhardt et al.'s finding.
Further evidence for the electrogenicity of basolateral membrane HCO 3 transport in the intact tubule is the voltage sensitivity of bicarbonate movement. Alpern measured cell pH microfluorimetrically in the in vivo perfused rat proximal convoluted tubule and showed that increasing peritubular K concentration from 5 to 50 mEq/L (a maneuver that causes cell depolarization) causes a rapid cell alkalinization that is reversible when peritubular K concentration is reduced back to 5 mEq/L. While these studies suggest the presence of a voltage-sensitive H/OH/HCO 3 transport mechanism on this membrane, they were also consistent with a K/HCO 3 cotransporter. To examine this possibility, the studies were repeated in the presence of 2 mM peritubular barium. Barium inhibits the basolateral membrane K conductance, preventing changes in peritubular K concentration from affecting cell voltage. In addition, by inhibiting the K conductance, barium itself leads to a cell depolarization. Barium addition causes cell alkalinization, and prevents any subsequent effect of changing peritubular K concentration on cell pH. These results are consistent with the presence of a voltage-sensitive H/OH/HCO 3 transport mechanism in the proximal tubule cell. While these studies did not specifically address which membrane the transport mechanism is on, the studies by Burckhardt et al. demonstrated a HCO 3 conductance on the basolateral, but not on the apical membrane.
While initially it was felt that Burckhardt et al.'s studies represent a simple HCO 3 conductance on the basolateral membrane, Boron and Boulpaep suggested a more complex transport mechanism. These investigators used ion selective microelectrodes in the in vitro perfused salamander proximal tubule and found that lowering the peritubular HCO 3 concentration causes a rapid cell depolarization, indicative of a HCO 3 conductance. However, peritubular acidification also led to cell acidification and a decrease in intracellular Na activity, and lowering peritubular Na concentration causes cell depolarization, cell acidification, and a decrease in cell Na activity. The cell depolarization in response to lowering peritubular Na concentration is in the wrong direction for a Na conductance and is more suggestive of a Na-coupled anion current. In further studies these investigators found that all of the above changes occur in the absence of Cl and are blocked by SITS. Considered together these studies provided the evidence suggesting a directly coupled Na/HCO 3 cotransport mechanism that transports more HCO 3 than Na ions and, thus, is electrogenic.
The first suggestion that such a transport mechanism exists in the mammalian nephron was made by Biagi and Sohtell, who measured cell voltage in the in vitro perfused proximal convoluted tubule and proximal straight tubule. These investigators found that lowering peritubular HCO 3 or Na concentration leads to a spike depolarization that is blocked by SITS. Once again, the cell depolarization occurring in response to lowering peritubular Na concentration is in the wrong direction for a simple Na conductance.
Subsequent studies performed by Alpern and Yoshitomi et al. measured cell pH in the in vivo perfused rat proximal tubule and confirmed the existence of an electrogenic Na/HCO 3 cotransporter. Alpern found that decreasing peritubular pH causes cell acidification. Additionally, lowering peritubular Na concentration from 147 to 25 mEq/L (replacing Na with either choline or tetramethylammonium) causes cell pH to decrease by 0.2 pH units, an effect that persists in the complete absence of Cl.
The above studies are consistent with three possible mechanisms of coupling between Na and HCO 3 : parallel Na and HCO 3 conductances, amiloride-sensitive Na/H antiport, or SITS-sensitive Na/HCO 3 cotransport. To determine if the results are attributable to parallel Na and HCO 3 conductances, Alpern examined the Na-dependence of basolateral membrane H/HCO 3 permeability. If the HCO 3 conductance were Na-independent, complete Na removal should have no effect on basolateral membrane H/HCO 3 permeability. The results showed that complete removal of Na from luminal and peritubular fluid inhibits basolateral membrane H/HCO 3 permeability by greater than 90%, demonstrating direct coupling between Na and HCO 3 . In subsequent studies, SITS, but not amiloride blocked the effect of peritubular Na on cell pH. Lastly, Alpern demonstrated that the effect of cell depolarization (raising peritubular K concentrations) on cell pH was prevented if lumen and peritubular capillaries were perfused with Na-free solutions. Taken together these studies suggest a directly coupled, electrogenic Na/HCO 3 cotransport mechanism similar to that found in the salamander.
Yoshitomi et al. found similar results, measuring cell voltage, pH and Na activity using ion sensitive microelectrodes. Lowering peritubular HCO 3 concentration causes a rapid cell depolarization, a decrease in cell pH, and a decrease in cell Na activity. Decreasing peritubular Na concentration also causes a rapid cell depolarization and a decrease in cell pH. SITS inhibits the effects of both lowering peritubular pH and peritubular Na concentration. Lastly, luminal and peritubular Na removal inhibits the effect of changing peritubular HCO 3 concentration on cell voltage and cell pH. In subsequent studies, Sasaki et al., and Lopes et al. found similar results in the rabbit proximal straight tubule and the Necturus proximal tubule, respectively. Thus, all of these studies consistently demonstrate an electrogenic, SITS-sensitive Na/HCO 3 cotransport mechanism on the proximal tubule basolateral membrane.
