Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiopulmonary bypass (CPB) is one of the most important biomedical inventions in the history of health care, rivaling the development of roentgenography and hemodialysis in its clinical impact. The scope of its application is far reaching. Its birth paralleled the evolution of an entire surgical subspecialty, and without its use, surgeons would be intimidated by the prospect of intracardiac repair.
Despite the belief that the need for CPB would be significantly diminished because of the surge in interest in off-pump coronary artery bypass grafting (CABG), the predictions have not materialized. Off-pump trends peaked at 24% in 2003, steadily declining thereafter to a stabilized percentage of 19% in 2011. In many practices, complex multiple arterial reconstruction and minimal-access surgery such as mini-thoracotomy mitral valve surgery are more comfortably and accurately approached using longer periods of CPB and optimized myocardial protection. Therefore, every surgeon must have a comprehensive understanding of all aspects of CPB, from the physiology of gas exchange to the molecular mechanisms characteristic of biocompatibility.
The evolution of CPB reflects ingenuity bred of necessity. A parallel discovery that contributed to the feasibility of extracorporeal circulation was the isolation of the natural anticoagulant heparin. Dr. John H. Gibbon, Jr. ( Fig. 63-1 ), a “receptive and talented surgeon with foresight … who could envision an intact surviving patient beyond the isolated organ studies,” was the inventor of the Gibbons-IBM oxygenator. In 1953, this device was successfully used to correct an atrial septal defect. Although it was initially successful, three subsequent attempts at intracardiac repair were fatal, resulting in a self-imposed moratorium on its clinical use. Nevertheless, the feasibility of this approach had been demonstrated.

The first clinically successful film and bubble oxygenators brought blood in direct contact with respiratory gases to equilibrate with them. Complications related to blood trauma eventually led to a decline in their popularity, and they are rarely used today. Membrane oxygenators were introduced in the 1950s after the observation by Kolff and Berk that venous blood was oxygenated while flowing through a cellophane dialysis tube in contact with O 2 -containing dialysate. The first membranes were relatively impermeable to gases, requiring huge surface areas and massive priming volumes. Formed of silicone rubber, they were designed in either an extraluminal