Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
All anesthesiologists who care for patients undergoing cardiopulmonary bypass (CPB) should be intimately familiar with the details and function of the extracorporeal circuit (ECC) or “heart-lung machine,” and be involved in the establishment of protocols and the conduct of CPB during cardiac surgery. Communication and teamwork are of paramount importance to the safe conduct of CPB.
The goal of CPB is to provide adequate gas exchange, oxygen delivery, and systemic blood flow with adequate perfusion pressure while minimizing the detrimental effects of bypass.
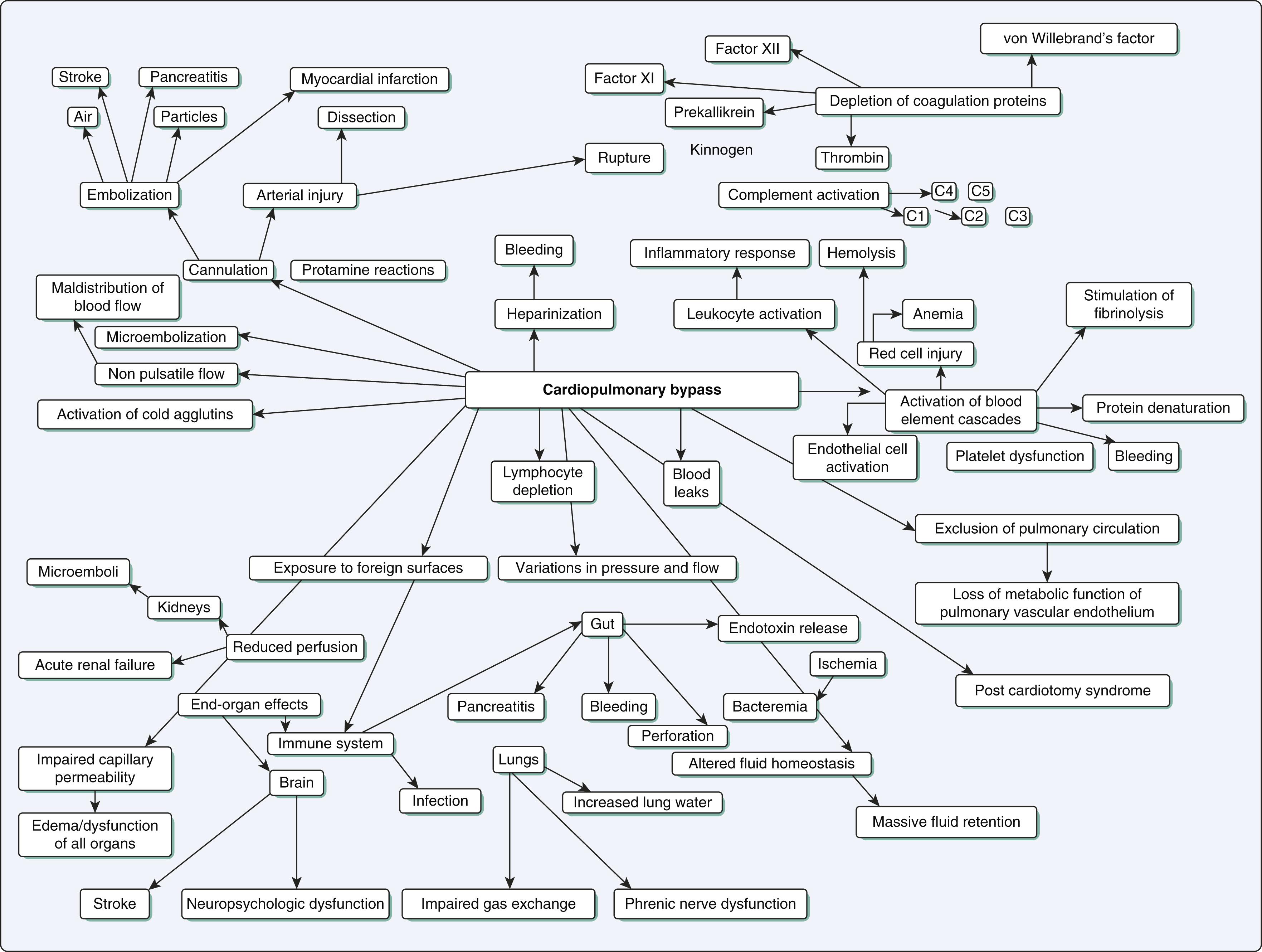
CPB is associated with a number of profound physiologic perturbations including altered flow and pulsatility, hematocrit and osmotic pressure; abnormalities of coagulation and blood cells, cooling and warming, micro- and macroembolization; and abnormal stress, inflammatory, metabolic, and hormonal responses.
The central nervous system, kidneys, lung, heart, and gastrointestinal tract are especially vulnerable to ischemic events associated with CPB. Embolization, hypoperfusion, and inflammatory processes are common pathophysiologic mechanisms responsible for organ dysfunction after CPB.
Advanced age is the most important risk factor for stroke and neurocognitive dysfunction after CPB.
Acute renal injury from CPB can contribute directly to poor outcomes.
Myocardial stunning represents injury caused by short periods of myocardial ischemia that can occur during CPB.
Gastrointestinal complications after CPB including pancreatitis, gastrointestinal bleeding, bowel infarction, cholecystitis, and liver failure are relatively uncommon, but associated with high morbidity and mortality.
Pulmonary complications such as atelectasis and pleural effusions are common after cardiac surgery with CPB.
Organ dysfunction cannot definitively be prevented during cardiac surgery with off-pump techniques.
Evidence-based perfusion should be conducted, but controversy remains regarding the optimal management of blood flow (and pulsatility), arterial pressure, temperature, and hematocrit during CPB. Perfusion should be adequate to maintain adequate oxygen delivery. Ideally mean arterial pressures should be maintained at individually determined autoregulatory ranges during CPB; however, this is not currently available for clinical use. Some suggest that a mean arterial pressure (MAP) of more than 70 mm Hg may benefit patients with cerebral and/or diffuse arthrosclerosis. Arterial blood temperatures should never exceed 37.5°C.
Anesthesiologists have an important role in assisting with preparation for initiation, conduct, monitoring, and withdrawal of CPB.
Transesophageal echocardiography (TEE) can play an important role in optimizing conduct of CPB.
Blood conservation is paramount, and an effective system involves proper equipment selection for the size of the patient, careful coagulation management, and the use of advanced techniques such as acute normovolemic hemodilution, retrograde and antegrade priming, ultrafiltration, and autotransfusion.
Before cross-clamping the aorta and inducing cardiac arrest, the team should observe the patient for at least 1 to 2 minutes to assure adequate performance of CPB.
Cardioplegia must be delivered accurately to prevent myocardial damage, and new pump delivery systems provide a better operator interface for effective delivery.
During bypass, excessive and rapid warming of blood with the bypass heat exchanger must be avoided (1) to prevent gas coming out of solution risking embolism, and (2) to avoid excessive heating of the brain with potential neurologic damage.
Gaseous and particulate microemboli enter the CPB circuit from entrainment in the venous inflow to the circuit and also through the cardiotomy suction system. None of the currently available CPB systems remove all of the emboli.
Special attention must be given to management of unique patient populations, including those with sickle cell disease, Jehovah’s Witness faith, and pregnancy, as well as those with coexisting diseases such as cerebrovascular disease, cirrhosis, renal insufficiency, and COVID-19.
Adverse events during CPB are not uncommon. The most serious events include iatrogenic aortic dissection and massive gas embolism. Failure of electrical supply to the operating room is another critical event. Anesthesiologists play an important role, in collaboration with perfusionists and surgeons, in risk avoidance, diagnosis, and management.
Modern ECC machines are equipped with a number of alarm systems and redundant backup systems to overcome primary system failures.
Cardiopulmonary bypass (CPB) is considered one of the major contributions to medicine. It was made possible by many basic science advances and the contributions of numerous investigators. The history of the development of cardiac surgery, CPB, and cardiac anesthesia was reviewed in the fifth edition of Kaplan’s Cardiac Anesthesia .
Gross, Crafoord, Blalock, Brock, Bailey, and many others introduced closed heart surgery in the 1930s and 1940s. Successful open heart surgery became possible with the development of the heart-lung machine for total CPB. Clinical CPB was first successfully used by John Gibbon Jr, in Philadelphia, Pennsylvania in 1953. However, it did not become recognized as a practical approach to cardiac surgery until 1955 when two groups in Minnesota, led by John Kirklin at the Mayo Clinic, and CW Lillehei at the University of Minnesota, began routine successful cardiac surgery using CPB. Its use then spread worldwide to become an essential component of cardiac surgery. Since that time, many improvements in the heart-lung machine and the conduct of CPB have occurred.
Familiarity with the use of CPB is one of the defining characteristics of the practicing cardiac anesthesiologist. Many anesthesiologists have played, and continue to play, an important role in the development and improvement in the conduct of CPB, which is used thousands of times each day throughout the world with remarkable success. Cardiac anesthesiologists should have a detailed knowledge of CPB and should be intimately involved in assisting with its conduct in each of their patients.
Improvements in the design of the CPB circuit and a greater understanding of the physiologic insult of CPB have contributed to the relative safety of modern cardiac surgery. Despite advancements in technology and knowledge during the past several decades, a variety of minor and major complications are still observed following CPB.
CPB is a nonphysiologic perfusion system, which alters normal homeostasis. “Cardiac output” during CPB is the pump flow rate, which can be set at any level desired but is limited by the amount of venous return. Systemic and venous pressures are partially dependent on the patient’s autonomic tone but can be manipulated by increasing or decreasing venous drainage and by administering fluids, vasopressors, or vasodilators. Thus the circulation during CPB is controlled mainly by the perfusionist but also by the anesthesiologist.
CPB triggers an “explosion” of highly adverse events that may cause or contribute to complications ( Fig. 25.1 ). These include microemboli (gas and particulate matter), exposure of blood to foreign surfaces that activates the inflammatory and coagulation systems, altered temperature with active or passive cooling followed by active warming, hemodilution (with anemia and reduced oncotic pressure), reinfusion of shed blood and transfusion of blood products, hemodynamic alterations (abnormal flow rate with nonpulsatile flow, abnormal arterial and venous pressures), ischemia and reperfusion (especially of heart, lungs, and gut), and hyperoxia.
As long as blood flow and depth of anesthesia remain stable, mean arterial pressure (MAP) is dependent on systemic vascular resistance (SVR). There is considerable patient-to-patient variation in SVR during CPB. At the start of CPB, there is usually a fall in systemic blood pressure. This may be due to reduced intravascular blood volume or to a decrease in SVR from decreased blood viscosity secondary to hemodilution induced by the pump-priming fluid. In addition, decreased vascular tone occurs secondary to dilution of circulating catecholamines, temporary tissue ischemia, and altered calcium and magnesium levels in the priming fluid.
However, as CPB progresses, there is usually a steady increase in MAP due to increasing SVR if flow rates are kept constant. This is due to decreased vascular cross-sectional area from closure of portions of the microvasculature; vasoconstriction brought on by hypothermia, increasing levels of circulating catecholamines, arginine vasopressin (AVP), endothelin, and angiotensin II; and increases in blood viscosity secondary to hypothermia and rising hematocrit (due to urine output, translocation of fluid into the interstitial compartment, or hemoconcentrator use). This overall pattern of gradual increases in SVR may be transiently interrupted after infusion of cardioplegia solutions that can lead to transient decreases in SVR and MAP. A more consistent decrease in SVR and MAP usually occurs with release of the aortic cross-clamp and reperfusion of the heart. Despite cardioplegia and hypothermia, there is some degree of ongoing cardiac metabolic activity and utilization of myocardial energy stores during the ischemic period. This results in coronary vasodilation with a marked increase in coronary blood flow and decrease in arterial pressure. In addition, when the heart is reperfused, accumulated metabolites are washed out into the general circulation. Some of these metabolites, most notably adenosine, are potent vasodilators that decrease SVR.
The distribution of systemic flow is highly influenced by total flow. As total flow decreases, first flow to skin and muscle, then flow to splanchnic organs, renal flow, and finally (only at extremely low total flow) cerebral blood flow (CBF) is reduced.