This question has also been addressed in basolateral membrane vesicles. Akiba et al. found that addition of HCO 3 to the extravesicular fluid increases the rate of Na uptake. SITS has no effect on Na uptake in the absence of HCO 3 , but inhibits the HCO 3 -dependent increment. Addition of valinomycin (a K ionophore) in the presence of an inwardly directed K gradient causes an internal positive voltage. This maneuver has no effect on Na uptake in the absence of HCO 3 , but accelerates the uptake in the presence of HCO 3 , an effect that is in the wrong direction for a Na conductance.
Grassl and Aronson demonstrated in rabbit cortical basolateral membrane vesicles that pH gradients drive Na uptake. Whereas the effect is small in the absence of exogenous CO 2 /HCO 3 , it is large in its presence. In addition, a valinomycin-generated positive interior voltage drives Na uptake, but only in the presence of HCO 3 . Lastly, using acridine orange to measure H transport, Grassl and Aronson found that Na gradients ([Na] in >[Na] out ) drives HCO 3 out of vesicles leading to acidification of the intravesicular space. Na uptake is blocked in a dose-dependent fashion by DIDS and is unaffected by amiloride. In the studies of Akiba et al. and Grassl and Aronson, Cl was absent from all fluids. Thus, these studies demonstrate the presence of an electrogenic, stilbene sensitive, Na-coupled HCO 3 transport mechanism. Grassl et al. found similar results in rat cortical basolateral membranes.
Stoichiometry : Based on the electrogenicity demonstrated above, it was concluded that the basolateral membrane Na-dependent HCO 3 transporter carries a greater number of HCO 3 than Na ions. If the free energy for HCO 3 efflux is calculated, it can be predicted that a Na/2HCO 3 co-transporter will not be able to function in the HCO 3 efflux mode, unless directly coupled to energy. Thus, it was predicted that the stoichiometry is a minimum of 3 HCO 3 :1 Na. Yoshitomi et al. directly examined this question by comparing the SITS-sensitive voltage response to a 10-fold reduction in peritubular HCO 3 concentration with the response to a 10-fold reduction in peritubular Na concentration, and found the former to be 2.95-fold greater. They also demonstrated that a reduction in peritubular HCO 3 concentration causes a 3.1-fold greater HCO 3 than Na flux out of the cell. Thus, both studies suggest a stoichiometry of 3 HCO 3 :1 Na.
A 3:1 stoichiometry was confirmed by Soleimani et al. in basolateral membrane vesicles by demonstrating that in the presence of valinomycin (to increase K permeability) the HCO 3 and K concentration gradients required for no net Na movement yield a stoichiometry of 1 Na:3 HCO 3 :2 negative charges.
Base species transported : Thus far in the discussion this basolateral membrane transporter has been described as a Na/HCO 3 cotransport mechanism, based on a strong transporter dependence on the presence of HCO 3 . Krapf et al. examined the dependence of the transporter on exogenous CO 2 /HCO 3 in the in vitro perfused rabbit proximal convoluted tubule utilizing the fluorescent measurement of cell pH. These investigators found that in the absence of exogenous CO 2 /HCO 3 , the transporter functions at 1/3 of its control rate. While these results could be interpreted as suggesting that the transporter is able to run in the absence of CO 2 /HCO 3 , it is also possible that metabolism was producing sufficient CO 2 and HCO 3 to run the transporter at a slow rate. In agreement with this latter interpretation, the transporter flux measured in the absence of exogenous CO 2 /HCO 3 is completely inhibited by acetazolamide, a carbonic anhydrase inhibitor, or by cyanide, a metabolic inhibitor. There should be no requirement for carbonic anhydrase if the transporter carries H or OH. In addition, the transporter is unaffected by cyanide when studied in the presence of exogenous CO 2 /HCO 3 . Thus, these results agree with the vesicle studies and suggest that the transporter has an absolute dependence on HCO 3 .