format (blood flowing on the outside of the tube with gas on the inside) or, less often, in an intraluminal format (the reverse). The next generation of oxygenators were prepared with silicone-coated polypropylene, whereas currently many commercial oxygenators use polymethylpentene fibers. Other refinements have been subtle but significant, and we now have oxygenators with surface areas of over 2.0 m 2 configured with minimal blood volume. The resulting minimization of prime volume has contributed more than any other factor to blood conservation practice in modern-day cardiac surgery.
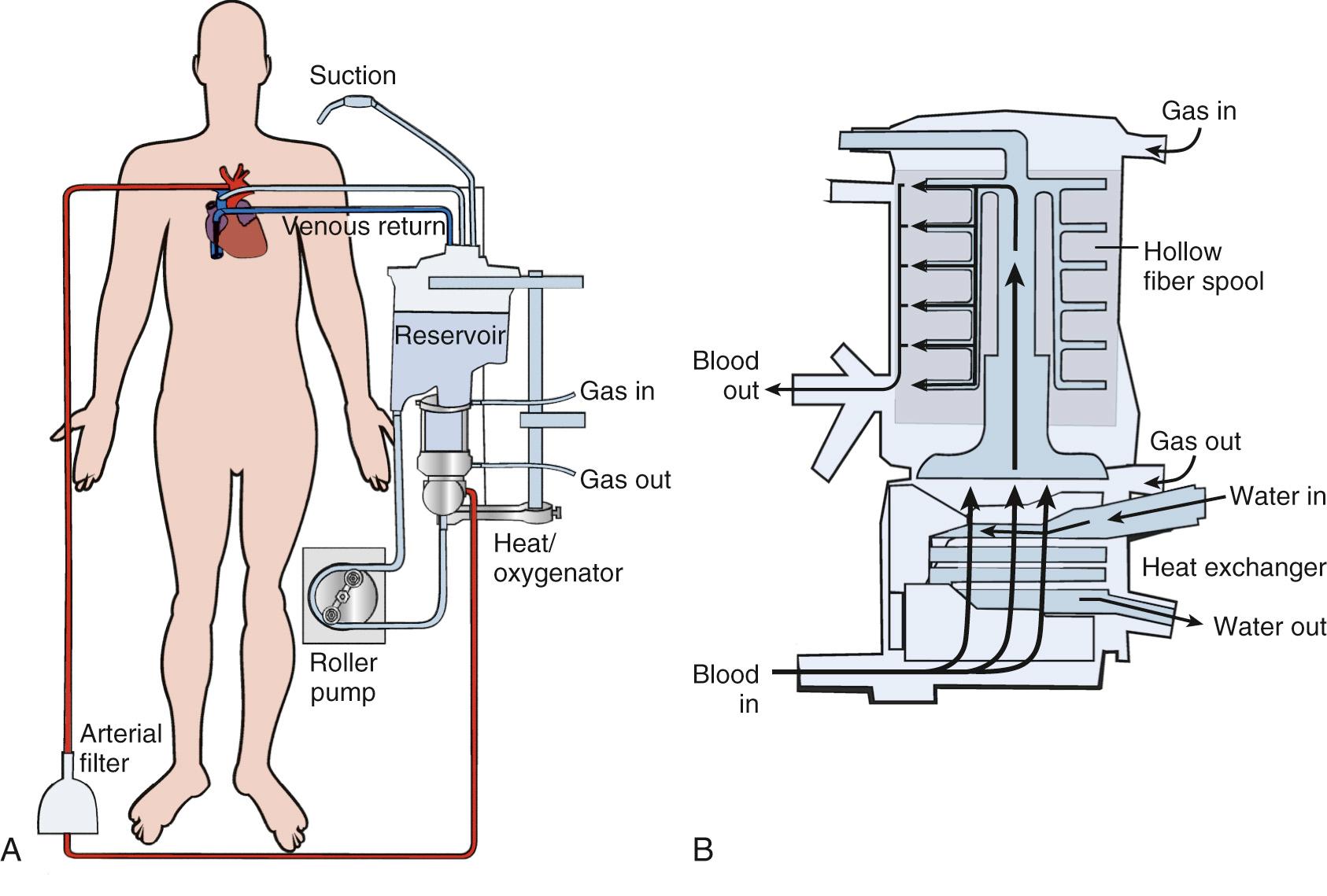
Inflow and outflow cannulas from the patient are attached to the oxygenator device with tubing composed of polyvinylchloride, polyurethane, or silicon rubber. Blood enters the venous reservoir from the venous circulation. Through separate inflows on the reservoir, blood can also be returned from the pericardial well (cardiotomy blood) and from cardiac and aortic vents. A pump is used to divert the venous reservoir blood to the oxygenator. After the blood passes through the integrated heat exchanger and oxygenator, it is circulated through an arterial filter and bubble trap and returned to the patient through the arterial cannula ( Fig. 63-2 ). Cardioplegia setups are often intimately incorporated into the CPB circuit.
Diffusion of gases at the blood-membrane interface can be predicted by Fick's law, which states that the rate of diffusion is proportional to the partial pressure gradient of the gas in the direction of diffusion. The rate of gas transfer is also inversely proportional to the distance through which it must pass (the thickness of the membrane). The rate also depends on the property of diffusivity of the membrane biomaterial. Currently used membranes are permeable to O 2 , but they are often less permeable to CO 2 . The problem of poor diffusion of CO 2 was solved by the introduction of microporous membranes. These surfaces allow transient blood-gas interfacing at pore structures smaller than blood cells; however, the hydrophobic nature of the membrane results in changes in blood surface tension, blocking actual contact between the two phases. As a result, the interface in microporous membranes behaves like a very thin stagnant film of plasma water that offers little resistance to gas exchange. Progressive protein accretion at the pores occurs over time, resulting in a finite functional capacity, detected by worsening efficiency in gas transfer, which is why oxygenators must be replaced with long CPB runs ( Fig. 63-3 ).