One of the major physiologic derangements introduced by CPB is loss of pulsatile arterial blood flow , since most CPB circuits generate nonpulsatile flow. The benefits of pulsatile flow include transmission of more energy to the microcirculation, which improves tissue perfusion, lymphatic flow, and cellular metabolism, and reduction of adverse neuroendocrine responses (mainly vasoconstrictive that emanate from baroreceptors, the kidneys, and the endothelium). There are a number of ways of generating pulsatile flow, but these have significant limitations including the amount of pulsatile hydrodynamic energy that actually can be transmitted into the patient. Furthermore, there is considerable controversy about the merits of and need for pulsatile perfusion as compared to conventional nonpulsatile perfusion. , Data on the impact of nonpulsatile flow during CPB have been conflicting. An evidence-based review by Alghamdi and Latter in 2006 concluded that existing data were insufficient to support recommendations for or against pulsatile perfusion to reduce the incidence of complications following CPB. However, the recent European guidelines on CPB recommend its use in patients at high risk for adverse lung and renal outcomes.
Exposure of blood to nonphysiologic surfaces and shear stresses causes alteration of proteins and formed elements of blood, activation of coagulation, and hemolysis. Changes in the coagulation cascade, platelets, and the fibrinolytic cascade are discussed in Chapter 13, Chapter 28 . Red blood cells (RBCs) become stiffer and less deformable during CPB, which may interfere with microcirculatory blood flow and increase susceptibility to hemolysis. The RBCs are exposed to foreign surfaces and shear stresses during CPB that may cause their destruction. The degree of hemolysis is increased by both higher flow rates and the accompanying increase in shear forces, and by gas-fluid interfaces in the extracorporeal circuit (ECC). As RBCs are lysed, the free hemoglobin produced binds to haptoglobin. When the amount of free hemoglobin generated exceeds the binding capacity of haptoglobin, serum-free hemoglobin concentrations increase and are filtered by the kidney, resulting in hemoglobinuria. Cardiotomy suction also contributes importantly to hemolysis during CPB.
CPB affects primarily neutrophils (polymorphonuclear leukocytes [PMNs]) and, to a lesser degree, monocytes. Shortly after the onset of CPB, circulating PMNs decrease markedly due to sequestration in various vascular beds. Blockage of vessels by PMN clumping or microcirculatory derangements induced by substances released from PMNs may contribute to organ dysfunction after CPB. Circulating PMN levels increase dramatically with rewarming. Neutrophils released from the pulmonary circulation and younger cells released from the bone marrow contribute to this neutrophilia. Effects of CPB on the host defense functions of PMNs remain controversial.
Exposure of proteins to gas-liquid interfaces causes denaturation . Protein denaturation leads to altered enzymatic function, altered solubility, lipid release into the blood stream, and RBC membrane alterations that promote capillary sludging and microcirculatory dysfunction. Hemodilution at the start of CPB causes plasma protein concentration and hence colloid osmotic pressure (COP) to fall, particularly if no colloids are added to the CPB circuit. There is controversy about the need and benefits of avoiding the fall in COP by using albumin or artificial colloids (eg, dextrans, starches) in the priming solution. Increased interstitial fluid in many tissues and organs is common during and following CPB. If extreme, this can cause pathologic capillary leak syndrome (eg, adult respiratory distress syndrome [ARDS], or cerebral edema).
Until recently, fluid fluxes at the microcirculatory level were attributed mainly to the factors described by Starling. These include the permeability of the membrane (“filtration coefficient”), the fluid pressure gradient across the membrane that are additive to the decrease in the oncotic pressure across the membrane, and clearance by the lymph flow. CPB shifts this balance toward accumulation of interstitial fluid by affecting several of these variables. Membrane permeability is increased by activation of a systemic inflammatory response (SIR) and intermittent ischemia/reperfusion potentially leading to organ injury. Plasma oncotic pressure falls due to the use of asanguinous priming fluids. Inadequate venous drainage may increase mean capillary hydrostatic pressure, whereas immobility, lack of pulsatile flow, and loss of negative intrathoracic pressure impede lymphatic flow. In addition the endothelial glycocalyx (EG) has been recognized as playing an important role in modifying the effect of these Starling factors. Shredding of the EG has been demonstrated during CPB, which likely increases capillary permeability and perhaps induces capillary leak syndrome.
Both ionized calcium and total and unfilterable fractions of magnesium commonly fall, whereas potassium levels may fluctuate widely, during CPB. The latter may be related to diuretics, catecholamines, β-blockers, potassium-containing cardioplegia, and renal dysfunction (RD). It is important to maintain normal levels of these ions to preserve normal muscle and cardiac function and prevent arrhythmias.
Hypothermia is a powerful stimulator of the stress and endocrine response to CPB. It also decreases oxygen consumption, and increases both blood viscosity and SVR. Thus, it may impair oxygen delivery and release to tissues partly depending on pH and Pa co 2 management. In contemporary CPB practice, patients are typically hemodiluted to hematocrits of 20% to 30% during CPB due to asanguineous pump priming. Although oxygen-carrying capacity is decreased from hemodilution, oxygen delivery may paradoxically increase as a result of decreased viscosity and enhanced microcirculatory flow. In general, the viscosity remains stable if hematocrit (expressed as percentage) matches temperature (in degree Celsius) during hypothermia. The optimal degree of hemodilution during hypothermic CPB has not been determined. Recent studies have demonstrated an association between severe CPB hemodilution (hematocrits below 20%–24%) and morbidity and mortality. Clinicians should avoid both excessively high (increased blood viscosity and decreased microcirculatory flow) and low (inadequate oxygen content) hematocrits during CPB.
Hypothermia has a profound effect on biochemical reactions. The Q10 for chemical reactions is a measure of changes in rate of reaction for each 10°C increase in temperature. For human tissues, Q10 is approximately 2. As a result, for each 10°C decrease in body temperature, the rate of reaction (i.e., metabolic rate or oxygen consumption) is roughly halved. This also affects drug pharmacokinetics and dynamics. Hypothermia also changes the oxygen-hemoglobin dissociation curve. As temperature decreases, the affinity of oxygen for hemoglobin increases (i.e., the oxygen-hemoglobin dissociation curve shifts to the left). This is associated with a lower partial pressure of oxygen in the tissues, and consequently oxygen delivery to the tissues is limited. Furthermore, as temperature decreases, gases become more soluble in liquid. For a given partial pressure, more gas will be dissolved in the plasma. The higher solubility of oxygen partially offsets the left shift in the oxygen-hemoglobin dissociation curve. Neutral water is water in which the [H + ] is equal to the [OH − ]. At 37°C, the pH of neutral water is 6.8. At 25°C, the pH of neutral water is 7. The neutral pH of water increases linearly by 0.017 units for each degree Celsius decrease in temperature. This affects optimal management of pH and Pa co 2 during CPB.
As the perfusate temperature is increased to rewarm the patient, variable circulatory responses are observed depending on the anesthetics used, patient hematocrit, underlying disease, and other factors. SVR and MAP increase frequently during initial rewarming from 25°C to 32°C, but then usually decrease as temperature increases above 32°C.
CPB is associated with a marked exaggeration of the stress response associated with all types of surgery. This is manifested by large increases in circulating epinephrine, norepinephrine, AVP, adrenocorticotropic hormone, cortisol, growth hormone, and glucagon. Elevated catecholamines may adversely affect regional and organ blood flow patterns. Catecholamines also increase myocardial oxygen consumption, which may adversely affect the balance of myocardial oxygen supply and demand at the time of reperfusion. Other stress hormones also increase catabolic reactions that increase energy consumption and potentially cause tissue breakdown and impaired wound healing.
Hyperglycemia is invariably encountered during CPB, especially in patients with diabetes mellitus. Contributors to hyperglycemia include decreased insulin production, insulin resistance (enhanced by stress hormones and hypothermia), decreased consumption (related to insulin resistance and hypothermia), increased glycogenolysis and gluconeogenesis (related to stress hormones), increased reabsorption of glucose by the kidney, and administration of glucose in some intravenous and cardioplegia solutions. Observational studies have demonstrated an association between post-CPB hyperglycemia and increased morbidity and mortality. Although measurement and control of blood glucose values during and following CPB is strongly recommended, the optimal “target range” for serum glucose concentrations, and means of achieving this level remain controversial.
Renin, angiotensin II, and aldosterone levels all tend to rise during CPB. Some patients display the so-called sick-euthyroid syndrome with reduced triiodothyronine (T3), thyroxine (T4), and free thyroxin levels, but normal thyroid-stimulating hormone levels. The etiology of this phenomenon is unclear, but it provides the rationale for administration of thyroid hormone in some patients with a low cardiac output syndrome postoperatively.
All patients undergoing cardiac surgery will experience a systemic inflammatory response (SIR). Although the trauma of major surgery per se induces inflammation, the CPB circuit accentuates this response. SIR represents unphysiologic activation of the innate immune system resulting in a whole-body response resembling that associated with sepsis and trauma. There is a spectrum of responses ranging from near-universal evidence of very mild inflammation (fever, leukocytosis), to more significant clinical signs (tachycardia, increased cardiac output, decreased SVR, increased oxygen consumption, increased capillary permeability), to potentially frank organ dysfunction (cardiac, renal, pulmonary, gastrointestinal [GI], hepatic, central nervous system), and very rarely, to multiple organ dysfunction syndrome (MODS) and death.
Normal inflammation is a localized protective response composed of cellular and humoral components. When the localized inflammatory response becomes excessive, it may spill over to the rest of the body. The same response occurs when the injury is systemically widespread (e.g., CPB), in which case a generalized inflammatory response leads to diffuse end-organ damage. Nonspecific activators of the inflammatory response include surgical trauma and tissue injury, blood loss or transfusion, and hypothermia. However, CPB may independently activate the inflammatory response by several unique mechanisms. Contact activation occurs when blood interacts with foreign surfaces of the ECC. This activates the complement, coagulation, kallikrein-bradykinin, and fibrinolytic systems, as well as white blood cells and platelets. The use of cardiotomy suction contributes importantly to this response. Blood aspirated via cardiotomy suction becomes contaminated with tissue factor (TF), TF activator, and fibrin degradation products. Ischemia-reperfusion injury refers to damage to tissue caused when blood supply returns to the tissue after a period of ischemia. The absence of oxygen and nutrients from blood creates a condition in which the restoration of circulation results in inflammation and oxidative damage from the oxygen rather than reestablishment of normal function. The reintroduction of oxygen via restored blood flow promotes the formation of oxygen-free radicals, which damage cellular proteins, DNA, and plasma membranes. White blood cells (WBCs) carried to the area also release a host of inflammatory substances such as interleukins (ILs). As noted earlier, these activated leukocytes can build up in small capillaries to cause obstruction and more ischemia. Transient splanchnic hypoperfusion/ischemia during CPB damages GI mucosa to induce endotoxemia , which is a lipopolysaccharide in the cell wall of gram-negative bacteria. Endotoxin binds with lipopolysaccharide-binding protein to stimulate the release of tumor necrosis factor (TNF) from macrophages, thus potentially triggering SIR. Gaseous and particulate microemboli also induce an inflammatory response.
Once the inflammatory response is triggered, components of the immune and coagulation systems and various cells are activated which propagate the SIR. The complement system is activated by exposure of blood to foreign surfaces, endotoxin released from the GI tract, and heparin-protamine complexes. Its activation leads to the release of various substances (e.g., C3a, C3b, and C5a) that increase production of cytokines and leukotrienes, as well as capillary permeability and leukocyte-endothelial adhesion. Cytokines are released from activated monocytes, macrophages, lymphocytes, and the endothelium leading to both pro- and antiinflammatory effects. Nitric oxide (NO) production is upregulated, contributing to vasodilation, increased vascular permeability, and potential end-organ dysfunction. The release of leukotrienes are potent vasoconstrictors, and platelet-activating factor (PAF) contributes to activation of clotting, as well as inflammation. Tissue factor is expressed by many cells during CPB and initiates coagulation and release of cytokines. Activation of the Kallikrein-bradykinin system amplifies the inflammatory response and increases vascular permeability. Collagenases , gelatinases , and metalloproteases are released. Endothelins are released by endothelial cells and are potent vasoconstrictors.
The coagulation-fibrinolysis cascade is closely intertwined with the inflammatory response and both are activated by cardiac surgery and CPB. These lead to both bleeding and thrombotic-embolic complications. The endothelium is an active participant in a variety of physiologic and pathologic processes. It plays a major role in regulating vascular tone, membrane permeability, coagulation and thrombosis, fibrinolysis, and inflammation. It attracts and directs the passage of leukocytes into areas of inflammation through the expression of adhesion molecules. CPB causes extensive activation and dysfunction of the endothelium due to ischemia/reperfusion, exposure to inflammatory mediators, surgical manipulation, and hemodynamic shear stresses. Expression of adhesion molecules mediates the binding of neutrophils to the endothelium and translocation into the interstitium. Resultant neutrophil degranulation attacks the endothelial barrier function to induce capillary leak and tissue edema. Interaction of activated endothelium and neutrophils with the endothelium leads to tissue damage and end-organ dysfunction via microvascular occlusion or release of toxic metabolites and enzymes. B and T lymphocytes decrease in number and function following CPB, which may cause immunosuppression and increase the risk for infections. Through the production and release of leukotrienes, serotonin, chemokines, Platelet factor 4 (PF4), and other substances, platelets contribute to the inflammatory response.
