Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiovascular disease in children, while at times sharing some similarities, is quite different from that of adults. Common adult cardiovascular disease is typically the culmination of years of hypertension, atherosclerosis from hypercholesterolemia, valvulopathy and vasculopathy from progressive calcification, and adverse effects from tobacco abuse and obesity. These events and more predispose to coronary artery disease and heart failure. Cardiovascular disease in the pediatric patient is more commonly due to congenital, genetic abnormalities ( Table 48.1 ). Many cases of cardiac disease may begin developing in utero and result in lifelong cardiovascular dysfunction. However, like adults, pediatric patients are susceptible to various infectious and inflammatory etiologies of cardiovascular disease as well as cardiomyopathy from exposure to chemotherapeutic agents and traumatic heart injuries. This chapter is aimed at providing an overview of these various forms of pediatric cardiovascular disease entities.
| Disorder | Potential Cardiac Involvement * |
|---|---|
| Alagille syndrome | Pulmonary valve and peripheral pulmonary artery stenosis, occasionally other CHD |
| Apert syndrome | 10% have CHD |
| Becker muscular dystrophy | Dilated cardiomyopathy beginning in the right ventricle; also found in female carriers, though typically less severe |
| Cat eye syndrome | Tetralogy of Fallot, total anomalous pulmonary venous return |
| Charge association | Most commonly tetralogy of Fallot |
| Complex I–V deficiencies | Cardiomyopathy |
| Costello syndrome | Hypertrophic cardiomyopathy, fatal arrhythmias |
| Cri-du-chat syndrome | CHD in 33%. PDA, ASD, VSD, PS |
| Down syndrome | 50% with CHD of those 50% with atrioventricular canal defects. Also, ASD and VSD |
| Duchenne muscular dystrophy | 90% with abnormal ECG. Heart failure, arrhythmias. Cardiomyopathy, typically in adolescents, increases anesthetic risk |
| Ehlers-Danlos syndrome | Valve insufficiency, CHD, conduction defects, aortic dissection, coronary artery disease in young adults |
| Ellis–van Creveld syndrome | CHD common, common atrium |
| Emery-Dreifuss muscular dystrophy | Cardiomyopathy, heart block |
| Epidermolysis bullosa | Mitral valve prolapse |
| Fabry disease | Ischemic cardiovascular disease, valve insufficiency, HOCM, valve insufficiency, conduction abnormalities |
| Friedreich ataxia | Cardiomyopathy |
| Glutaric acidemia type II | Severe HOCM |
| Homocystinuria | Premature coronary artery disease |
| Hunter syndrome | Coronary artery narrowing, thickened valves, mitral insufficiency |
| Hurler syndrome | Coronary artery narrowing, thickened valves, mitral insufficiency |
| Hurler-Scheie syndrome | Valve stenosis or insufficiency |
| Kabuki syndrome | CHD is common, right bundle branch block |
| Leigh disease | Hypertrophic cardiomyopathy |
| Leopard syndrome | Conduction abnormalities, PS, HOCM |
| Lipoprotein lipase deficiency | Premature coronary artery disease |
| LCAD (long-chain acyl-COA dehydrogenase deficiency | Cardiomegaly, possible cardiomyopathy |
| Maroteaux-Lamy syndrome | Heart failure in second to third decade, aortic and mitral valve calcification |
| Melas syndrome | Cardiomyopathy, Wolff-Parkinson-White syndrome |
| Morquio syndrome | Mitral and aortic valve involvement |
| Myotonic dystrophy | 90% have conduction abnormalities. Mitral valve prolapse |
| Pompe disease | Cardiomegaly with heart failure, abnormal ECG |
| Prune belly (Eagle-Barrett) syndrome | CHD in 10% |
| Rubinstein-Taybi syndrome | 33% have CHD |
| Scheie syndrome (mucopolysaccharidosis) | Aortic stenosis or insufficiency |
| Sickle cell disease | Myocardial fibrosis from prior sickling |
| Smith-Lemli-Opitz syndrome | CHD, especially tetralogy of Fallot and VSD |
| Stickler syndrome | Mitral valve prolapse |
| Thalassemia | Possible cardiac involvement from hemosiderosis from chronic hemolysis or recurrent transfusions |
| Tuberous sclerosis | Cardiac rhabdomyoma |
| Turner syndrome | Bicuspid aortic valve, coarctation of the aorta |
| VATER association | Variety of CHDs |
| Very-long-chain acyl-COA dehydrogenase deficiency | Cardiomyopathy in virtually all. Pericardial effusion. |
* Other cardiac defects can be found in addition to those listed.