As opposed to the relatively facile transfer of O 2 gas through a membrane, when the O 2 is dissolved in plasma or blood, its diffusivity is 25 times less than that of CO 2 . This difference is due to the more facile dissolution of CO 2 in plasma and due to the linear shape of the CO 2 dissociation curve as compared to the sigmoid shape of the O 2 dissociation curve. The patient's PaCO 2 can be decreased by increasing the rate of fresh gas flow, whereas alteration of the PaO 2 can be achieved only by enhancing the rate of blood flow exposure to the surface. This latter can be accomplished either by increasing the overall flow or by enhancing the efficiency of exposure. To achieve this, the path length (the distance the blood travels as it passes the gas-exchange surface) must be maximized. This modification is limited by the parallel need to increase the priming volume. Second, disturbed flow patterns can be used to promote mixing and to bring deoxygenated blood closer to the exchange surface. Mechanisms that interfere with the development of fully developed flow patterns enhance diffusion by keeping the boundary layer narrow. In an oxygenator, this can be accomplished by making the surface irregular or by positioning elements in the flow stream to disrupt smooth flow and to enhance mixing. This is the rationale for the extraluminal hollow fiber design.
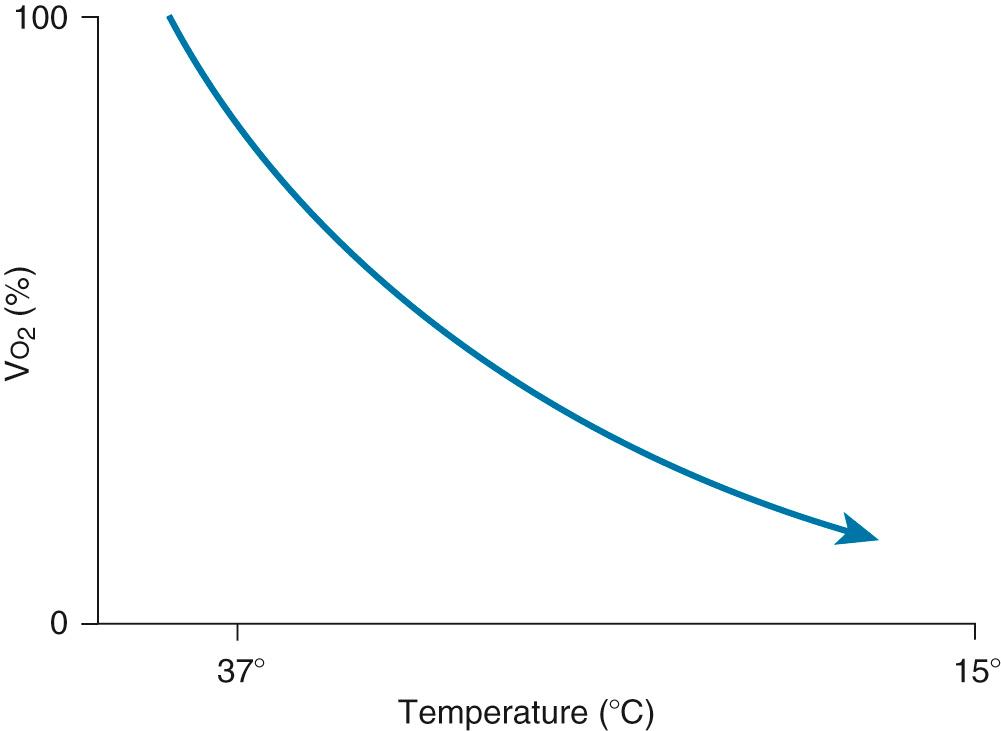
The feasibility and applicability of hypothermia for heart surgery was first suggested by Bigelow and colleagues in 1950. They demonstrated the safety benefit of hypothermia as a means to decrease O 2 consumption during inflow occlusion in an animal model. O 2 consumption decreases by 50% for every 10° C drop in temperature ( Fig. 63-4 ). Lower flows decrease collateral flow and rewarming of the heart from contact with adjacent tissues, and they provide a margin of safety if equipment fails.