The inflammatory response to major surgery itself and to CPB likely contributes to multiple organ injuries, coagulopathy, disseminated intravascular coagulation (DIC), infection risk, and death. In most patients, the early SIR resolves without significant injury as a result of discontinuation of the stimulus, dissipation of mediators, or the action of naturally occurring antagonists (e.g., IL-10). The variability in the expression, consequences, and outcome of the inflammatory response to CPB is the subject of much speculation. Factors likely to influence this variability include the preoperative condition of the patient, type and complexity of the surgery, and, perhaps most importantly, underlying genetic polymorphism.
Although some studies have found that modifying the ECC and practice of CPB (e.g., surface heparin coating, minimizing circuit size, leukofiltration, eliminating or reducing cardiotomy suction, and shortening bypass duration) and even elimination of CPB (e.g., off-pump coronary artery bypass graft [OPCAB]) reduce inflammatory markers, they do not eliminate the SIR to cardiac surgery and may not improve clinical outcomes.
During CPB, cardiac output and arterial pressure can be maintained at “normal” values. However, decreases in oxygen consumption and increases in serum lactate concentrations during CPB, as well as evidence of organ dysfunction post-CPB suggest that tissue perfusion may be impaired. Factors contributing to this may include constriction of precapillary arteriolar sphincters caused by catecholamines, angiotensin, vasopressin, thromboxane, endothelin, and decreased release of NO, increases in interstitial fluid volume (edema), decreased lymphatic drainage, loss of pulsatile flow, “sludging” in the capillaries due to hypothermia, altered deformability of RBCs, microaggregation and adhesion of WBCs, platelets, and fibrin onto the endothelium, and microemboli (gas, lipids, cellular aggregates), some related to the use of cardiotomy suction. Suggested ways to optimize microcirculatory function during CPB include administration of vasodilators, use of pulsatile perfusion techniques, hemodilution to a hematocrit between 20% and 30%, use of microfiltration, minimizing return of unprocessed cardiotomy suction blood directly into the CPB circuit, and other antiinflammatory strategies.
The pharmacokinetics and pharmacodynamics of anesthetic drugs are altered significantly during CPB. The causes of these changes include hemodilution with subsequent reduced plasma protein concentrations, hypothermia, lung isolation resulting in drug sequestration, and other mechanisms listed in Table 25.1 . Cardiac anesthesiologists are required to have a good understanding of CPB-related alterations of the pharmacology of anesthetic drugs, in order to avoid under- or overdosing, particularly during initiation of CPB and rewarming. Barry et al. published a systematic review of the impact of CPB on the pharmacology of anesthetic agents.
| Intravenous anesthetics | Effects of hemodilution |
| Altered plasma protein binding | |
| Volume of distribution | |
| Altered drug clearance | |
| Acid-base disturbances | |
| Lung isolation | |
| Drug sequestration | |
| Systemic inflammatory response | |
| Inhalational anesthetics | Altered blood/gas partition coefficient |
| Altered tissue solubility | |
| Oxygenator design | |
| Hypothermia | |
| Impaired cerebral function |
CPB alters the pharmacology of intravenous anesthetics, opioids, and neuromuscular blocking agents. Hemodilution during CPB results in the reduction of intravenous anesthetic and opioid concentrations. However, serum protein concentrations such as albumin are also reduced due to hemodilution, resulting in higher fractions of unbound and pharmacodynamically active intravenous anesthetics and opioids. The lower serum protein concentration, therefore, offsets the effect of reduced intravenous anesthetic concentrations due to hemodilution. Hepatic metabolism and renal clearance of anesthetic drugs will be slowed during CPB, primarily due to hypothermia and diminished organ perfusion, which results in increased elimination half-lives and increased blood levels. Remifentanil is inactivated via temperature and pH dependence of Hofmann elimination; the lower the temperature, the slower the inactivation of the drug with a reduction of about 30% for each 5°C drop in temperature. If the patient is cooled to 32°C, there will be an initial hemodilution-induced reduction in plasma levels which is offset by the temperature-induced reduction in metabolism after about 20 to 30 minutes. During CPB, opioids and muscle relaxants are sequestered in the lungs. Therefore, during weaning from CPB and subsequent lung perfusion, plasma levels of these drugs will increase. Accidental awareness is higher in cardiac surgery when compared with noncardiac surgery. Furthermore, there is an association of accidental awareness with the use of muscle relaxants in cardiac anesthesia supporting the use of short-acting neuromuscular blocking agents and monitoring their effect throughout the case.
Results of a recent meta-analysis demonstrated that volatile anesthetics during CPB reduce mortality and the incidence of myocardial infarction. Furthermore, they resulted in a lower need for inotropic medications and a shorter time to extubation. A recent multicenter international survey demonstrated that 36% of anesthesiologists use volatile anesthetics routinely as the only maintenance during CPB. Volatile anesthetics can cross the microporous polypropylene hollow fiber membrane oxygenator and it has been demonstrated that the oxygenator exhaust line concentration of volatile anesthetics correlates well with the concentration in arterial blood of the patients. Volatile anesthetic agents do not pass through Poly (4-methyl-1-pentene) (PMP) plasma tight diffusion membrane oxygenators, and thus TIVA must be used if these oxygenators are used during CPB. Hemodilution decreases the blood/gas partition coefficient of volatile anesthetics (resulting in a rapid onset and offset of anesthetic action) and hypothermia increases it, resulting in neutralization of their opposite effects during most phases of CPB. During rewarming, the increase in temperature results in a faster onset/wash-in of volatile anesthetics. Requirements for volatile anesthetics are temperature dependent with less demand during hypothermia. This has been demonstrated by the usage of depth of anesthesia monitors.
Gaseous microemboli (GME) are ubiquitous during CPB and have been suspected as a contributor to the inflammatory response and ischemic organ injury, especially to the brain. Their presence has been documented by ultrasound studies, and sources and methods of reducing their occurrence have been extensively studied. Kihar and Oihashi studied the complexity of determining air bubbles by echocardiography. Emboli are more common in open-chamber surgery and with unclamping of the aorta. GME have been associated with various maneuvers by perfusionists (e.g., injections into the circuit). However, the significance of GME (vs particulate emboli) is controversial.
Cerebral dysfunction, which ranges from subtle neurocognitive dysfunction to frank stroke or coma, is not infrequent following CPB. In the Multicenter Study of Perioperative Ischemia Research Group (McSPI) study of 2417 patients undergoing CABG between 1991 and 1993, 6.1% experienced adverse cerebral outcomes. These were classified into two groups: 3.1% were type I (stroke, transient ischemic attack [TIA], or persistent stupor or coma) and 3.0% were type II (deterioration in intellectual function, confusion, agitation, disorientation, memory deficits or seizures without evidence focal injury). The cause of these abnormalities is multifactorial and includes hypoperfusion, macroemboli, microemboli, and the inflammatory response to CPB. The causes of cerebral dysfunction and strategies to minimize adverse cerebral outcomes are discussed in detail in Chapter 31 .
Although postoperative delirium (POD) and postoperative cognitive dysfunction (POCD) are distinct disorders, similarities in their likely mechanisms, risk factors, and long-term sequelae suggest they may be part of an underlying neurobiologic “injury continuum.” Both delirium and POCD are referred to as types of “neurocognitive dysfunction.” The 2018 recommendations for the nomenclature of cognitive change associated with anesthesia and surgery recommend that preexisting cognitive impairment and POD, as well as early (first 30 days), intermediate (30 days–12 months), and late (beyond 12 months) POCD be included under the overarching term of “perioperative neurocognitive disorders.” They recommend the severity to be indicated as “mild” or “major” based on the Diagnostic and Statistical Manual for Mental Disorders , fifth edition, criteria. Abnormalities detected in the first 30 days are termed “delayed neurocognitive disorders” (“DNCDs”), those present between 30 days and 12 months are termed “postoperative NCDs” (“POCDs”), and those present beyond 12 months are termed “NCDs.” The general topic of perioperative neurocognitive disorder recently was addressed by an international workshop.
POD is prevalent and is associated with increased morbidity, hospital length of stay (LOS), costs, and even acute and long-term mortality. It is particularly common after cardiac surgery. , , The Sixth Perioperative Quality Initiative (POQI-6) consensus conference produced a consensus statement of POD prevention. They recommended identifying surgical patients at high risk for POD, informing patients of their risk, use of a delirium-screening tool postoperatively in high-risk patients, and implementing multicomponent nonpharmacologic interventions to prevent POD in older high-risk patients.
The reported incidence of POD after cardiac surgery varies from about 8% to 55%. Leenders et al. found an incidence of 0% in patients < 50 years old, 8% in patients 50 to 69 years old, 14.5% in patients 70 to 79 years old, and 54% in patients > 80 years old. In a retrospective analysis of prospectively collected data, Kotfis et al. found an incidence of 21.4% in patients 65 years old and 31.5% in patients 80 years old.
The following eight risk factors for POD were mentioned in more than 10 studies: older age, cardiac status, personality traits, cerebrovascular or peripheral vascular disease, metabolic syndrome, preoperative cognitive impairment, type of surgery, and duration of surgery. Others have identified other risk factors. Smulter et al. found that the duration of mixed venous oxygen < 75% was an independent predictor of POD. A retrospective analysis of prospectively collected data on patients who underwent CABG with monitoring of oxygen delivery during CPB found that patients who developed POD had a lower nadir oxygen delivery.
A randomized controlled trial (RCT) demonstrated a decreased incidence of POD in those managed with a higher MAP (80–90 mm Hg) versus a lower level (60–70 mm Hg) during CABG. Another study found that maintaining MAP during CPB above the lower limit of autoregulation was associated with substantial decreases (45%) in POD. On the other hand, Hori et al. also found a possible association of POD with a MAP above the upper limit of autoregulation. In a single-center study of 8917 undergoing on-pump and 1843 off-pump CABG between 1996 and 2001, the incidence of POD was 2.8% following off-pump coronary artery bypass (OPCAB) and 7.8% following on-pump CABG. This was substantiated by a review of the Society of Thoracic Surgeons (STS) database for CABG surgery done between 2009 and 2015, which observed a somewhat higher incidence of POD following on-pump versus off-pump CABG (24% vs 19%) and a substantial increase in incidence of POD as CPB time exceeded 100 to 140 minutes in the on-pump group.
Most studies have reported that the occurrence of POD is associated with increased morbidity and LOS, and some have found it to be associated with increased hospital mortality. Hughes et al. state that patients with POD are more likely to have functional decline and dependency in activities of daily living after discharge, and that POD is one of the strongest predictors of cognitive impartment after surgery. The long-term consequences of POD are less clear. In a systematic review of the long-term effects of POD after cardiac surgeries, Crocker et al. concluded that POD was strongly associated with a greater likelihood of readmission to the hospital, as well as decreased cognition, functional decline, lower health-related quality of life, and death.
Of particular relevance to this discussion of postoperative neurocognitive dysfunction is the possible relationship between POD and subsequent POCD/dementia. Two studies have addressed this question. , Both reported that at 1 month post-surgery, patients who had experienced POD had worse POCD, but by 1 year it was similar; however, those who experienced POD may have had a decline in some functional domains (motor skills, executive function, or processing speed). Lingehall et al. reported a prospective longitudinal study on the incidence of postoperative dementia in patients > 70 years old (mean: 76.5 years) followed-up for up to 5 years after cardiac surgery involving CPB. None had dementia preoperatively, but 8% had mild cognitive impairment (MCI) preoperatively. Fifty-six percent of all patients developed POD, and 26% developed postoperative dementia by 5 years. Dementia developed in 41% of those who experienced POD and in only 8% of those who did not. Dementia developed in 89% of those with preexisting MCI and in 21% without evidence of preoperative MCI. Multivariable logistic regression found that older age, POD, and MCI were associated with dementia occurrence. In another prospective observational study, Blokzijl et al. found that POD was a risk factor for failure to recover from early POCD at 6 months ( P = .015).
The American Society for Enhance Recovery have published a consensus statement on prevention of postoperative delirium.
