Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
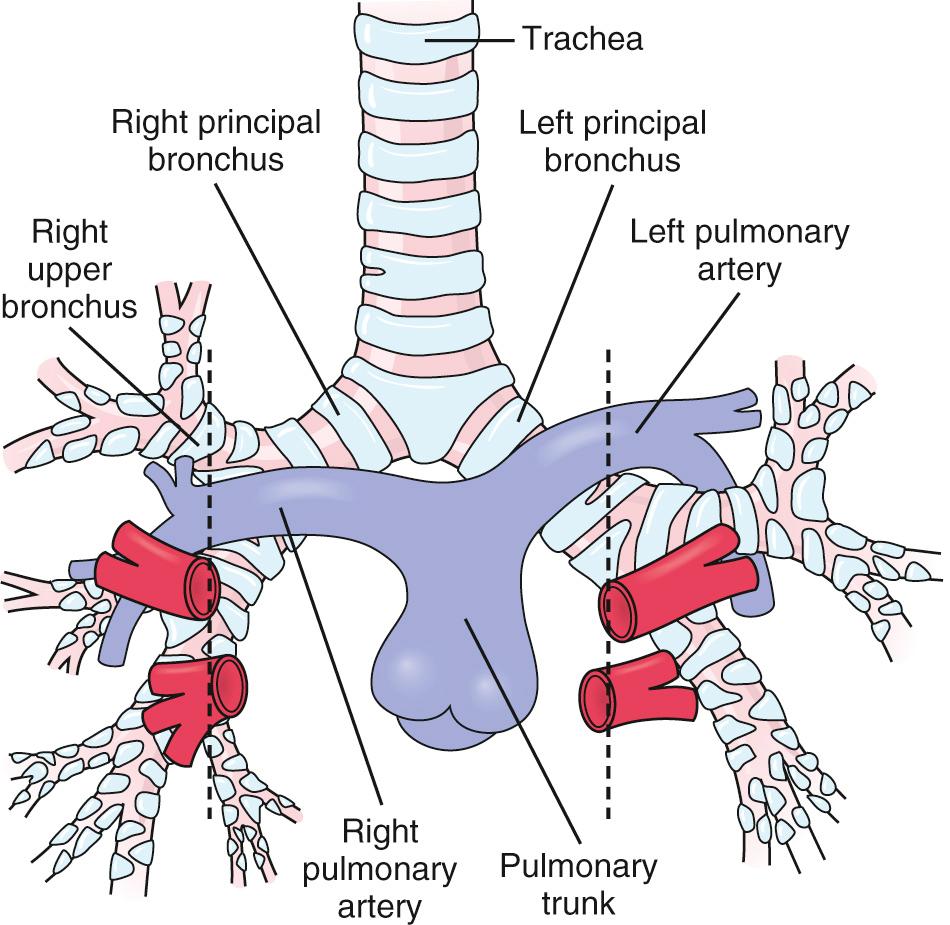
The development of morphologically right-sided structures on one side of the body, and morphologically left-sided structures on the other side, is termed lateralization or situs . The normal arrangement, in which right-sided structures are on the right side of the body and vice versa, is known as situs solitus . The term visceroatrial situs is often used to refer to the situs of the viscera, or noncardiac organs, and atria when their situs is in agreement. In normal atrial arrangement or atrial situs solitus , the left atrium is on the left side and right atrium is on the right side. In atrial situs inversus , the left atrium is on the right side and right atrium is on the left side. The morphology of the lungs and the relation between the tracheobronchial tree visible on chest radiograph and the pulmonary arteries are useful in determining situs. A morphologically right lung typically has three lobes, and a morphologically left lung typically has two lobes. Furthermore, the right lung tends to have an eparterial bronchus, or one that branches superior to the first lobar division of the pulmonary artery, in contrast to the left-sided lung, which has a hyparterial bronchus, or one that branches inferior to the first lobar division of the respective pulmonary artery ( Fig. 76.1 ).
Visceral situs solitus occurs when the abdominal organs are lateralized normally with the spleen and stomach on the left side and liver on the right side. The assessment of abdominal situs can be made by physical examination and other routine tests, such as radiography and ultrasound. The liver is palpable on the left in situs inversus with the stomach bubble on the right side. Ultrasound of the abdomen will confirm these findings along with demonstration of other structures such as the kidneys and spleen. If the spleen is not visible on routine abdominal ultrasound, an abdominal CT or MRI may be indicated to demonstrate the presence or absence of a spleen. A nuclear scan is rarely indicated. Another finding that is generally present if the spleen is absent or nonfunctional is Howell-Jolly bodies on the peripheral smear, because these are usually removed by a normal spleen.