Hypothermia is associated with marked changes in blood pH and P co 2 levels. With a rise in temperature, CO 2 becomes less soluble in blood, and there is a greater tendency for the dissolved CO 2 to come out of solution (increased gaseous phase). Similarly, CO 2 solubility increases with decreasing temperature. When blood from a hypothermic patient (e.g., 24° C) is introduced into a CO 2 electrode for measurement, it is first warmed to 37° C (increasing the amount of CO 2 in the gaseous phase), and therefore the measured partial pressure of CO 2 is higher than it was in reality at the cooler temperature. The correction for P co 2 is based on a calculated decrease of 4.5% per degree Celsius. On the other hand, the pH increases 0.015 units per degree Celsius drop in temperature. This shift in pH is partially related to the influence of buffers such as the imidazole moiety of histidine, but it is also related to the dynamics of the Henderson-Hasselbalch equation, pH = pK + [HCO 3 − ]/(0.03 × P co 2 ).
Because alkalosis and hypocarbia trigger a decrease in cerebral blood flow (CBF), some investigators have suggested the addition of CO 2 during hypothermia to compensate and to keep the pH unchanged (pH-stat strategy). In 1987, Murkin and coworkers confirmed that pH-stat management results in a greater ratio of CBF to cerebral metabolic rate (CMRO 2 ) than alpha-stat management (i.e., no active correction of pH with hypothermia). Most agree that a pH-stat strategy is probably preferable in children, where increased CBF increases the rate of cooling and thus increases the chance of achieving uniform cerebral hypothermia. The rate of brain oxygen depletion during deep hypothermic circulatory arrest is also considerably slower with pH-stat strategy. As a result, pH-stat substantially prolongs the interval between the onset of arrest and the exhaustion of brain oxygen stores and may be associated with better clinical outcomes in this group.
In contrast, in adults, an alpha-stat strategy may provide greater cerebral protection during hypothermia on CPB. Alpha-stat management is certainly easier to accomplish, and a justification is that most cellular mechanisms are capable of maintaining intracellular pH despite fluctuations in extracellular conditions. Because excess CO 2 is added in a pH-stat strategy, brain acidosis may occur during rewarming, and, combined with decreased O 2 delivery after CPB, this may augment CNS injury in adults. Most importantly, as mentioned, a pH-stat strategy results in excessive CBF, which may increase embolic load. Finally, pH-stat strategy is associated with a decreased ability to maintain autoregulation at low pressures. Three randomized controlled trials have demonstrated a small but present benefit of alpha-stat strategy in terms of neurologic and neurocognitive outcome in adults, especially when CPB time exceeded 90 minutes and therefore evidence-based guidelines now support that patients undergoing moderate hypothermic CPB should be handled with an alpha-stat pH management (Class I, Level A).
In the 1960s, crystalloid (dextrose 5% in water, or D5W) in prime was introduced as an alternative to routine whole blood prime. An increase in efficiency of oxygenation and a decrease in end-organ complications were seen with this approach. Now hemodilution is commonly practiced in virtually all adult and pediatric cardiac surgeries, with the hematocrit (Hct) maintained between 20% and 25% during CPB. Hemodilution has a major effect on blood viscosity, primarily at the capillary level where the radius is small and the shear rate is low. Low flow at the capillary level increases viscosity of the blood, further increasing the resistance, but this effect is counterbalanced by the effect of hemodilution.
The drop in O 2 content at most levels of moderate hemodilution may be compensated by augmented cardiac output (CO), so total O 2 delivery (CO × O 2 content of blood) is increased. Hypothermia complicates the effects of hemodilution, as the decreased temperature causes increased viscosity and induces vasoconstriction.