Berger et al. reviewed neurocognitive dysfunction after cardiac surgery, and Bhamidipati et al. reviewed cognitive outcomes after CABG. In a systematic review and meta-analysis of 156 studies involving patients undergoing CABG surgery, Greaves et al. reported the prevalence of cognitive impairment. They found a prevalence of 19% preoperatively, 43% at up to 4 days postoperatively, 39% at up to 1 month, 25% at up to 4 months, 19% at up to 6 months, 25% at up to 1 year, 38% at 1 to 3 years, and 39% at 3 to 5 years. In a prospective observational study, Blokzijl et al. reported of persisted cognitive dysfunction in 142 patients following CABG. At 3 days postoperatively, 60% displayed early cognitive dysfunction, while at 6 months 33% exhibited long-term dysfunction. Of those with early cognitive function (at 3 days), at 6 months 46% had recovered, while 54% had persistent cognitive dysfunction. Older age, lower educational level, and POD were associated with persistent POCD.
Key contributors to POCD include inflammation, embolization, endothelial dysfunction, cerebrovascular disease, and perhaps preexisting abnormal neuronal/synaptic function (e.g., latent Alzheimer’s syndrome). The conduct of CPB may influence the incidence of POCD. Key factors may include management of arterial pressure, hematocrit, glucose, and temperature, with both high and low values likely being detrimental. Lingehall et al. identified older age, preoperative MCI, and POD as independent risk factors for developing dementia post-CPB. A prospective, single-center observational study of patients who underwent elective cardiac surgery found that the 6-minute walk distance was an independent predictor of the incidence of POCD measured at 14 days using the Mini-Mental State Examination score. Wilberg et al. reported that postoperative serum levels of tau and GFAP were elevated after cardiac surgery in patients experiencing POCD. A small, single-center, observational study of patients who underwent CABG found no association between short-term episodes of decreased regional cerebral oxygen saturation (measured using near-infrared spectroscopy [NIRS]) (which occurred in 34%) and POCD at 10 days (which occurred in 37%). ,
Thromboembolism from the ascending aorta associated with surgical manipulation and cannulation is believed to be an important contributor to stroke after cardiac surgery and use of embolic protections devices have been advocated by some. However, a recent multicenter RCT found that use of two such devices was not associated with a reduced incidence of evidence of diffusion-weighted magnetic resonance imaging (MRI) cerebral infarcts, clinical strokes, POD, or mortality.
The role of type of the anesthesia is unclear. Based on preliminary data, Berger et al. suggested that excessively deep anesthesia (e.g., low bispectral index values) may be associated with worse POD and POCD. This question was recently review by Lomivorotov et al. who indicated that that several studies have reported the association of using volatile anesthetics with improved biochemical markers of brain injury and postoperative neurocognitive function, but emphasized the need for additional studies. A systematic review and meta-analysis of RCTs found that patients who received volatile agents had lower S100B levels and better mini-mental state scores than those who received total intravenous anesthesia. The implications of these results need to be evaluated with large RCTs using neuropsychological testing late postoperatively. Shaefi et al. observed no difference in neurocognitive function at 6 months following cardiac surgery between those receiving normoxia and hyperoxia during surgery.
The debate on arterial pressure management during CPB will be discussed later. One RCT found less decline in mini-mental status scores in patients managed with a higher (80–90 mm Hg, average: 84 ± 11 mm Hg) versus lower (60–70 mm Hg, average: 65 ± 8 mm Hg) MAP during CPB for CABG. However, a recent RCT comparing maintaining arterial pressure during CPB above the lower limit or cerebral autoregulation versus usual practice found no statistically significant difference in a composite of neurological outcome, or delayed neurocognitive recovery. In a provocative preliminary study, Smith et al. measured both regional cerebral perfusion with MRI and cognitive function in patients who underwent on-pump cardiac surgery. Baseline cerebral perfusion was lower in these patients compared with matched control patients with cardiac disease, but at 6 weeks cerebral perfusion had increased and matched that in the control group, and both groups had similar MRI perfusion at 1 year. Increases in perfusion in the cardiac surgery patients were associated with improved psychomotor speed, but not in verbal or visual memory or executive function. These data support the hypothesis that cardiac surgery requiring CPB could have beneficial effects on cerebral perfusion and cognitive function in some patients despite the potential adverse effects that have been reviewed.
The Fifth International Perioperative Neurotoxicity Working Group recently provided recommendations for optimizing postoperative “brain health,” including the following.
All patients older than 65 should be informed of the risks of perioperative neurocognitive disorder, including confusion, inattention, and memory problems after undergoing surgery.
Baseline cognition should be objectively evaluated with a brief screening tool during preoperative evaluation in all patients older than 65 and in any patient with risk factors for preexisting cognitive impairment.
Avoid centrally acting anticholinergics, benzodiazepines, and meperidine.
Avoid relative hypotension.
Maintain normothermia.
Monitor age-adjusted end-tidal minimal alveolar concentration.
Use electroencephalography-based intraoperative brain monitoring to titrate anesthetic management in older adults.
Strive to optimize cerebral perfusion.
Clinically apparent strokes occur in about 1% to 10% of patients undergoing cardiac surgery with CPB depending on the type of surgery and other risk factors. Its incidence is lowest with CABG (ranging from 1% to 4%), highest with multivalve (∼9.5%) and mitral valve surgery (∼8.5%), and intermediate with aortic valve (3%–5%) and aortic root or arch surgery (4%). Most strokes appear within the first two days, with about 30% to 50% intraoperatively (ie, on awakening). A recent meta-analysis of 36 articles which included 174,969 patients undergoing cardiac surgery found an incidence of early stroke (on awakening) of 0.98% (95% confidence interval, 0.79–1.23) and of delayed stroke of 0.93% (95% confidence interval, 0.77–1.11). The operative mortality was 28.8% following early stroke and 17.9 following delayed stroke, compared with 2.4% in those without stroke. Off-pump was inversely associated with early stroke, while previous stroke was associated with delayed stroke. The incidence of silent (covert) nonclinical strokes is likely significantly higher than clinical strokes. After surgical aortic valve replacement (AVR), the incidence of silent (ie, “covert”) nonclinical strokes, detected using diffusion-weighted magnetic resonance imaging (DW-MRI) scanning may be at least two times higher than clinically recognized strokes.
Risk factors include history of CVA, peripheral vascular disease, diabetes, hypertension, prior cardiac surgery, urgent surgery, prolonged CPB, and high blood transfusions. Other risk factors that have been mentioned include advanced age, severe carotid stenosis, aortic atheroma, perioperative atrial fibrillation, female sex, renal failure, and open-chamber or combined procedures. Intraoperative strokes are usually thought to be of embolic origin (from aorta, valves, air), and uncommonly due to hypoperfusion, while postoperative strokes may be related to cardiogenic emboli due to atrial arrhythmias or low cardiac output states. Daniel et al. reported the incidence of stroke in over 10,000 patients undergoing isolated CABG between 2002 and 2009 was 1.4%. With on-pump CABG, the risk of stroke was significantly higher when a double clamp technique (cross-clamp and partial occluding clamp) was used (odds ratio [OR], 2.6; 95% confidence interval, 1.03–6.67; P = .044). Interestingly, they observed no statistically significant difference in stroke rate in cases performed off-pump whether or not a clamp was used. On the other hand, Misfeld et al. in a meta-analysis of over 11,000 cases from eight studies found a lower incidence of neurologic complication (0.5% vs 1.4%) when “no-touch” technique was used in off-pump CABG. The STS online adult cardiac surgery risk calculator provides an estimate of risk of stroke following various types of cardiac surgery. Hu et al. based upon an analysis of a nationwide cohort study of 43,694 patients in Taiwan, found that the CHA 2 D S2 -VASc (an acronym for congestive heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65 to 74 and sex category (female)) score was highly predictive of operative ischemic stroke and death. The risk of stroke was 4.4% if the score was 0 and 17.8% if score was ≥6.
The etiology and consequences of isolated seizure following cardiac surgery are not well understood. , , There has been an increased incidence associated with administration of antifibrinolytics (especially tranexamic acid [TXA] ). In the McSPI study, the incidence of seizures in the absence of evidence of focal neurologic injury following isolated CABG was 0.3%. In a single-center study of 2578 patients undergoing cardiac surgery, all of whom received epsilon-aminocaproic acid (EACA; 150 mg/kg loading and 25 mg/kg/h during the surgery), 1.0% experienced postoperative seizures. The incidence was 0.1% following CABG, 1.0% following isolated valve, 3% following valve plus CABG, and 5% following aortic surgery. Identified risk factors included deep hypothermic circulatory arrest, aortic calcification or atherosclerosis, and critical preoperative state. Patients who experienced seizures had a nearly fourfold increase in mortality (29% vs 6%). In another single-center report of 1188 patients undergoing cardiac surgery between 2005 and 2006, the overall incidence of seizures was 2.9%. This incidence was 0.4% in patients undergoing isolated CABG and 4.7% in patients undergoing valve or high-risk surgery. The incidence was 1.2% in those receiving aprotinin and 4.6% in those receiving TXA . However, the incidence was not different between these two agents in those undergoing isolated CABG. Manji et al. retrospectively reported the results of analysis from a single-center database of 5958 patients who underwent cardiac surgery between 2003 and 2009. The incidence of seizures was 0.9%. Identified risk factors for seizures included preoperative cardiac arrest, Acute Physiology and Chronic Health Evaluation (APACHE) score > 20, prior cardiac surgery, open-chamber surgery, CPB duration > 150 minutes, preoperative neurologic disease, and administration of TXA. Regarding the latter, the risk of seizures was found to be associated with dose: 0.2% if no TXA, 0.4% if >1 g, 0.9% with about 3 g, and 1.2% to 1.8% if 6 g was administered. Patients who experienced seizures had an increased incidence of neurologic complications (strokes or delirium; 20% vs 3%), prolonged intensive care unit (ICU) LOS, and mortality (8.7% vs 1.4%). A study from two other academic centers found that the incidence of seizures increased when they converted from use of aprotinin to TXA (61–269 mg/kg). Of the 669 patients who received TXA, 24 (3.6%) developed seizures; 21 of these had undergone open-chamber surgery, 11 had renal dysfunction (RD), and 21 had received more than 100 mg/kg of TXA. The seizures occurred an average of 4.7 hours postoperatively. Only three had evidence of new cerebral ischemic injury. The incidence of seizures reduced to previous level when they decreased the dose of TXA to the Blood conservation sing Antifibrinolytics in a Randomized Trial (BART) protocol. A single-center observational study compared 1-year outcome in a sequential group of 275 patients who received TXA (perhaps >100 mg/kg) in 2006, followed by 329 who received EACA; all the patients in both groups had undergone open-chamber procedures. These authors reported an incidence of seizure of 7.6% in those receiving TXA and 3.3% in those receiving EACA. A recent propensity-matched study compared 2062 patients who received a low dose of TXA (15–50 mg/kg), which was only administered immediately post-CPB, with 2062 other patients who did not receive TXA. These authors found no statistically significant increase in seizures in those who received TXA compared with those who did not (2.7% vs 2.2%; P = .32). Ninety-four percent (94%) of these patients had undergone open-ventricle procedures.
Peripheral neuropathies are encountered following cardiac surgery just as with all surgical procedures. Ulnar neuropathy is likely associated with preexisting abnormalities and problems with positioning and padding. Brachial plexus injury, especially involving the ulnar distribution, has been attributed to excessive spreading of the sternum with compression of nerves between the clavicle and first rib. It may also occur related to cannulation of the internal jugular vein. Lower extremity neuropathy may be related to positioning, harvesting of the saphenous veins, surgery in the groin, and ischemic neuropathy following prolonged cannulation of the femoral arteries. Phrenic nerve injury was more common in the past, attributed to topical cooling of the heart. It may last for many months. Permanent injury may occur due to direct surgical damage. Ischemic optic neuropathy is a rare but dreaded complication for which risk factors have been difficult to define. The incidence appears to be declining. In the most recent audit from the Mayo Clinic only one case occurred in over 44,000 cardiac surgical procedures (0.22/10,000) done between 1995 and 2017; the authors of this report stated this was similar to the incidence of 1.4/10,000 reported from a National Inpatient Sample between 1998 and 2013.