Congenital heart disease (CHD) has a birth prevalence of approximately 12 to 14 per 1000 live births ( ). The severity of CHD has been broken down into mild, moderate, and severe ( Box 48.1 ). Nearly 25% of those born with CHD will have complex CHD requiring an invasive intervention within the first year of life ( ). When caring for a patient with CHD for noncardiac surgery, it is imperative that the anesthesiologist understands each patient’s specific physiology at that time. Children with univentricular physiology, particularly in the first interstage period (Norwood, described later), are at the highest risk for perioperative cardiac arrest ( ). Patients with severe CHD (see Box 48.1 ) have 8.4 and 7.3 times the odds of 30-day and overall mortality, respectively, compared with matched peers without CHD when undergoing noncardiac surgery. Additional pathologies such as pulmonary hypertension, arrhythmias, and cardiomyopathy put CHD patients at risk for adverse perioperative events. Each patient should be assessed for the appropriateness for spontaneous bacterial endocarditis (SBE) prophylaxis ( Box 48.2 ) and be provided with the appropriate antibiotic if they meet criteria ( Table 48.2 ). Specific CHD and perioperative management for CHD surgery are covered extensively elsewhere in this text. The following is provided as an overview for common forms of CHD.
| Mild | Small VSD Small PDA Mild pulmonary stenosis BAV without AS or AI Small ASD |
| Moderate | Mild to moderate AS or AI Moderate PS or PI Noncritical coarctation Large ASD Complex forms of VSD |
| Severe | All cyanotic congenital heart defects* AVSD Large VSD Large PDA Critical or severe AS Severe PS Critical coarctation |
| *Cyanotic CHD | d-Transposition of the great vessels Tetralogy of Fallot Hypoplastic right ventricle Tricuspid atresia Pulmonary atresia with intact ventricular septum Ebstein anomaly (severe) Double inlet left ventricle AVSD (unbalanced) Hypoplastic left heart Aortic atresia Mitral atresia Double outlet right ventricle AVSD (unbalanced) Truncus arteriosus Total anomalous pulmonary venous return |
AI, Aortic insufficiency; AS, aortic stenosis; ASD, atrial septal defect; AVSD, atrioventricular septal defect; BAV, bicuspid aortic valve; PDA, patent ductus arteriosus; PI, pulmonary insufficiency; PS, pulmonary stenosis; VSD, ventricular septal defect.
Prosthetic cardiac valve or cardiac valve repaired with prosthetic material
Previous infectious endocarditis
Congenital heart disease (CHD)
Unrepaired cyanotic congenital heart disease, including palliative shunts and conduits
Completely repaired CHD with prosthetic material or device, whether placed surgically or via catheterization, during the first 6 months after the procedure
Repaired defects with residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device
Cardiac transplantation recipients who develop cardiac valvulopathy
| Situation | Agent | Adults | Children |
| Oral | Amoxicillin | 2 g | 50 mg/kg |
| Unable to take oral medication | Ampicillin | 2 g IM or IV | 50 mg/kg IM or IV |
| OR | |||
| Cefazolin or ceftriaxone | 1 g IM or IV | 50 mg/kg IM or IV | |
| Allergic to penicillins or ampicillin—oral | Cephalexin | 2 g | 50 mg/kg |
| OR | |||
| Clindamycin | 600 mg | 20 mg/kg | |
| OR | |||
| Azithromycin or clarithromycin | 500 mg | 15 mg/kg | |
| Allergic to penicillins or ampicillin and unable to take oral medication | Cefazolin or ceftriaxone | 1 g IM or IV | 50 mg/kg IM or IV |
| OR | |||
| Clindamycin | 600 mg IM or IV | 20 mg/kg IM or IV | |
| Single dose 30–60 minutes before starting procedure | |||
Heart murmurs remain the most common reason for referral to a pediatric cardiologist. However, <1% of those referred are identified as having a pathologic murmur ( ; ). Many of these pediatric murmurs are identified as innocent, or physiologic, murmurs. These can typically be classified into one of four categories: still vibratory murmur, pulmonary flow murmur, supraclavicular systemic flow murmur, and venous hums. Many high-output states like anemia, fever, arteriovenous malformation, etc., can also result in heart murmur ( ).