Soleimani and Aronson performed further studies that suggest that carbonate (CO 3 2− ) is also a substrate. They found that with a constant extravesicular HCO 3 concentration, increasing carbonate concentration increases Na uptake into basolateral membrane vesicles. Sulfite, a structural analog of carbonate, also stimulates Na uptake in the presence of CO 2 /HCO 3 , but not in the absence of CO 2 /HCO 3 , an effect that is blocked by the disulfonic stilbene, DNDS. Lastly, using an equilibrium approach similar to that described above, a stoichiometry of 1 Na:1 sulfite:1 HCO 3 was derived, with carbonate and sulfite competing for a similar site. The last issue addressed in these studies was whether three separate binding sites exist or whether Na and carbonate formed a NaCO 3 – ion pair that then interacts with a single site, while HCO 3 interacts at another site. Multiple pieces of evidence are against the ion pair model. First, lithium, which is more likely to form an ion pair with carbonate than is Na, is a less effective substrate for the transporter. Secondly, oxalate and phosphate, anions that participate in ion pair formation with Na, do not interact with the transporter. Lastly, harmaline, an inhibitor of Na transporters, competes with Na on the Na/3HCO 3 transporter. Such an interaction would be unlikely if the transporter possessed only two transport sites with both binding negatively charged substrates. Thus, it appears that the transporter is actually a Na/HCO 3 /CO 3 cotransporter rather than a Na/3HCO 3 cotransporter.
Romero et al. cloned the cDNA for the electrogenic Na/3HCO 3 transporter from Ambystoma tigrium . This cDNA, referred to as NBC1 ( N a B icarbonate C otransporter e lectrogenic 1 ) encodes a 1035 amino acid protein, the same size as and ~80% identical to the human and rat sequence. The human and rat mRNAs are larger than the amphibian kidney mRNA, with the human and rat mRNA ~7.5 kb in length and the amphibian mRNA 4.2 kb. The difference is due to an extensive 3-prime non-coding region in the human and rat transcript.
Human NBCe1 is highly expressed in both the kidney and pancreas, the two differing only in their N-terminal sequence. The kidney transcript is expressed predominantly in the kidney, while the pancreas transcript is expressed in multiple tissues. In kidney the protein localizes to the basolateral membranes of the proximal tubule. More detailed reviews of HCO 3 -coupled transporters have been published and the transporters are discussed in Chapter 54 .
Many segments along the nephron possess basolateral membrane Na/H antiporters that likely participate in housekeeping functions. Boron and Boulpaep found that the salamander proximal tubule possesses Na/H antiporters on apical and basolateral membranes. Ives et al. and Sabolic and Burckhardt did not find any Na/H antiporter activity in basolateral membrane fractions from rabbit renal cortex. While Grassl and Aronson found some pH-dependent Na uptake in the absence of HCO 3 , it is not inhibited by amiloride. In the in vivo perfused rat proximal convoluted tubule Alpern found that the effect of basolateral Na changes on cell pH is not inhibited by amiloride and in the in vitro perfused rabbit proximal convoluted tubule Krapf et al. found similar results. Kurtz found an amiloride-sensitive basolateral membrane Na/H antiporter in the S3 proximal tubule and Geibel et al. reported a basolateral membrane Na/H antiporter in S1 and S2 proximal tubule segments dissected from the most juxtamedullary portions of the nephron. By immunohistochemistry, Biemesderfer et al. found inhomogeneous labeling of the proximal tubule basolateral membrane with anti-NHE1 antibodies. Taken together these studies suggest that a Na/H antiporter may be expressed on the basolateral membrane of juxtamedullary proximal tubule, but not likely in cortical proximal tubules. More recently evidence for regulation of a basolateral membrane Na/H antiporter in the S3 segment was demonstrated by examining the effect of aldosterone on intracellular pH recovery rate. The basolateral membrane NHE1 appears to be regulated by both genomic (1h via a mineralocorticoid receptor) and non-genomic (2–15 min, likely via a glucocorticoid receptor) mechanisms. At lower concentration ranges (10 −12 M aldosterone) intracellular [Ca] is increased and NHE1 activity is stimulated, while at higher concentrations (10 −6 M) intracellular [Ca] decreases and NHE1 activity is inhibited.
Edelman et al. first demonstrated the presence of basolateral membrane Cl/HCO 3 exchange in Necturus proximal tubule using Cl-sensitive microelectrodes. Guggino et al. confirmed these results and, in addition, found that the transporter is coupled to Na. Lowering basolateral Cl concentration causes intracellular Cl activity to decrease. Removal of peritubular HCO 3 leads to an increase in cell Cl activity, while changes in peritubular pH in the absence of exogenous HCO 3 have no effect on intracellular Cl activity. When cell pH is modulated by NH 3 /NH 4 + addition and removal, cell Cl activity changes in a manner consistent with Cl/HCO 3 exchange, repeating these maneuvers in the absence of exogenous CO 2 /HCO 3 causes a marked decrease in the response of intracellular Cl activity. Lowering peritubular Na concentration leads to an increase in intracellular Cl activity. In addition, cell acidification by NH 3 /NH 4 + addition and removal causes a decrease in Cl activity that is blocked if Na is simultaneously removed from the peritubular fluid. Taken together these findings suggest a NaHCO 3 /Cl exchange mechanism. On the basis of other studies that fail to find basolateral membrane electrogenic Cl movement, it was concluded that the transporter is a Na(HCO 3 ) 2 /Cl exchanger. A similar transporter has been found in many invertebrate and vertebrate cells and, in these cells, is felt to be an important mechanism responsible for cell pH defense against an acid load.