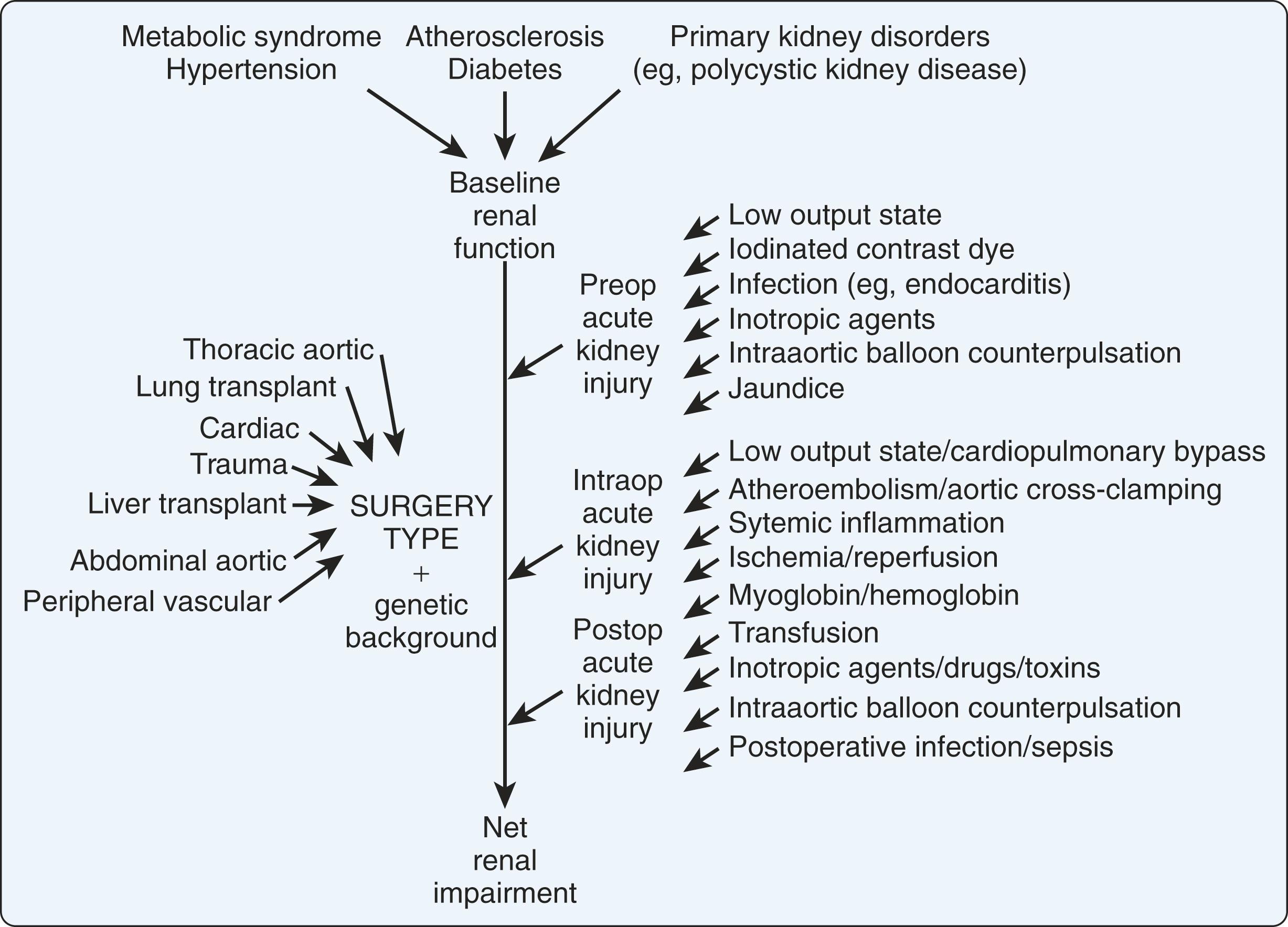
RD, or acute kidney injury (AKI) ranges from a rise in creatinine and release of renal tubular proteins to severe renal failure requiring renal replacement therapy (RRT), so-called “cardiac surgery-associated AKI or CSA-AKI.” It remains a persistent and prevalent problem following cardiac surgery (15%–30%) and is associated with increased morbidity and short- and long-term mortality. , Even mild elevations of serum creatinine are associated with increased morbidity and mortality, while need for postoperative RRT is associated with mortality as high as 60%. In a single-center study of patients who developed cardiac surgery related AKI, 84% regained baseline renal function, whereas 11% were dialysis dependent (see Chapter 37 ).
In a systematic review of 32 studies of patients having undergone cardiac surgery, the incidence of AKI was 22% (interquartile range [IQR]: 14%–34%), and RRT was 3.1% (IQR: 2%–5%). A more recent single-center study of 7633 patients undergoing cardiac surgery with CPB between 2008 and 2019 reported an incidence of AKI of 22.4% (17.5% in isolated CABG, 26.2% in valve, and 32.7% in CABG plus valve). In this latter study, the 30-day mortality in patients with AKI was 12.5% versus 0.9% in those without.
Features of cardiac surgery and CPB generally accepted to contribute to AKI include the inflammatory response; ischemia/reperfusion; embolization (both micro- and macroemboli of gas, and atheroma); hypotension; low cardiac output; elevated inferior vena cava pressure; damage-associated molecules (e.g., high mobility group proteins, hemoglobin, myoglobin, and uric acid); transfusion of blood products; and administration of nephrotoxic drugs (e.g., IV antibiotics). CPB per se is commonly considered as one of the contributors to CSA-AKI. However, a consensus conference concluded that off-pump CABG is associated with a decreased incidence of RD at 30 days, but no decrease in the need for RRT.
Identified risk factors for AKI include prior cardiac surgery, intra-aortic balloon pump (IABP) need, higher blood loss during surgery, older age, duration of surgery, diabetes mellitus, lower left ventricular ejection fraction (LVEF), lower baseline glomerular filtration rate (GFR), and intraoperative blood product transfusion. , In a study of AKI after heart transplant, Jocher et al. reported that CPB time > 170 minutes and use of vasopressors and inotropes post-transplant were risk factors. AKI has been associated with the severity of atherosclerosis of the ascending aorta, the magnitude and duration of MAP below the lower limit of autoregulation in the brain, and a critically low systemic oxygen delivery (<280 mL/min/m 2 ) during CPB. , Intravascular hemolysis and hemoglobinuria can also cause acute tubular necrosis. One study found that increased duration of rewarming > 37°C on CPB was associated with a 51% increase of AKI, but that the duration of rewarming at lower temperatures was not. Preexisting renal failure has been demonstrated to be associated with higher hospital mortality compared with patients without preexisting renal failure (10.9% vs 1.4%). Page 9
Several approaches to predicting the occurrence of postoperative AKI have been described with variable success. Preoperative score cards that include age, gender, baseline GFR and LVEF, diabetes, and type of surgery have successfully predicted postoperative AKI. Another model including baseline GFR, change in GFR upon arrival in ICU, change in GFR in first 12 hours, preoperative hemoglobin, packed red blood cells (PRBC) given intraoperatively, and CPB time predicted 12 hours postoperative AKI. Others have found an association of low urinary oxygen partial pressure in the early postoperative period, intraoperative and postoperative urinary KIM1 and postoperative urinary IL-18 levels, circulating levels of endothelial microparticles at 12 hours postoperatively, Cardiac Surgery Urinary Neutrophil Gelatinase-Associated Lipocalin score, and serum-free hemoglobin with the development of AKI.
Vandenberghe et al. reviewed the diagnosis of CSA-AKI. They noted that even though it typically is diagnosed based on a rise in creatinine and reduced urine output (Kidney Disease Improving Global Outcomes [KDIGO] criteria), creatinine is slow to rise; therefore, various biomarkers that permit earlier recognition and therapeutic interventions are coming into practice. Prominent biomarkers include neutrophil gelatinase-associated lipocalin, tissue inhibitor of metalloproteinases-2 (TIMP-2), and insulin-like growth factor–binding protein 7 (IGFBP7). Meersch et al. observed that a urinary TIMP-2 ∗ IGFBP7 level of 0.3 ng/mL 4 hours post-CPB was predictive of CSA-AKI. McIIroy et al. found that the combination of elevated urinary biomarkers (cysteine-c, kidney injury molecule-1, chemokine ligand 2, or IL-18) and a rise in serum creatinine at 3 hours was superior in predicting hospital mortality or RRT.
Renal hemodynamics during and after cardiac surgery demonstrate strong evidence for renal medullary ischemia and hypoxia during CPB, and that monitoring for this and manipulating the conduct of CPB to minimize this effect could reduce the incidence of AKI. Evidence of renal ischemia during CPB was documented by Lannemyer et al., who observed an increase in renal vasoconstriction (∼20%), decrease in percent of systemic flow to the kidney (∼28%) and renal oxygen delivery (∼20%), an increase in renal oxygen extraction (∼40%), and the release of protein makers of tubular injury. After CPB, renal oxygenation was further impaired. Changes in management such as increasing arterial flow rates from 2.4 to 3.0 L/min/m 2 progressively improved renal oxygenation in patients during CPB. The renal resistive index also may be elevated in patients with AKI, which may reflect an increase in intra-capsular pressure (i.e., injury-related “renal compartment syndrome”). The renal resistive index can be measured using transesophageal echocardiography (TEE) and has been found to be elevated early post-CPB in some patients; this could be an early predictor of AKI.
Urine output is a crude indicator of renal function, but there is no correlation between the amount of urine output during CPB and the incidence of postoperative renal failure. Urine output is greater when MAP is higher, when pulsatile perfusion is used, and when mannitol is added to pump-priming fluids.
Meersch et al. reviewed the prevention of AKI , while many studies have looked at various methods to reduce the incidence of CSA-AKI. Maintaining oxygen delivery during CPB ≥ 280 to 300 mL/min/m 2 compared with conventional arbitrary flow reduced the incidence of stage 1, but not of stages 2 to 3 AKI. Numerous drugs including statins, levosimendan, dopamine, and dexmedetomidine have been studied to evaluate their effects on CSA-AKI, but none have been found to have a consistently significant positive effect. Techniques such as leukodepletion filters during elective valve surgery also have had minimal effects on the incidence of AKI.
In summary, although CPB per se may contribute to AKI, other preoperative and intraoperative surgical factors (e.g., low hematocrit and transfusion of RBCs) and postoperative course and care, also contribute to it ( Fig. 25.2 ). , Studies comparing off-pump with on-pump CABG have found less RD, but not less renal failure or RRT by avoiding use of CPB. Thus the development of renal failure appears to depend more on the preoperative renal function and postoperative hemodynamic status than on various manipulations during CPB, although maintaining adequate oxygen delivery is likely important.
Some degree of myocardial injury and cell necrosis, as evidenced by release of troponin, occurs during CPB and may result in myocardial stunning and dysfunction. However, frank myocardial infarction is relatively uncommon; cardiac enzyme changes which identify myocardial infarction and particular myocardial injury after cardiac surgery have recently been described more in detail.
With the placement of the aortic cross-clamp after the start of CPB, myocardial tissue hypoxia develops resulting in acidosis, increased lactate as adenosine triphosphate (ATP) is consumed, and Ca 2+ accumulation. In severe ischemia the muscle cell membrane is disrupted and intracellular components leak into the extracellular compartment. Mitochondria supply ATP to cardiomyocytes, and they also have been associated with activation of cell death pathways, modulating the balance between death and survival. The mitochondrial permeability transition pore (mPTP) sits in the inner mitochondrial membrane as a nonselective channel. A closed mPTP protects the cardiomyocytes during acidosis. However, mPTP opening results in uncoupling or impairment of oxidative phosphorylation, which results in intracellular ATP depletion and cell death.
There are different kinds of myocardial dysfunction after ischemic reperfusion: stunning, no-flow phenomenon, reperfusion arrhythmias, and lethal reperfusion injury. Myocardial stunning is a mechanical dysfunction, which is reversible (see Chapter 6 ). No-reflow describes the situation that a previously ischemic region cannot be reperfused. Reperfusion arrhythmias are usually treatable, and lethal reperfusion injury is mediated by intracellular calcium overload, reactive oxygen species (ROS), and inflammation.
Myocardial protection has been based on the inhibition of the opening of the mPTP and also on activation of the opening of the ATP-dependent potassium (KATP) channel. It has been shown that myocardial ischemia can induce protection through G-protein–coupled cell surface receptors (reperfusion injury salvage kinase [RISK] pathway) and also via TNF-alpha receptors (survivor activating factor enhancement [SAFE] pathway). Beyond cardiomyocytes, other myocardial protection targets include the endothelium, platelets and leucocytes, autonomic myocardial innervation, and extracellular vesicles.