The optimal Hct for CPB remains a topic of significant controversy. A post hoc assessment of the impact of low Hct on admission of cardiac patients to the intensive care unit (ICU) by Spiess and colleagues showed an inverse correlation between admission Hct and the risk of Q-wave myocardial infarction (MI). On the other hand, studies have demonstrated that an Hct of less than 20% may be associated with abnormal distribution of blood flow to organs, and that an Hct of less than 15% may lead to maldistribution of coronary flow away from the subendocardium in the presence of residual coronary stenosis. Retrospective observational studies of consecutive patients undergoing isolated CABG have demonstrated that a lower minimum Hct is associated with a significantly increased risk of renal injury and mortality. These relationships have also been demonstrated in patients with low Hct during CPB who did not receive any transfusion so as to differentiate that this was not an effect of blood products that these patients might otherwise receive. As excessive hemodilution also increases CBF, it may cause a parallel increase in microembolization and thus may theoretically be a contributor to neurologic damage after CPB and the increased risk of stroke observed with lower Hct on CPB.
Most centers prime the CPB circuit with a solution of balanced salt with or without the addition of a colloid solution such as pentastarch. Infants and children often need blood added directly to the prime, depending on the anticipated Hct after initiation of bypass (calculated from standard nomograms). Other additives to the prime may include calcium, mannitol, and pharmacologic agents such as heparin and aprotinin. The patient's circulating volume and the pump prime volume mandate the minimum priming volume. Although the prime volume averages 1 liter, experienced practitioners can achieve as low as 200 mL in pediatric circuits.
Flow is generally kept in the range of 2.2 to 2.5 liter/min/m 2 to provide a margin of safety during CPB, because systemic blood flow distribution and O 2 consumption remain normal at this level. At normothermia, a target mean blood pressure of 50 to 70 mm Hg is used. (As a rule of thumb in adults, the mean pressure chosen should be equal to the patient's age.) The perfusionist can control the pressure by increasing or decreasing flow or by the addition of vasoconstrictors or vasodilators (inhalational anesthetics). At lower temperatures, a mean pressure of 35 mm Hg is still generally accepted as safe, but these findings are controversial.
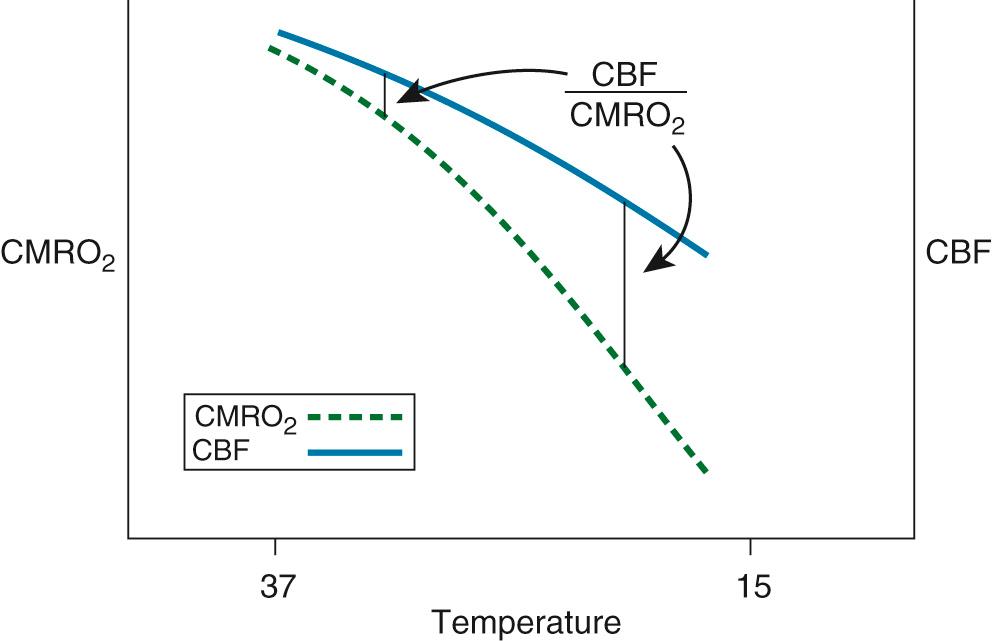
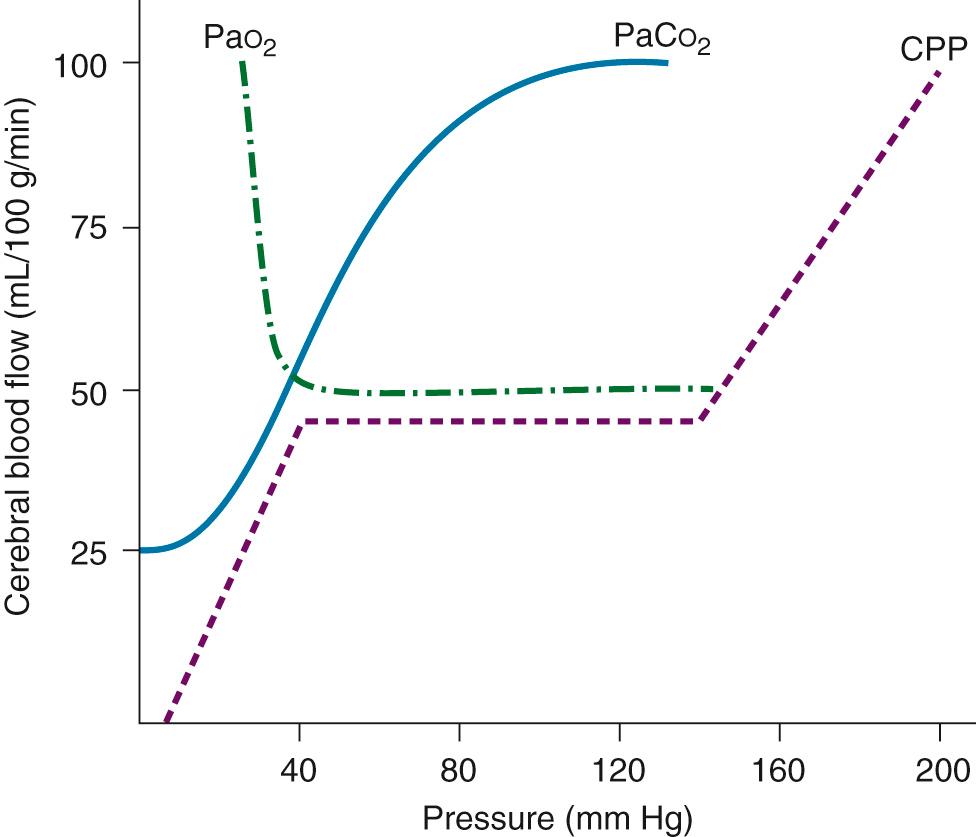
Although the brain comprises only 2% of body weight, its metabolic needs demand 15% of the cardiac output, extracting as much as 25% of the delivered O 2 . Temperature is the most important element influencing CBF during CPB. As the temperature is dropped, the CMRO 2 decreases exponentially and the CBF decreases linearly ( Fig. 63-5 ). As a consequence, the ratio of CBF to CMRO 2 increases, resulting in “luxuriant” flow, further facilitated by hemodilution.