Still vibratory murmur is a brief, low-pitched, midsystolic murmur heard best over the left-lower sternal border while supine, as it can diminish when upright. It appears to be caused by blood flow in the left ventricular outflow tract and is most commonly heard in children between 2 and 7 years of age ( ). Pulmonary flow murmurs are harsh, medium-pitched ejection murmurs heard best over the left-upper sternal border created by blood flow over a normal pulmonary valve. Supraclavicular systemic flow murmurs are described as harsh, crescendo-decrescendo, medium-high-pitched murmurs of the supraclavicular region with radiation to the carotids. Venous hum murmurs are continuous, low-pitched murmurs heard best just posterior to the sternocleidomastoid muscles of the neck, as they are derived from superior caval blood return ( ) ( Fig. 48.1 ).
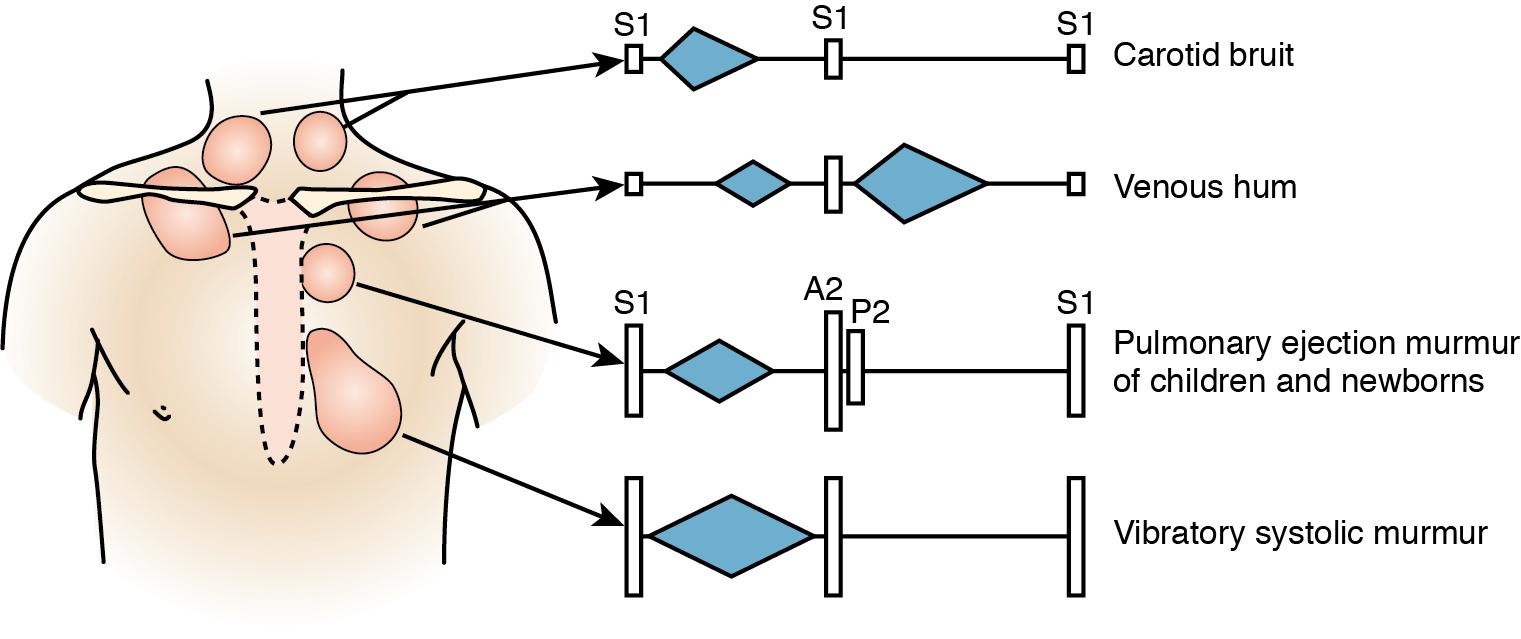
There are, however, grave consequences for misdiagnosing a murmur as innocent or physiologic when in reality it may be pathologic. Therefore in 2014 the first pediatric criteria were derived for ordering transthoracic echocardiography ( ). Subsequently, a multicenter study found that out of 2655 transthoracic echocardiograms performed, 71% were appropriate as per the guidelines, 12% may have been appropriate, 12% were rarely appropriate, and 5% were unclassifiable ( ). For those referred for presumed pathologic murmur (an appropriate criteria for echocardiography), 40% had abnormal findings, whereas there were still 44% with normal studies (17% had incidental findings). For those referred for presumed innocent murmurs (a rarely appropriate criteria), 92% had normal findings, but there were 3% who had abnormal findings (5% with incidental findings). More recently, electronic stethoscopes have been used to record heart sounds. Some of these stethoscopes contain computer algorithms and artificial intelligence and are being investigated for diagnostic purposes. These results show a high sensitivity and specificity at similar levels as those reported for specialist auscultation ( ).