Dextrocardia is present when the base to apex axis of the heart is directed to the right side of the chest ( Fig. 76.2 ). Another possible location for the heart is mesocardia, where the apex is in the midline. Situs solitus is the normal location of the atria and abdominal organs as described previously. Situs inversus is present when the right atrium is on the left, the left atrium is on the right, and the liver and stomach are similarly reversed. Situs ambiguous, with the liver and stomach in the midline, is seen in neonates with either bilateral right-sidedness or left-sidedness, also commonly referred to as heterotaxy syndrome . Babies with bilateral right-sidedness tend to have asplenia, and their atrial and pulmonary morphologic features are characteristic of bilateral right atria and lungs. Babies with bilateral left-sidedness tend to have polysplenia and bilateral morphologic left atria and lungs. These conditions are invariably associated with a multitude of other intracardiac abnormalities.
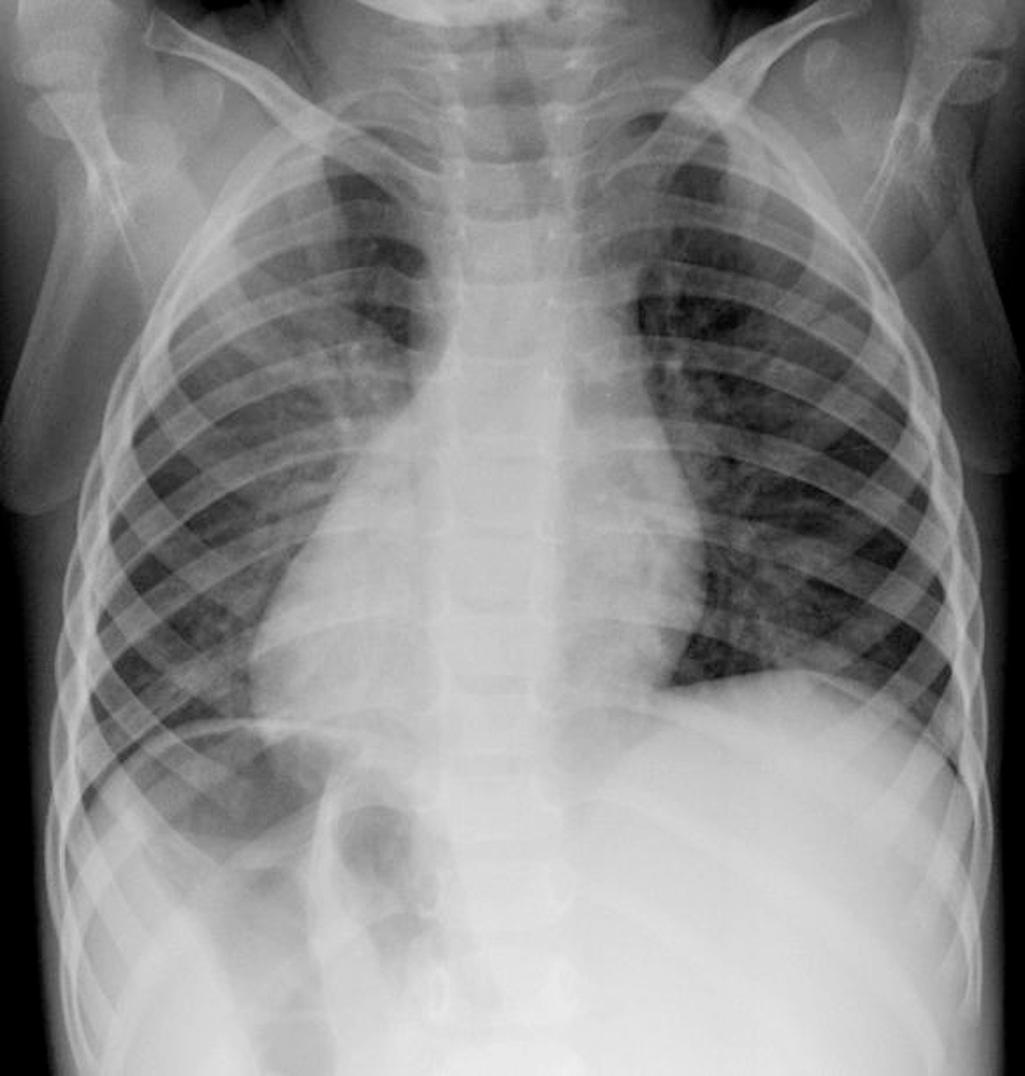
Dextrocardia with situs inversus is referred to as the mirror image; dextrocardia may be a part of Kartagener syndrome or immotile cilia syndrome but is generally associated with normal cardiac anatomy. It is frequently detected by clinical exam with the heart sounds auscultated in the right chest and incidental chest radiograph demonstrating dextrocardia (see Fig. 76.2 ). In contrast, babies born with dextrocardia and situs solitus frequently have associated congenital heart defects, ranging from simple septal defects to complex lesions such as l -transposition of the great arteries. The same is true for those neonates with situs inversus of their abdominal organs with levocardia (normal cardiac position with base to apex axis to the left) who almost always have associated cardiac defects.