Alpern and Chambers examined whether such a transport mechanism is present on the basolateral membrane of the rat proximal tubule by studying the effect of peritubular Cl on cell pH. In tubules perfused in the absence of luminal and peritubular Cl, peritubular Cl addition causes cells to acidify by 0.14 pH units, an effect that is blocked by peritubular SITS. In addition, if Cl is added to the peritubular perfusate in the complete absence of luminal and peritubular Na, cells acidify by only 0.02 pH units. These studies suggest the presence of a small amount of Na-independent and a much larger amount of Na-dependent Cl/HCO 3 exchange activity.
However, the above results are also consistent with a basolateral membrane Cl conductance functioning in parallel with the electrogenic Na/3HCO 3 cotransporter. Preisig and Alpern addressed this issue by examining the Cl-dependence of the basolateral membrane H/HCO 3 permeability. If changes in peritubular Cl only affect cell pH by changing voltage and secondarily modifying Na/3HCO 3 cotransporter activity, Cl removal should not affect basolateral membrane H/HCO 3 permeability. However, complete removal of Cl from luminal and peritubular solutions leads to a 35% decrease in basolateral membrane H/HCO 3 permeability. Because complete removal of Na from luminal and peritubular fluids leads to a greater than 90% inhibition of basolateral membrane H/HCO 3 permeability, taken together these results suggest that at least 25% of H/HCO 3 permeability is mediated by a Na and Cl-coupled H/HCO 3 transporter. The remaining fraction of H/HCO 3 permeability, which is not inhibited by Na removal, may represent a Cl/HCO 3 exchanger. Sasaki and Yoshiyama found similar results using microelectrodes to measure intracellular Cl and pH in the rabbit proximal straight tubule perfused in vitro . Decreases in peritubular Cl concentration cause a cell alkalinization and decreases in peritubular pH lead to an increase in cell Cl activity, with both effects inhibited by peritubular SITS. Total Na removal from luminal and peritubular fluids prevents most of the effect of bath pH on cell Cl activity. And, lastly, removal of luminal and peritubular Cl slows the cell pH response to peritubular acidification by 26% and slows the cell pH response to lowering peritubular Na concentration by 19%. These results demonstrate a Na-dependent Cl/HCO 3 exchanger. Kurtz and Nakhoul et al. have reported basolateral membrane Na-independent Cl/HCO 3 exchange in the S3 segment of the rabbit proximal tubule perfused in vitro .
While studies in the intact tubule seem to consistently demonstrate the presence of a Na-dependent Cl/HCO 3 exchanger, studies in basolateral membranes have been much less consistent. Grassl et al. found that a pH gradient (pH in >pH out ) can drive Cl uptake in rabbit cortical basolateral membrane vesicles. In the presence of CO 2 /HCO 3 , a similar pH gradient leads to a 6-fold greater accumulation of Cl. This effect is not inhibited by voltage clamping the vesicles, but is inhibited by DIDS. Grassl et al. did not find Na-dependence of HCO 3 -driven Cl uptake. In subsequent studies examining the effect of Cl and Cl gradients on HCO 3 -stimulated Na uptake, results were not consistent with a Na(HCO 3 ) 2 /Cl exchanger.
In rat basolateral membrane vesicles, Grassl et al. demonstrated that symmetrical Cl addition to the inside and outside of vesicles stimulates HCO 3 -dependent Na uptake and that a Cl gradient (Cl in > Cl out ) further stimulates uptake. Chen and Verkman used a Cl-sensitive fluorescent dye to examine the effect of pH gradients on Cl uptake into rabbit cortical basolateral membrane vesicles. These authors demonstrated that pH gradients (pH in > pH out ) stimulate Cl uptake, an effect that is enhanced by the presence of CO 2 /HCO 3 and inhibited by H 2 DIDS. Na gradients (Na in > Na out ) also stimulate Cl uptake, but only in the presence of CO 2 /HCO 3 .
Based on all of the above data, it appears that the basolateral membrane contains a Cl/HCO 3 exchanger that demonstrates less transporter activity in intact S1 and S2 proximal tubules compared to the S3 proximal tubule. While vesicle studies are clearly consistent with this observation, there is controversy as to the existence of Na-dependent Cl/HCO 3 exchange.
Preisig and Alpern measured basolateral membrane H permeability, using an approach similar to that described for apical membrane H permeability. The permeability, 0.67 cm/sec, was similar to that found in the apical membrane.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here