Common forms of CHD are typically acyanotic and functionally biventricular. However, there are many forms of CHD that start out as cyanotic defects requiring ductal patency, but that can be surgically palliated to result in a functionally biatrial, biventricular heart. Several common forms of biventricular CHD and potential residual abnormalities after surgical palliation are presented in Fig. 48.2 .
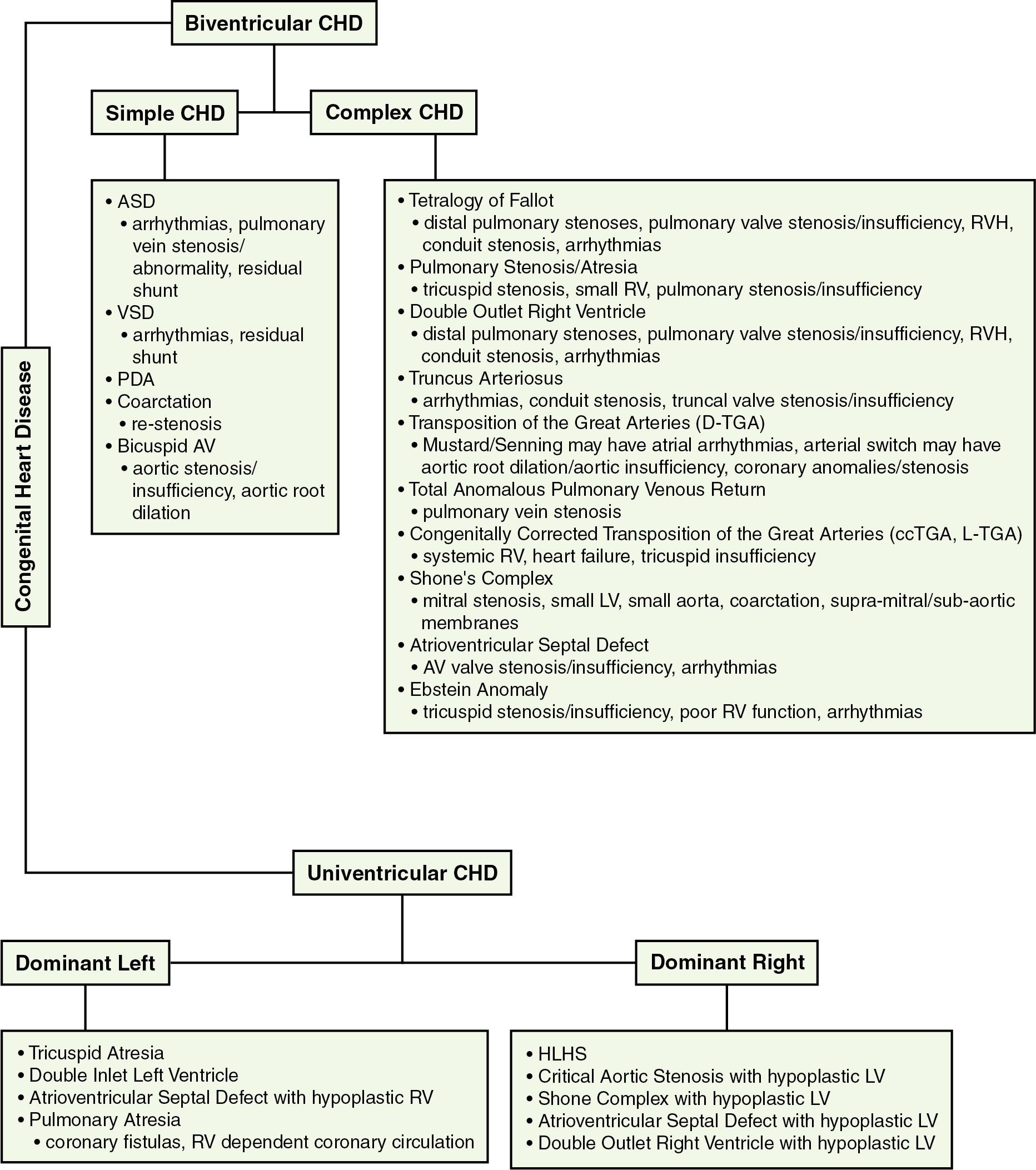
Simple congenital heart defects do not result in cyanosis and in fact may go undetected for a prolonged period. Secundum-type (most common) atrial septal defects (ASDs) will often present in the toddler ages after auscultation of a flow acceleration murmur across the pulmonary valve from right-sided volume overload. Unlike ASDs, the murmur from ventricular septal defects (VSDs), coarctation, and patent ductus arteriosus (PDA) lesions are more easily auscultated at birth or during infancy. Large ASDs and VSDs may be detected earlier in the infant stage because of pulmonary overcirculation, heart failure symptoms (e.g., tachypnea, poor feeding, poor growth), and frequent pulmonary infections. Coarctations may become recognized within the first week of life as partial pressure of oxygen (Pa o 2 ) increases, leading to ductal closure and clinically relevant aortic stricture. It is not uncommon for preterm neonates to maintain a PDA despite higher Pa o 2 and interventions such as indomethacin. The resultant diastolic flow reversal makes feeding difficult, as gut ischemia may result, predisposing neonates to necrotizing enterocolitis ( ). Repairs for these simple lesions are fairly straightforward and at times may be amenable to catheterization-based techniques (ASD/VSD device closures, coarctation stenting, PDA coiling/plugs). Otherwise, septal defects may require surgical patch closure and coarctations, and PDAs may require surgical resection ± augmentation and ligation, respectively.
Complex biventricular CHD typically results in cyanosis at birth, and some may require ductal patency. Some of the most severe forms may require neonatal intervention and potentially even emergent intervention. Sometimes the same defect spans a spectrum of severity and thus intervention may be postponed. Some common complex CHD types are shown in Fig. 48.2 .