Asplenia syndrome or bilateral right-sidedness should be suspected whenever complex cardiac defects are identified in association with total anomalous pulmonary venous connection. Because there is no morphologic left atrium, pulmonary venous drainage is rarely directly to the patient's left-sided atrium. Similarly, in this condition, the coronary sinus, which lies posterior to the normal left atrium, is usually absent. Systemic venous return is also abnormal, with the presence of bilateral superior vena cava. The left superior vena cava may enter directly into the roof of the left-sided morphologic right atrium or the coronary sinus, whereas the right superior vena cava generally enters the right atrium in a normal fashion. The right inferior vena cava is present, and there might be an additional left inferior vena cava. Another relatively common variety encountered in cardiac position is dextrocardia with an associated common atrium and a single atrioventricular valve, resulting in either a common ventricle or a large ventricular septal defect. Severe pulmonary stenosis or atresia is common in this particular defect. Surgical palliation beginning as a neonate and terminating in a palliated univentricular circulation is the norm for over 80% of asplenia patients. Survival at 5 years in a live-born cohort of asplenia infants is 53% and is worse in those with stenotic pulmonary veins. In addition to their complex congenital heart disease, these babies are often immunologically compromised because of the lack of a spleen. Severe, life-threatening septic events are a considerable concern and daily, lifelong administration of a prophylactic antibiotic to reduce the risk for bacterial sepsis is critically important.
Absence of the hepatic segment of the inferior vena cava, so-called interrupted inferior vena cava, is an excellent clue to the presence of polysplenia or bilateral left-sidedness in a neonate with ambiguous abdominal situs. Because the baby has no true right atrium, the course of the inferior vena cava is abnormal. Drainage of the inferior vena cava is through the azygos or hemiazygos veins and into the superior vena cava. With bilateral left atria, the pulmonary veins usually return to the heart in a bilateral fashion to their closest atrium (right-sided pulmonary veins return to the right-sided atrium). The presence of polysplenia is often associated with levocardia, normally related great arteries, a common atrium, and two ventricles with a ventricular septal defect. The pulmonary valve in these cases is usually normal. A quarter of fetuses with polysplenia may have bradyarrhythmias in utero, including sinus bradycardia and complete heart block. Survival to birth in fetal bradyarrhythmia associated with polysplenia is nearly 80% in fetuses where termination is not chosen upon diagnosis. Survival at 5 years in a live-born cohort is 86% in polysplenia. Although the patient has multiple small spleens, splenic function can be either normal or abnormal regardless of the presence of Howell-Jolly bodies on blood smear, which as previously stated may indicate hyposplenism. Assessment of splenic function and identification of the subset requiring daily prophylactic antibiotics are important prior to discharge home from the hospital following birth.
Arteriovenous malformations (AVMs) are an abnormal, direct connection between arteries and veins via a network of vessels called the nidus that lack an intervening capillary bed. Depending upon the type of cerebral AVMs, manifestation in the neonatal period with signs of congestive heart failure, including a hyperdynamic precordium and hepatomegaly, may result. Most of these are vein of Galen malformations, although neonates with large pial malformations and congestive heart failure have been described. Vein of Galen malformations can be classified as choroid and mural type, of which the choroidal type usually presents in the neonatal period.
Even large vein of Galen malformations may not result in fetal heart failure, because the low resistance of the malformation is offset by the utero-placental circulation. Thus, fetal cardiac failure is an ominous sign. As systemic vascular resistance increases at birth, progressive blood flow is diverted into the malformation from the systemic circulation. Congestive heart failure, right heart dilation, lactic acidosis, persistent pulmonary hypertension of the newborn, and diastolic runoff in the ascending aorta or arch on echocardiogram are clues to the diagnosis. Presence of cranial bruit, a louder pulmonary component to the second heart sound, and a systolic ejection murmur of increased aortic and pulmonary artery flow are also typically present. The chest radiograph shows cardiomegaly. Early heart failure presentation of vein of Galen malformations is associated with worse outcomes. An ECG may demonstrate right ventricular hypertrophy and ST-T wave abnormalities due to coronary artery steal. In addition to flow reversal in the aorta, an echocardiogram will frequently demonstrate a dilated, noncompliant right ventricle with systemic or suprasystemic pulmonary artery pressures and a hyperdynamic left ventricle. Ductal flow may be right to left resulting in significant cyanosis. Diagnosis is confirmed with CT or MRI.
Arteriovenous malformations in the neonatal period usually present as congestive heart failure, and initial medical management goals consist of stabilizing systemic cardiac output. Recently, milrinone and inhaled nitric oxide have been utilized along with loop diuretics. Half of patients are unable to be medically managed and are referred for a procedural palliation.
Endovascular embolization has replaced surgery as the mainstay of treatment of vein of Galen malformation. The goal is reduction in the size of the shunt and resultant heart failure symptoms. Complete occlusion is not required during the initial procedure. Outcomes have improved and a neurologically normal to mild developmental delay is expected. Neonates showed the worst mortality rate of 52%, following endovascular treatment with only 36% of survivors being neurologically normal. If symptoms can be managed medically, survival after treatment from 5 months to 2 years increases to more than 90%, with 78% of survivors classified as neurologically normal.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here