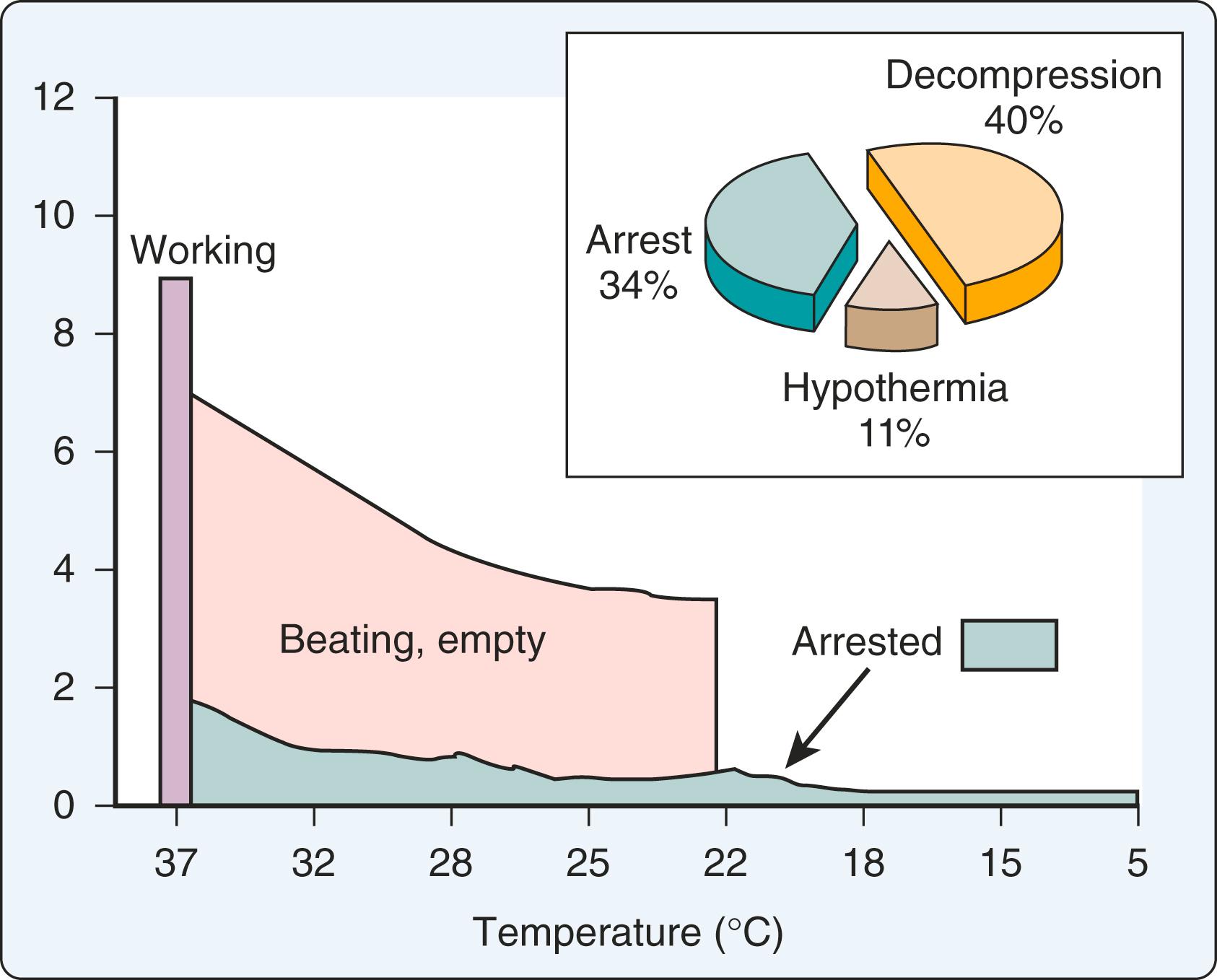
In order to protect the myocardium from ischemia and reperfusion injury, hypothermia and cardioplegia have been shown to be beneficial. Optimizing the metabolic state of the myocardium is fundamental to preserving its integrity. The major effects of temperature and functional activity (i.e., contractile and electrical work) on the metabolic rate of the myocardium have been well described. With the institution of CPB, the emptying of the heart significantly reduces contractile work and myocardial oxygen consumption (Mv o 2 ). Nullifying this cardiac work reduces the Mv o 2 by 30% to 60%. With subsequent reductions in temperature, the Mv o 2 further decreases, and with induction of cardiac arrest and hypothermia, 90% of the metabolic requirements of the heart can be reduced ( Fig. 25.3 ).
The composition of the various cardioplegia solutions used during cardiac surgery varies as much between institutions as it does between individual surgeons. Cardioplegia can be classified into blood-containing and nonblood-containing (i.e., crystalloid) solutions. Crystalloid cardioplegia has fallen out of favor, and blood cardioplegia in various combinations of temperatures and routes of delivery is the most used solution. However, even within the category of blood cardioplegia, the individual chemical constituents of the solution vary considerably with respect to the addition of numerous additives. Table 25.2 outlines the various additives to cardioplegia solutions along with their corresponding rationale for use. Blood cardioplegia has the potential advantage of delivering sufficient oxygen to ischemic myocardium to sustain basal metabolism, or even augment high-energy phosphate stores, as well as possessing free radical scavenging properties. Although hypothermic cardioplegia is the most commonly used temperature, numerous investigations have examined tepid (27°C–30°C) and warm (37°C–38°C) temperature ranges for the administration of cardioplegia. Although hypothermia clearly offered some advantages to the myocardium in suppressing metabolism, it may have some detrimental effects.
| Principle | Mechanism | Component |
|---|---|---|
| Reduce O 2 demand | Hypothermia | Blood, crystalloid, ice slush, lavage |
| Perfusion | ||
| Topical/lavage | ||
| Asystole | KCl, adenosine (?), hyperpolarizing agents | |
| Substrate supply and use | Oxygen | Blood, perfluorocarbons, crystalloid (?) |
| Glucose | Blood, glucose, citrate-phosphate-dextrose | |
| Amino acids | Glutamate, aspartate | |
| Buffer acidosis | Hypothermia (Rosenthal factor), intermittent infusions | |
| Buffers | Blood, tromethamine, histidine, bicarbonate, phosphate | |
| Optimize metabolism | Warm induction (37°C), warm reperfusion | |
| Reduce Ca 2+ overload | Hypocalcemia | Citrate, Ca 2+ channel blockers, K channel openers (?) |
| Reduce edema | Hyperosmolarity | Glucose, KCl, mannitol |
| Moderate infusion pressure | 50 mm Hg |
GI complications are relatively uncommon (∼1.2%, range: 0.3%–6.1%) following cardiac surgery, but are associated with morbidity and high mortality (∼34%, range: 9%–87%). , , These complications include GI bleeding (∼33% of GI complications and ∼0.4% of all cardiac surgery cases, respectively), mesenteric ischemia (13% and 0.16%), pancreatitis (3% and 0.13%), cholecystitis (9% and 0.11%), peptic ulcer (6% and 0.07%), diverticulitis (3% and 0.04%), liver failure (2% and 0.02%), and others (21%).
Multiple risk factors have been identified, but the most prominent preoperative factors include age > 70, low cardiac output, peripheral vascular disease, and chronic renal failure. The most prominent intraoperative factors include reoperative procedures, CABG plus valve procedures, duration of CPB, transfusion, and administration of vasopressors. A complicated postoperative course (e.g., sepsis, mediastinitis, bleeding, and prolonged mechanical ventilation > 24–48 h) is frequently associated with GI complications. The presumed cause of these complications is splanchnic ischemia that may be related to abnormal systemic hemodynamics (low flow, low MAP, lack of pulsatility), SIR, and microemboli.
Clinical and animal studies suggest that, although global splanchnic blood flow appears to be preserved during high-flow CPB, splanchnic flow will be compromised early in the hierarchy of regional blood flow whenever systemic flow is reduced. Administration of vasoconstrictors such as phenylephrine, norepinephrine, and vasopressin likely further reduces splanchnic blood flow. Furthermore, increased intestinal permeability, decreased gastric or intestinal mucosal pH and increased mucosal P co 2 , decreased mucosal blood flow, and endotoxemia all suggest that mucosal ischemia can occur during CPB. Although SIR likely contributes to GI ischemia, conversely GI ischemia may play a primary role in the development of SIR syndrome and injury of other organs. Thus the GI tract is both the cause and a target of SIR in cardiac surgery.
Hyperbilirubinemia (>2.50–3.0 mg/dL) is encountered in approximately 25% (range: 9%–40%) of cardiac surgery patients, with an associated mortality of approximately 4%. In a recent systematic review and meta-analysis of 10 studies of 6100 patients, the incidence of hyperbilirubinemia (defined as >3 mg/dL in seven of the studies) was 23%. Preoperative risk factors included elevated right atrial (RA) pressure, bilirubin, and aspartate aminotransferase (AST), while intraoperative risk factors included CPB time, aortic cross-clamp time, and amount of blood transfused. Postoperative hyperbilirubinemia was associated with increased in-hospital mortality.
Liver failure occurs in 0.03% to 0.1% of cardiac surgery patients with an extremely high associated mortality of 56% to 78%. As with renal failure, hepatic dysfunction appears to be more dependent on hemodynamic status before and after CPB than on any direct effect of CPB. The risk of postoperative jaundice increases in the setting of high RA pressures, persistent hypotension after CPB, or significant transfusion.
Mesenteric ischemia is relatively rare (0.15%), but associated with a very high mortality (60%–90%). A retrospective cohort study compared 108 patients who developed mesenteric ischemia after cardiac surgery with 3:1 matched patients who did not. They observed that serum lactate concentrations > 3 mmol/L at the end of surgery, and lactate that remained >3 mmol/L at 24 hours postoperatively, were associated with a substantial increase in risk of mesenteric ischemia (four- to eightfold).
As with other adverse effects attributed to CPB following cardiac surgery, most studies have not found a reduced incidence of GI complications when CABG is done off-pump. Chapter 37
Pulmonary complications and acute lung injury are common after cardiac surgery. During CPB, the lungs are deprived of pulmonary blood flow, and receive reduced bronchial arterial flow. Thus the lungs become somewhat ischemic. Although less frequent than in the past, postoperative pulmonary complications remain a leading cause of morbidity and mortality. , , , Manifestations range from the nearly ubiquitous decline in Pa o 2 /Fi o 2 ratio to ARDS (incidence: 0.4%–3%, mortality: 15%–70%). Other pulmonary complications include pulmonary effusions, pulmonary edema, transfusion-related acute lung injury (TRALI), pneumonia (incidence: 2%–10%, mortality: up to 43%), diaphragmatic dysfunction, and phrenic nerve paralysis.
Etiologies of these complications include an inflammatory response, activation of coagulation, pulmonary ischemia and reperfusion, left heart failure, atelectasis from absent ventilation during CPB, mechanical injury from entering the pleural cavities, blood transfusion, and perioperative lung management. Preoperative lung disease, poor left ventricular (LV) function, age and genetic makeup comprise other risk factors.
A single-center study of 4262 patients undergoing cardiac surgery between 2015 and 2017 reported an incidence of respiratory failure of 21%. The authors developed a risk score for respiratory failure that included body mass index (BMI) ≥ 30, diabetes, chronic lung disease, home oxygen, recent pneumonia, GFR < 60, previous cardiac intervention, NYHA classification ≥ 3, CPB duration, cardiogenic shock, mechanical support, total blood products, and American Society of Anesthesiologists (ASA) score ≥ 4. Liu et al. developed a nomogram to predict ARDS after cardiac surgery that included diabetes, preoperative albumin, CPB time, and APCHE II score after surgery, with an area under the curve (AUC) of 0.785.
Sanfilippo et al. reviewed the prevention and management of ARDS post-cardiac surgery; techniques to minimize lung injury associated with CPB remain controversial. Lung protective ventilation (low tidal volume, positive end-expiratory pressure [PEEP], recruitment maneuvers, and minimizing Fi o 2 prior and following CPB) has been advocated. Typically, ventilation is interrupted, and the lungs are exposed to atmospheric pressure during CPB based on the assumption that ventilation is not required, as well as to facilitate surgical exposure. The possible benefits of applying continuous positive airway pressure (CPAP) or intermitted ventilation during CPB continue to be debated. Two meta-analyses of 17 and 15 RCTs involving 1169 and 748 patients, respectfully, recently were reported. , Both found that CPAP and/or ventilation during CPB temporarily may improve oxygenation, but there is little evidence that they have important clinical benefit beyond the immediate post-CPB period. A survey of practice of mechanical ventilation in 56 Italian cardiac surgery centers revealed significant heterogeneity. When not on CPB, 91% used a tidal volume of 6 to 8 mL/kg, 77% used PEEP of 3 cm H 2 O, and 71% used an Fi o 2 of 50% to 80%. While on CPB, 75% stopped ventilating, 16% applied PEEP, and 9% continued to ventilate.