Tetralogy of Fallot is one such lesion that can span a spectrum of severity. Some “pink tets” may have minimal right ventricular outflow tract (RVOT) obstruction and small, restrictive VSDs. Others may be born with complete pulmonary atresia with the pulmonary blood flow arising from major aortopulmonary collateral arteries (MAPCAs). In severe forms without and sometimes with MAPCAs, prostaglandin infusion may be necessary to maintain ductal patency. Surgical repair options range in complexity, just as the disease ranges in severity from valve-sparing RVOT and/or pulmonary artery augmentation and VSD closure to right ventricular (RV) to pulmonary artery conduit insertion and unifocalization of major aortopulmonary collaterals. Long-term survival after repair is 94.5% at 25 years, with sudden death, arrhythmias, and heart failure observed as causes of death ( ). Reintervention on the RVOT within 20 years occurred in 14% ± 4% for those with pulmonary stenosis and 57% ± 16% for those with pulmonary atresia ( ).
Transposition of the great arteries (d-TGA) is a complex form of CHD that may require emergent neonatal intervention. Without adequate atrial-level left-to-right shunting, even in the presence of a PDA and VSD, neonates may develop unacceptable hypoxia necessitating a catherization with balloon atrial septostomy. Depending on atrial-level shunting and the size of the PDA, prostaglandin may or may not be required. Prior to 1975, d-TGA was palliated with atrial-level baffling to deliver the systemic venous blood to the mitral valve and the pulmonary venous blood to the tricuspid valve, but this maintained the RV as the systemic ventricle. Jatene and colleagues introduced the arterial switch operation in 1975, whereby the aorta is removed from the pulmonary trunk and moved posteriorly over the left ventricle (LV) with coronary reimplantation, and the pulmonary artery is removed from the aortic trunk and positioned over the right ventricle ( ). Survival at 25 years postrepair is 96.7% with a reintervention rate of 3.8% ( ). Pulmonary stenoses and aortic dilation are mid to late sequelae of the arterial switch operation.