Autoregulation of CBF is also related to changes in perfusion pressure. At normothermia, a mean pressure of 50 mm Hg is the threshold at which the brain autoregulates flow, but with hypothermia (26° C), the threshold drops to 30 mm Hg ( Fig. 63-6 ). At deep hypothermia (<20° C), there is a loss of pressure-flow autoregulation, as severe temperature reductions impair cerebral vascular relaxation and changes in cerebral perfusion pressure alone result in corresponding proportional changes in CBF. Other factors that influence CBF and CMRO 2 include blood viscosity, intracranial pressure and central venous pressure (CVP), and the blood gas status (pH, Pa co 2 , Pa o 2 ). CBF varies linearly with the Pa co 2 , in the range of 20 to 80 mm Hg, whereas a Pa o 2 of less than 50 causes cerebral vasodilation, which overrides pressure-flow autoregulation.
The two commonly used types of pumps for CPB involve either roller or centrifugal fluid propulsion. With the former, noninterrupted contact of the rollers with the tubing in the track results in the nonpulsatile nature of the flow ( Fig. 63-7 A ). Flow is calculated from the RPM of the roller pump knowing the diameter of the tubing and thus precisely the volume displaced. A low compression will result in inadequate flow, whereas excessive compression may aggravate hemolysis and tubing wear. Other complications associated with the use of the roller pump include (1) cavitation caused by excess pressure and (2) spallation (the release of particles from the inner surface).
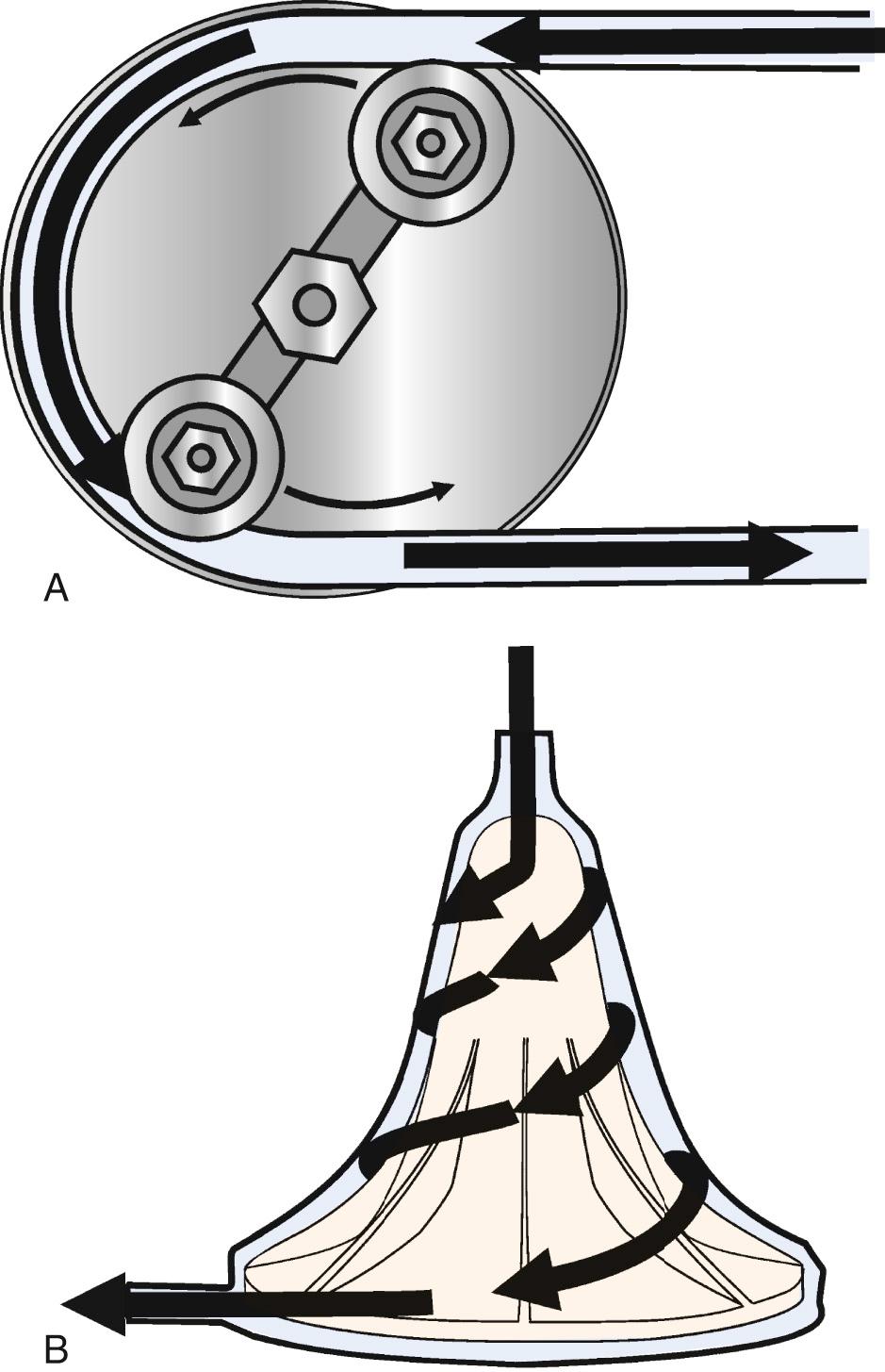
Centrifugal pumps (see Fig. 63-7 B ) are a popular alternative to the roller pump, particularly in pediatric cardiac surgery. A rotating impeller spins at 2000 to 5000 revolutions per minute on a small bearing, or the blades are magnetically suspended. This creates a vortex that draws blood into the pumphead and thrusts it to the oxygenator. The advantages of this device include its relatively small priming volume and its reliability (with fewer technical failures as compared to a roller pump device). Whereas with a roller pump, venous drainage to the reservoir from the patient is dependent on gravity drainage, centrifugal pumps are not constrained by this action. The flow in a centrifugal pump is preload and afterload dependent, and it is not predictable solely based on the calculated revolutions per minute, so an in-line flowmeter is essential. Aside from their expense ($150 per case), these devices are susceptible to air locks, thus requiring vigilance by the perfusionist. On the other