Since some of the adverse effects of traditional CPB are attributed to lung ischemia due to interruption of pulmonary perfusion, some have advocated simultaneous lung perfusion and ventilation (autologous oxygenation) during cardiac surgery. However, a recent meta-analysis evaluating oxygenated blood perfusion of the lungs during CPB concluded that the benefits are uncertain and may be associated with increased mortality. Lung injury and its prevention and management are further discussed in Chapter 33 .
CPB was initially introduced based upon physiologic principles, animal experimentation, and clinical applications (trials). Many improvements have occurred over the years, but much of current practice is based upon personal biases, clinical impression, and experience of individual cardiac surgical groups. This has resulted in major differences in practice among various teams conducting CPB. With the emphasis on practicing “Evidenced Based Medicine” (EBM), many prestigious groups have made recommendations including the American Society of Extracorporeal Perfusionists (AmSECT), and the combined European Association for Cardio-thoracic Surgery, the European Association of Cardiothoracic Anaesthesiology, and the European Board of Cardiovascular Perfusion. , Page 12
All teams providing CPB are encouraged to read the AMSECT ( Box 25.1 ) and European guidelines. Teamwork and communication are essential to successful conduct of CPB. The European guidelines emphasize that verbal communication between team members in the operating room be standardized and always acknowledged. These guidelines further recommend that there be written standard operating procedures for the conduct of CPB and development of protocols, use of checklists and “time-outs,” conduct of formal reviews immediately post-procedure, and periodic simulation practice involving all members of the team. The importance of teamwork on patient outcome has been emphasized by Wahr et al.
Standard 1: Development of institutionally based protocols
Standard 1.1: As a mechanism for applying each standard to clinical practice, an institution or service provider shall develop and implement an operating procedure (protocol) for each of the standards.
Standard 1.2: The protocol shall be approved by the chairman of cardiac surgery or his/her designee, director of perfusion or equivalent, and other relevant clinical governance committees if available. Reviewed and revised annually or more frequently when deemed necessary.
Standard 3.1: A patient-specific management plan for the cardiopulmonary bypass (CPB) procedure shall be prepared and communicated to the surgical team either during the preoperative briefing or prior to beginning the procedure.
Standard 5: Checklist
Standard 5.1: The Perfusionist shall use a checklist for each CPB procedure.
Guideline 5.1: The Perfusionist should use checklists in a read-verify manner where critical steps that should have been performed are confirmed. Completion of the checklist should be performed by two people, one person being the primary Perfusionist responsible for operation of the heart-lung machine during the intraoperative period.
Guideline 5.2: The Perfusionist should utilize a checklist throughout the entire perioperative period (e.g., setup, pre-bypass, initial onset of bypass, prior to cessation of bypass, post bypass, and/or any return to bypass).
Standard 6: Safety devices (Make sure your groups are utilizing these.)
Standard 6.1: Pressure monitoring of the arterial line, cardioplegia delivery systems and venous reservoir (when augmented venous drainage is utilized), shall be employed during CPB procedures.
Standard 6.2: A bubble detector shall be employed during CPB procedures
Standard 6.3: A level sensor shall be employed during CPB procedures utilizing a (hard-shell) reservoir.
Standard 6.4: Temperature monitoring of the arterial outflow from the oxygenator shall be employed during CPB procedures.
Standard 6.5: An arterial line filter shall be employed during CPB procedures.
Standard 6.6: A one-way valve in the vent line shall be employed during CPB procedures.
Standard 6.7: A method for retrograde flow avoidance when using a centrifugal pump shall be employed during CPB procedures.
Standard 6.8: An anesthetic gas scavenge line shall be employed whenever inhalation agents are introduced into the circuit during CPB procedures.
Standard 6.9: Hand cranks shall be readily available during CPB procedures.
Standard 6.10: A backup gas supply shall be available.
Standard 6.11: A backup battery supply for the CPB machine shall be available.
Guideline 6.1: A ventilating gas oxygen analyzer should be employed during CPB procedures.
Guideline 6.2: A level sensor should be employed during CPB procedures utilizing a soft shell reservoir.
Standard 7: Monitoring
Standard 7.1: Patient arterial blood pressure shall be monitored continually during CPB.
Standard 7.2: Arterial line pressure shall be monitored continually during CPB.
Standard 7.3: Arterial blood flow shall be monitored continually during CPB.
Standard 7.4: Cardioplegia dose, delivery method, line pressure (antegrade), coronary sinus pressure (retrograde), and ischemic intervals shall be monitored continually during CPB.
Standard 7.5: Patient and device temperatures shall be monitored continually during CPB.
Patient (e.g., nasopharyngeal, rectal, bladder, esophageal)
Heart-lung machine (arterial, venous, and cardioplegia)
Heater cooler (H 2 O temperature)
Standard 7.6: Blood gas analyses shall be monitored continually or at regular intervals during CPB.
Standard 7.7: Hematocrit (or hemoglobin) shall be monitored continually during CPB.
Standard 7.8: Oxygen fraction and gas flow rates shall be monitored continually during CPB.
Standard 7.9: The percentage of venous line occlusion of the venous occluder shall be monitored continually.
Standard 7.10: Venous oxygen saturation shall be monitored continually during CPB.
Standard 8: Anticoagulation
Standard 8.1: The Perfusionist, in collaboration with the physician-in-charge, shall define the intended treatment algorithm for anticoagulation management (heparin) and an alternative algorithm for when heparin is not suitable, including acceptable ranges for activated clotting time (ACT).
Guideline 8.1: The surgical care team should determine the target ACT by considering relevant factors, including variability in the measurement of ACT attributed to the device’s performance characteristics.
Guideline 8.2: Patient-specific initial heparin dose should be determined by one of the following methods: Weight, Dose Response Curve (automated or manual), Blood Volume, Body Surface Area.
Guideline 8.3: Anticoagulation monitoring should include the testing of ACT. Additional monitoring tests may include heparin level measurement (e.g., heparin/protamine titration or unfractionated heparin level), partial thromboplastin time, thromboelastograph, thrombin time, anti-Xa.
Standard 9: Gas exchange
Standard 9.1: Gas exchange shall be maintained during CPB according to protocol.
Guideline 9.1: Point-of-care testing should be considered to provide accurate and timely information for blood gas analysis.
Guideline 9.2: Oxygen delivery and consumption calculations should be utilized to evaluate and optimize gas exchange.
Oxygen delivery: D o 2 = 10 × CI × Ca o 2
Oxygen consumption: V o 2 = 10 × CI × (Ca o 2 − Cv o 2 )
Standard 10: Blood flow
Standard 10.1: Target blood flow rates shall be determined prior to CPB according to protocol.
Standard 10.2: The Perfusionist shall work closely with the surgical care team to maintain targeted blood flow rate during CPB.
Standard 11: Blood pressure
Standard 11.1: The Perfusionist, in collaboration with the physician-in-charge, shall define and communicate the intended treatment algorithm for blood pressure management prior to CPB, including acceptable ranges for blood pressure.
Standard 12. Protamine and cardiotomy suction
Standard 12.1: Cardiotomy suction shall be discontinued at the onset of protamine administration to avoid clotting within the CPB circuit.
Standard 13: Blood management
Standard 13.1: The Perfusionist shall participate in efforts to minimize hemodilution and avoid unnecessary blood transfusions.
Standard 13.2: The Perfusionist shall minimize the CPB circuit size to reduce prime volume.
Standard 13.3: The Perfusionist shall calculate and communicate to the surgical team prior to initiating CPB, a patient’s predicted post-dilutional hemoglobin or hematocrit.
Standard 15: Staffing and on-call
Guideline 15.1: The “n + 1” staffing model should be utilized at all times, where “n” equals the number of operating/procedure rooms in use at any given time at a single site.
Guideline 15.2: An on-call Perfusionist should be present and clinically ready for unscheduled and emergency procedures within 60 min of being called.
Standard 16: Duty hours
Standard 16.1: In order for the Perfusionist to ensure proper provision of care, he/she shall receive an adequate rest period between scheduled work hours.
Guideline 16.1: The Perfusionist should receive a minimum of 8 h rest period for every 16-h consecutive work period.
Anesthesiologists play a key role in the conduct of CPB; they should be knowledgeable about its conduct; and collaborate with the surgeon and perfusionist in the planning, monitoring, and management of CPB. Anesthesiologists have extensive information about the patient’s cardiac disease and coexisting conditions, as well as important physiologic data from the pre-CPB phase in the operating room. The cardiac anesthesiologist’s role during CPB includes continued provision of anesthesia, monitoring, and providing recommendations regarding the conduct of CPB, assessing adequacy of patient perfusion, as well as detecting and helping manage common problems such as low or high arterial pressure, inadequate venous return or cardiac decompression, high venous pressure, continued excessive loss of volume, low mixed venous or arterial oxygen levels, and high arterial line pressure (from the heart-lung machine). They should assist with communication between the perfusionist and surgeon (if needed), and they should continue to monitor the conduct of the surgical procedure including cannulation and decannulation, decompression of the heart, administration of cardioplegia, and assist with the detection and management of major complications. In summary, they should serve as a consultant to the surgeon and perfusionist, but whether the anesthesiologist affects outcome after cardiac surgery remains a controversial subject. ,
Despite the severe trespass of normal physiology during CPB as currently practiced, patients do remarkably well. Studies comparing outcome of CABG performed off-pump versus on-pump suggest that much of the adverse effects that had previously been attributed to CPB are in fact more related to the perturbations and stress of the surgical procedure super-imposed on the underlying cardiac disease and comorbidity in the patients.
Evidence-based perfusion uses the best available evidence to guide the conduct of CPB. It is assumed that optimal perfusion results in the best outcome that can be achieved if adequate systemic blood flow, pressure, and oxygen delivery are maintained to all organs. Further specific focus includes minimizing microembolization, activation of inflammation and coagulation, and the stress response during CPB. In the absence of clear consensus regarding optimal perfusion during CPB, the assessment of extracorporeal care and management is often restricted to the presence of postoperative organ dysfunction (see earlier). To support these goals, perfusion during CPB should ideally address the following aims.
The flow rate most commonly used during normothermic CPB is 2.2 to 2.8 L/min/m 2 , which approximates the cardiac index of a normothermic anesthetized patient with a normal hematocrit. It is rational to use higher flows in the presence of reduced hematocrit and is common to accept lower flows during hypothermia, but there is little clinical evidence to guide optimal flow rates in these circumstances. In healthy animal models, even at flows of 2.4 L/min/m 2 , muscle flow is significantly reduced during CPB, and as flow is progressively reduced, first splanchnic, then renal, and eventually (only at extremely low flows) cerebral flows are reduced.
The European guidelines suggest that pump flow be based on lean body mass in obese patients (BMI > 30). They also recommend that pump flow be guided by oxygenation, respiratory and metabolic parameters (e.g., Sv o 2 and lactates), and flow rate should be adjusted according to the arterial oxygen content in order to maintain a minimal threshold of oxygen delivery (D o 2 ) under moderate hypothermia.
The importance of pulsatile flow remains controversial and unresolved. It is more commonly used in Europe than in the United States. , , One major problem is the difficulty in transmitting significant pulsatile energy into the patient through the arterial cannula. A relatively simple means of generating pulsatile flow during CPB is with use on an IABP. The benefits of prophylactic IABP during cardiac surgery in high-risk patients remain controversial. , The success of long-term nonpulsatile flow as provided with current continuous flow left ventricular assist devices (LVADs) used for destination therapy, suggests that pulsatile flow is not critical to deliver end-organ perfusion (see Chapter 22 ).
The acceptable or optimal range of MAP during CPB has been debated since the advent of CPB. , If pressure is too low, perfusion of critical vascular beds may be compromised, especially if vascular disease is present. Conversely, excessive arterial pressure increases noncoronary collateral flow to the heart during aortic cross-clamping (hence “washing out” myocardial protection with cardioplegia), bronchial flow to the lungs (increases blood return to the left heart), and places strain on arterial clamps and suture lines. Hemodilution itself will cause a decrease in MAP due to its effect on viscosity and SVR. Target pressures have been chosen empirically on the basis of physiologic principles, expert opinion, observational studies, and local practice. They often incorporate factors such as patient age, usual preoperative blood pressure, coexisting disease (e.g., renal, diabetes), and evidence of vascular disease. Many teams maintain MAP at 50 to 60 mm Hg during adult CPB, assuming the lower limit of autoregulation of the brain is 50 mm Hg; however, recent clinical data have observed that the lower limit of autoregulation of the brain during CPB in adults may vary between 43 and 90 mm Hg (see later). The review article on CBF and metabolism during CPB published by Schell et al. more than 25 years ago still is relevant.