Univentricular heart defects may present as ductal-dependent lesions. Prostaglandins are required at birth to maintain ductal patency for systemic blood flow (hypoplastic left heart syndrome [HLHS]) or pulmonary blood flow (pulmonary atresia with intact ventricular septum [PA-IVS]). On the other hand, CHD such as tricuspid atresia may have both adequate systemic and pulmonary vascular perfusion, such that as pulmonary vascular resistance decreases, a pulmonary band may be required to limit pulmonary blood flow to prevent pulmonary overcirculation. Some common forms of univentricular CHD are shown in Fig. 48.2 .
Univentricular CHD with systemic LV occurs because of defects such as tricuspid atresia, double inlet LV, and PA-IVS. Without a patent tricuspid valve (tricuspid atresia), there is poor or no RV development. This can occur with normally related or transposed great vessels, and depending on which, a sufficient VSD is necessary to allow for pulmonary or systemic cardiac output, respectively. Double inlet LV is similar to tricuspid atresia except that the tricuspid valve is patent but opens into the LV, and transposed great arteries are more common than normally related great arteries. Pulmonary atresia with intact ventricular septum is a certain type of CHD that may be amenable to biventricular repair. Neonates often require cardiac catheterization to ascertain coronary anatomy, as coronary fistulas and RV-dependent coronary circulation may be present. In these instances, decompressing the RV by opening the pulmonary valve could result in catastrophic coronary malperfusion. With normal coronary anatomy and a tripartite RV (inlet, apical trabecular, and outlet segments), perforation of the atretic pulmonary valve can be attempted. Otherwise, a functionally univentricular pathway is undertaken.
Univentricular CHD with systemic RV occurs because of defects such as HLHS, critical aortic stenosis with hypoplastic LV, and double outlet RV with hypoplastic LV. Hypoplastic left heart syndrome can occur with any combination of aortic stenosis or atresia and mitral stenosis or atresia. Some patients born with HLHS may also have a restrictive atrial septum (large ASD required for left-to-right shunting of oxygenated pulmonary venous blood). Even with emergent septostomy, early survival is 80% versus 94% in those with a nonrestrictive septum, and 35-month survival is 62% versus 81% ( ).
Univentricular CHD results in a single systemic left or right ventricle with no subpulmonary ventricle. The result is parallel circulation with a single pump (RV or LV). The conversion from a circulation that is parallel to one that is in series is achieved through a sequence of palliative operations aimed at separating deoxygenated systemic venous blood from oxygenated pulmonary venous blood. The first-stage operation typically consists of some form of arteriopulmonary shunt, such as a modified Blalock-Taussig shunt (for HLHS) or PDA stent (for PA-IVS). In HLHS, an arch augmentation, Damus-Kaye-Stansel anastomosis of the aorta and pulmonary artery, and definitive atrial septostomy are performed in addition to an arteriopulmonary or RV-to–pulmonary artery shunt, a procedure commonly referred to as a Norwood operation. The second stage of univentricular palliation is the superior cavopulmonary anastomosis (Glenn operation). This typically occurs sometime between 2 and 6 months of age, but this is highly dependent on each medical center’s practice ( ). Factors such as pulmonary vascular resistance, pulmonary volume overload, shunt stenosis, and overall high interstage risk are a few of the contributing factors ( ). A cardiac catheterization is usually performed before the operation to ensure that appropriate hemodynamics are present. The procedure is accomplished by removing the superior vena cava from the right atrium and creating an end-to-side anastomosis between the superior vena cava and (most typically) the right pulmonary artery, as well as takedown of the arteriopulmonary or RV-to–pulmonary artery shunt. At this point, all pulmonary blood flow is passive and is solely supplied by superior vena cava blood. The third and final procedure is the completion of the total cavopulmonary anastomosis (Fontan operation), which typically occurs at around 18 months to 4 years of age ( ). Again, a cardiac catheterization usually precedes the operation to ensure hemodynamics are favorable to move forward with the operation. This is accomplished by removing the inferior vena cava from the right atrium and connecting it to the (most typically) right pulmonary artery, often via an extracardiac conduit. A small fenestration may be created between the Fontan conduit and the right atrium to ensure adequate cardiac output via right-to-left fenestration shunting in the setting of increased pulmonary pressures. Survival at 25 years after Fontan operation is around 76% ( ). For more information on CHD, please see Chapter 30 , Anesthesia for Congenital Heart Disease.