hand, this may be protective, because there is less chance of pumping large volumes of air into the patient via an inadvertent leak, and the lines cannot be overpressurized with distal obstruction, as occurs with roller pumps. These devices are also not valved, and if rotation stops without clamping the outflow, rapid retrograde flow from the arterial line occurs in milliseconds, potentially exsanguinating the patient. Finally, roller pumps can be operated manually, but a power outage with a centrifugal pump can be a disaster.
A recent meta-analysis comparing roller versus centrifugal pumps for CPB, which included 18 randomized controlled trials with 1868 patients (predominantly isolated CABG), identified no significant difference for hematologic variables, blood loss and transfusions, neurologic outcomes (albeit not amenable to pooled analysis), or mortality rate.
Cardiotomy blood is the extravascular blood collected in the thoracic wound during CPB. In general, blood from the wound cavity is aspirated through a sucker device and transferred to a cardiotomy reservoir. Because of the close proximity of tissues in the wound and the potential for particulate matter to be collected, a filter and a defoaming chamber are incorporated into the cardiotomy reservoir. Mechanical injury, such as hemolysis, may result from the air-blood interface at the sucker, as well as from the compressive effects of the roller pump. Other mechanical complications from cardiotomy suction include the formation of particulate emboli, including fibrin, macroaggregates of denatured proteins and lipoproteins, fat globules, platelet and leukocyte aggregates, calcium, cellular debris, talc, and suture material.
Cardiac venting involves the active aspiration of blood from the heart, thus creating a bloodless field to facilitate visualization. Venting in some form is necessary in virtually all cardiac operations involving CPB, primarily to aid visualization but also to avoid chamber distention. When the aorta is cross-clamped and the native coronary circulation is stopped, a variable amount of noncoronary collateralization remains, particularly related to bronchial artery flow and return from the thebesian veins. Blood flow can also occur through the heart as a result of continued transit of blood through the right heart, through the lungs, and into the left heart. Potential sites for cardiac venting include the pulmonary artery, the superior pulmonary vein, the left atrium, the left ventricle, and the ascending aorta.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here