Practice by experienced groups varies widely. A survey of 271 European centers in 2019 reported that 26% targeted 51 to 70 mm Hg, 17% targeted 71 to 90 mm Hg, 5% targeted 40 to 50 mm Hg, while 34% individualized the target. The scientific approach to the management of MAP during CPB was largely introduced by the landmark study by Govier et al. in 1984. They found, based on measurements of CBF during hypothermic CPB, no significant effect of variations of MAP between 35 and 85 mm Hg. However, in 1995 Gold et al. published an RCT of patients undergoing CPB that compared a high (80–100 mm Hg, actual average achieved about 70) versus a low (50–60 mm Hg, actual average achieved about 52) target during CPB. They found the incidence of the combined 6-month outcome of mortality and major cardiac or neurologic morbidity was lower in those managed with the higher MAP (4.8% vs 12.9%). However, the incidence of specific adverse outcomes was not different in the two groups.
In a more recent RCT, Siepe et al. compared the incidence of early (48 h) POD and POCD in 92 patients undergoing elective or urgent on-pump CABG managed with higher MAP (80–90 mm Hg, actual average: 84 ± 11 mm Hg) versus lower MAP (60–70 mm Hg, actual average: 65 ± 8 mm Hg) during CPB. The incidence of POD was much lower (0% vs 13%) and the drop in Mini-Mental State Examination scores were less (1.1 vs 3.9) in those managed with the higher pressure. Interestingly, the cerebral oxygen saturations measured using NIRS (i.e., cerebral oximetry) were nearly identical in the two groups. Studies such as these have led to the popular adoption of the use of higher perfusion pressures during CPB.
However, this debate was reenergized by a recent provocative study by Vedel et al. In their single-center RCT of 197 adult patients who underwent cardiac surgery during normothermic CPB, the authors compared patients managed with low MAP (40–50 mm Hg, mean: ∼45 ± 4) versus high MAP (70–80, mean: ∼67 ± 5). The latter was achieved by administering phenylephrine or norepinephrine. New evidence of cerebral injury on diffusion-weighted MRI was observed in 54% of all patients and was not different in the two groups. The volume of these new lesions also was similar, and the incidence of clinical evidence of stroke and new early and late postoperative cognitive decline also were not different in the two groups. The authors concluded that vasopressor-facilitated high-targeted MAP did not appear to be beneficial.
On the other hand, opposite findings were reported in a recent study by Sun et al. They reported on a retrospective analysis of prospectively collected continuous arterial blood pressure during cardiac surgery using CPB in 7457 patients. They found that the time MAP was 55 to 64 mm Hg and <55 mm Hg (compared with 65–74 mm Hg) during CPB was associated with an increased risk of stroke (2.1% and 3.8% vs 1.6%, respectively) and that each progressively longer 10 minutes that MAP was <55 mm Hg was an independent predictor of stroke. Other independent predictors of stroke risk included older age, history of hypertension, combined CABG/valve surgery, emergency surgical status, prolonged CPB duration, and postoperative new-onset atrial fibrillation.
It is difficult to reconcile the results of these studies. One was much larger in size, but retrospective and observational, while the other was smaller, but prospective and randomized. Differences in anesthetic technique and the conduct and management of CPB, which were not fully defined, and criteria for diagnosing stroke may explain some of these discrepant observations.
McEwen et al recently published a systematic review of 8 randomized controlled trials (RCTs) comparing low (50 to 70, average achieved 55 mm Hg) versus a high (> 60 to < 80 average achieved 74 mm Hg) targeted MAP during CPB involving 1116 patients. They found no difference in incidence of delirium, cognitive decline, stroke, ALI, or mortality in those managed with the low vs high MAP targets.
Rather than base the MAP target on an arbitrary level or on patient characteristics, it has been suggested that a more rational target would be the individual patient’s cerebral autoregulatory range. Over the past several years, the group at Johns Hopkins University has attempted to identify the cerebral autoregulatory range of MAP in patients during CPB. In 2012, they reported using transcranial Doppler (TCD) monitoring of the middle cerebral arteries and NIRS monitoring to identify the lower limit of cerebral autoregulation in patients undergoing CPB. They found the average lower limit of autoregulation was 66 mm Hg (95% prediction interval: 43–90 mm Hg) and notably that there was no relationship between preoperative MAP or a history of diabetes, hypertension, prior cerebrovascular accident and this lower limit of autoregulation. Their study demonstrated that real-time monitoring of autoregulation with cerebral oximetry index is possible, and may provide a more rational means for individualizing MAP during CPB. This group recently reported on a retrospective study of 614 patients who underwent cardiac surgery at three hospitals using TCD to identify the range of cerebral autoregulation. Monitoring could be used to identify the optimal MAP during CPB in 71% to 83% of patients. They found that optimal MAP during CPB was 78 ± 11 mm Hg and, as before, that optimal MAP was not influenced by age or history of hypertension or diabetes. Importantly, although the average optimal MAP during CPB was 78 ± 11 mm Hg, the lower limit of autoregulation in 17% of patients was above this range, which, if chosen, could lead to possible cerebral hypoperfusion. On the other hand, in 29% of patients, the upper limit of autoregulation was below this range, which, if chosen, could lead to possible cerebral hyperperfusion. Thus it is not possible to rely on the average optimal MAP for this population on all patients. This group also found that perfusion at a MAP below the lower limit of autoregulation could lead to increased morbidity (e.g., strokes) and mortality, whereas MAP above the upper limit of autoregulation may be associated with an increased incidence of POD. They have reported that keeping the MAP above the lower limit of autoregulation reduces the incidence of delirium, but did not reduce the incidence of a composite neurologic end-point or any of it components. They also reported that when patients had a MAP below the lower limit of autoregulation AKI was more common.
In summary, the optimal MAP during CPB remains unclear. A reasonable hypothesis is that this should be best guided by maintaining the MAP within the autoregulatory range of CBF. However, studies have shown that this may vary considerably in individual patients from traditionally accepted values and is not predictable. Although prior studies have suggested an association between a MAP below or above the limit of autoregulation during CPB and harm, and although it is reasonable to suspect that maintaining patient MAP within the patient’s autoregulatory range would improve outcome, no prospective studies have documented such a benefit. Such studies are ongoing and will be required before this technique should be advocated for routine clinical care. The Johns Hopkins group has used analysis of NIRS instead of the more-difficult-to-obtain TCD to identify the autoregulatory range clinically. Furthermore, the best method of increasing arterial pressure (e.g., increasing flow, increasing hematocrit, or use of vasoactive medications) is undetermined. The decision as to the level of arterial pressure to maintain during CPB in any patient must be left to the clinical judgment of the team, based upon an assessment of the benefits and risks of higher or lower perfusion pressures for each patient. The 2019 European guidelines recommend maintaining MAP between 50 and 80 mm Hg by administration of vasopressors or vasodilators. The impact of MAP during CPB on the CNS is further discussed in Chapter 31 .
Organ perfusion pressure is the difference between MAP and venous pressure. Normally the latter is low and relatively insignificant, but due to problems with venous cannulation and drainage, it may become unacceptably high and compromise organ perfusion, especially when maintaining relatively low MAPs. Avoiding elevated pressure in the jugular veins (i.e., in the superior vena cava [SVC]) is particularly important for cerebral perfusion and demands accurate monitoring of the venous pressure cephalad to the end of the venous cannula.
The optimal or lowest acceptable hematocrit during CPB remains unresolved. , A reduced hematocrit may improve microcirculation during hypothermia, but the current widespread use of tepid or normothermic CPB makes this unnecessary or even dangerous. Unfortunately, both severe anemia and red blood cell (PRBC) transfusion have been found to increase the risk of adverse postoperative outcomes. Observational studies in the last 14 years have suggested a critical hematocrit level of between 20% and 24% (hemoglobin of 7–8 g/dL) during CPB. This is likely influenced by the patient’s temperature, systemic blood flow, and vascular disease. Since administration of PRBCs has adverse consequences, efforts should be directed at minimizing the occurrence of a low hematocrit during CPB (instead of treating it with allogeneic RBC transfusion). This may be achieved by preventing and correcting preoperative anemia (use of preoperative anemia clinics), reducing prime volumes (employment of minimized circuits), use of retrograde or antegrade autologous priming, rigorous hemostasis, and retrieval of shed blood.
In a survey of 271 European centers in 2019, 37% reported that their transfusion trigger was hemoglobin ≤ 7g/dL, 22% used a trigger of < 8 g/dL, and 4% a trigger of < 9 g/dL, while 33% individualized the trigger for different patients. The European guidelines recommend that PRBCs be transfused during CPB if the hemoglobin value is <6.0 g/dL (Class I recommendation, level of evidence C); PRBCs should not be transfused during CPB if the hematocrit is >24%. They state that for hematocrit values between 18% and 24%, PRBCs may be transfused based on an assessment of the adequacy of tissue oxygenation (D o 2 is maintained at >273 mL/min/m 2 and cerebral oximetry is satisfied).
While these are not unreasonable suggestions, to make them recommendations seems problematic, especially the contraindication to giving PRBC if the hematocrit is >24%. The recent Society of Cardiovascular Anesthesiologists (SCA) Clinical Practice Improvement Advisory for Management of Perioperative Bleeding and Hemostasis in Cardiac Surgery Patients recommended that PRBCs be given when the hemoglobin is <7.5 g/dL, but not when the hemoglobin is more than 10 g/dL. Three recent studies found no benefit from a restrictive versus more liberal transfusion threshold in patients undergoing cardiac surgery. Encouraging teams to make decisions based on markers of inadequate oxygen delivery (e.g., hemodynamic instability, increased lactate levels, severe metabolic acidosis, or other signs of ischemia in various organs), rather than the hemoglobin concentration alone would seem more prudent. The most recently published multidisciplinary clinical practice guidelines for patient blood management from the STS/SCA/AmSECT/SABM task force identified 23 new or updated recommendations. Among them were several class I recommendations including preoperative identification of high-risk patients for transfusion, use of synthetic antifibrinolytic agents, retrograde autologous priming, reduced CPB circuit primes, and use of autotransfusion/cell salvage.
Until recently, CPB was conducted utilizing moderate hypothermia (28°C–32°C) both to reduce oxygen demands on the oxygenator and the risk of neurologic injury. In recent years, “tepid” (34°C–36°C) or normothermic bypass is more commonly employed to both reduce time spent in cooling and warming and to minimize the risks associated with rewarming. Current clinical outcome evidence does not strongly support one method or another. Despite lack of good clinical evidence of neuroprotective effect of moderate hypothermia during CPB (excluding deep hypothermia with circulatory arrest [DHCA]), many groups continue to use mild-to-moderate hypothermia for protection against unexpected or accidental periods of hypoperfusion and to permit lower flow rates or arterial pressure. There remains some controversy regarding location to measure temperature, how pH and Pa co 2 should be managed during hypothermia (pH stat or alpha-stat), and acceptable cooling and warming gradients. These issues have been addressed in the 2015 STS, SCA, and AmSECT Clinical Practice Guidelines for Temperature Management During CPB . Key recommendations include:
Oxygenator arterial outlet temperature is the recommended surrogate for cerebral temperature (class I recommendation, level C evidence)
Oxygenator arterial outlet temperature is assumed to underestimate cerebral temperature (class I, level C)
Nasopharyngeal or pulmonary artery (PA) temperatures are reasonable estimates of core temperature after weaning from CPB (class IIa, level C)
Arterial outlet temperature should be limited to <37°C during CPB to prevent cerebral hyperthermia (class I, level C)
Peak cooling temperature gradient between the oxygenator arterial outlet and venous inlet should not exceed 10°C to prevent the generation of gaseous emboli (class I, level C)
Peak warming temperature gradient between the oxygenator arterial outlet and venous inlet should not >10°C to prevent outgassing (class I, level C)
During warming, when oxygenator arterial outlet temperature ≥ 30°C, warming gradient between the oxygenator arterial outlet and venous inlet should be ≤4°C and/or warming rate ≤ 0.5°C/min
Because of the theoretic concerns with the danger of hyperoxia (production of toxic free radicals and aggravation of reperfusion injury), it is recommended to avoid high levels of PaO 2 . However, acceptable levels have not been defined and clinical evidence supporting the advantage of avoiding hyperoxia is not available. Recent studies have offered conflicting conclusions. , , The result of the Risk of Oxygen during Cardiac surgery (ROSC) prospective RCT may be helpful.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here