Pediatric cardiomyopathy has an overall incidence of 4.8 per 100,000 infants and 1.3 per 100,000 children less than 10 years of age ( ). Pediatric cardiomyopathy is a common cause of heart failure and heart transplantation in children, and although cardiomyopathies in general are typically classified as ischemic or nonischemic, nearly all pediatric cardiomyopathies are considered nonischemic ( ). Pediatric cardiomyopathies can be classified as dilated, hypertrophic, restrictive, noncompaction, arrhythmogenic, or unclassified. However, it is not uncommon to have mixed types and/or transition from one type to another ( ). A summary of the anesthetic considerations for the perioperative management of children with cardiomyopathies is presented in Box 48.3 .
| Cardiomyopathy | Features | Implications |
|---|---|---|
| Dilated | Four-chamber dilation Predominantly LV dilation Atrioventricular valve regurgitation Low ejection fraction/fractional shortening |
Consider neuromuscular comorbidities Maintain adequate preload Normal heart rate Avoid increased SVR Inotropes may be required |
| Hypertrophic | Dynamic LV outflow obstruction LV cavity obliteration Diastolic dysfunction Increased LV mass |
Maintain slower heart rate Avoid hypovolemia Normal to increased SVR Consider phenylephrine for ECG ST segment depression Avoid vasodilating agents |
| Restrictive | Near-normal ventricles Dilated atria Dilated pulmonary veins Diastolic dysfunction |
Maintain preload Maintain contractility Avoid bradycardia Avoid additional increases in PVR |
| Left ventricular noncompaction | Prominent myocardial trabeculations Myocardial hypokinesis |
Consider associated neuromuscular disorders |
| Arrhythmogenic | Fibro-fatty infiltration of RV Abnormal ECG RV failure |
Avoid exogenous catecholamines External defibrillation pads |
ECG, Electrocardiogram; LV, left ventricle; PVR, pulmonary vascular resistance; RV, right ventricle; SVR, systemic vascular resistance.
Pediatric dilated cardiomyopathy (DCM) accounts for nearly 54% of all pediatric cardiomyopathies, or 0.57 per 100,000 children per year in the United States ( ; ). Pediatric DCM is characterized by prolongation of total isovolumic time and a reduction in LV effective filling time on echocardiography, in addition to hypertrophy of myocytes, attenuation of myocytes, and endocardial smooth muscle cell hyperplasia on endomyocardial biopsy ( ). Children with DCM may present either as asymptomatic or with severe acute decompensated heart failure and cardiogenic shock ( ). Many will require hospitalization upon diagnosis. At diagnosis, 54% required intravenous inotropic support, 41% were placed on mechanical ventilation, 13% necessitated extracorporeal membrane oxygenation, and 11% underwent urgent heart transplantation ( ).
Between 35% and 40% of genetic DCM cases are thought to be a result of mutations in sarcomere genes and Z-band proteins ( ). There are also inflammatory causes of DCM, such as viral myocarditis. Between 35% and 48% of children with DCM show evidence of viral myocarditis on endomyocardial biopsies, with parvovirus B19, influenza, Epstein-Barr, HIV, coxsackie, herpes, and adenovirus having all been identified as causes ( ). Toxic etiologies of DCM in children most often are the result of anthracycline exposure as part of a chemotherapeutic plan ( ). In a study comparing children with familial DCM versus idiopathic DCM, there was no difference in risk of transplant or the combined death or transplant outcome ( ).
Many of the long-term DCM therapies for children mimic those used in adults, although studies aimed specifically at evaluating these therapies in children are limited. Mainstays of treatment include diuretics (loop, thiazide, and/or potassium-sparing), angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and beta-blocking agents ( ). In addition, disease-specific therapies for inborn errors in metabolism can abate cardiac decompensation, such as carnitine therapy for primary carnitine deficiency causes of DCM (Lee et al. 2017). Nearly 40% of children with DCM undergo heart transplantation or die within 2 years of their diagnosis ( ). However, overall survival at 1 and 5 years after diagnosis is 90% and 83%, respectively, with the use of cardiac transplantation ( ). Survival in the decade between 2000 and 2009 was better compared with the decade prior (1990–1999) ( ). According to a study by Singh and colleagues, even when controlling for the degree of LV systolic dysfunction, the cause of DCM, and the presence of heart failure symptoms at diagnosis, children with DCM in the early cohort were 40% more likely to die than were children in the late cohort ( ). More long-term follow-up shows that freedom from death or transplant was 62% at 10 years and 56% at 20 years ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here