Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
With a worldwide prevalence of over 100 million cases, valvular heart disease remains a significant human and clinical burden, necessitating over an estimated 400,000 interventions annually worldwide and estimated to surpass 850,000 by 2050 . Three factors account for this expected increase: (1) the most common type of valvular heart disease, aortic stenosis (AS) is strongly associated with aging, (2) with the general increases in life expectancy globally, the number of vulnerable persons is increasing rapidly, and (3) the introduction of new technologies, including improved prosthetic valves and new methods of insertion has allowed previously untreated/untreatable populations to benefit from surgery and other interventions. The diagnosis and treatment of valvular heart disease has seen significant change over the past century. The importance of this progress was exemplified by the recognition of the contributions of Drs Albert Starr and Alain Carpentier, surgeons who revolutionized the treatment of valvular heart disease through the development and demonstration of the feasibility of implantation of substitute mechanical and tissue valves, respectively , by the prestigious Albert Lasker Clinical Research Award in 2007. FLOAT NOT FOUND FLOAT NOT FOUND
Untreated valvular heart disease has serious consequences and effective medical therapies are not available. Therefore cardiac valve replacement and repair are now performed frequently. In the United States alone, cardiac surgeons and interventional cardiologists performed over 150,000 aortic valve replacements (AVRs) alone in 2019, and additional surgeries to replace or repair the mitral valve. Symptomatic patients with heart valve stenosis or regurgitation who are managed by replacement of a diseased valve with a synthetic or biological substitute heart valve prosthesis (HVP) typically experience increased survival, reduced symptoms, and enhanced quality of life for extended intervals. Importantly, until recently, approximately one-third of all patients were refused surgery because of “high risk” and comorbidities . The availability and use of implantable prosthetic heart valves (PHV) that do not entail open heart surgery [transcatheter aortic valve implantation (TAVI)] has grown rapidly over the past two decades . Data from the Society for Thoracic Surgeons show that in the United States, the number of TAVI procedures in 2020 (see later sections) surpassed the number of patients who underwent surgical aortic valve replacement (SAVR). This number will only increase, given the favorable clinical data permitting the broadening of indications for TAVI (to patients at lesser surgical risk), the lower hospitalization stay, and superior patient experience associated with less invasive procedures . TAVI may also be used in a so-called “valve-in-valve” approach to the treatment of diseased bioprosthetic implants. Thus the increasing availability of TAVI is ushering in a new era in the management of valvular heart disease.
This chapter summarizes the pathologic anatomy and clinicopathological considerations in valve replacement and repair surgery, encompassing pathology associated with a substitute valve as well as other pertinent cardiac and noncardiac pathology. It updates the chapter by Schoen and Butany in the fourth edition of this book . Discussion of the following related areas provides additional context: (1) clinical considerations and outcomes, (2) description of valve replacement devices, (3) approach to evaluating postsurgical hearts and substitute valves as pathological specimens and added information on transcatheter aortic valve procedures. Ongoing research and development that may affect the future of cardiac valve replacement and repair are also mentioned briefly in context. The discussion relates to open cardiac valve replacement via sternotomy and with cardiopulmonary bypass (CPB) as well as to pathologic considerations related to minimally invasive, transcatheter implantation, and repair procedures on cardiac valves.
Valvular disease causes secondary effects on the heart and other organs that vary with the valve involved and the nature, severity, and duration of the abnormality. In particular, the compensatory myocardial hyperfunction and hypertrophy induced by the hemodynamic burden can eventually lead to muscle dysfunction, heart failure, and occasionally sudden death (see Chapter 12 : Toxins and the Heart). In a patient with clinically overt valvular heart disease, surgery or other intervention is usually deferred until the risks of complications with prolonged medical management are greater than the new dangers associated with both the surgical procedure and a device ( Fig. 17.1 ) . Prognosis following surgery is determined by the quality of the myocardium, other cardiac and systemic pathology, and the consequences of prosthesis-associated complications. As mentioned above, the availability and evolution of TAVI has permitted definitive mechanical correction in previously high-risk and ineligible patients. In the last few years, the patients deemed suitable for these valves (by the FDA) have gone from the high-risk and extremely high risk groups only to the medium-risk and now the low-risk group also .
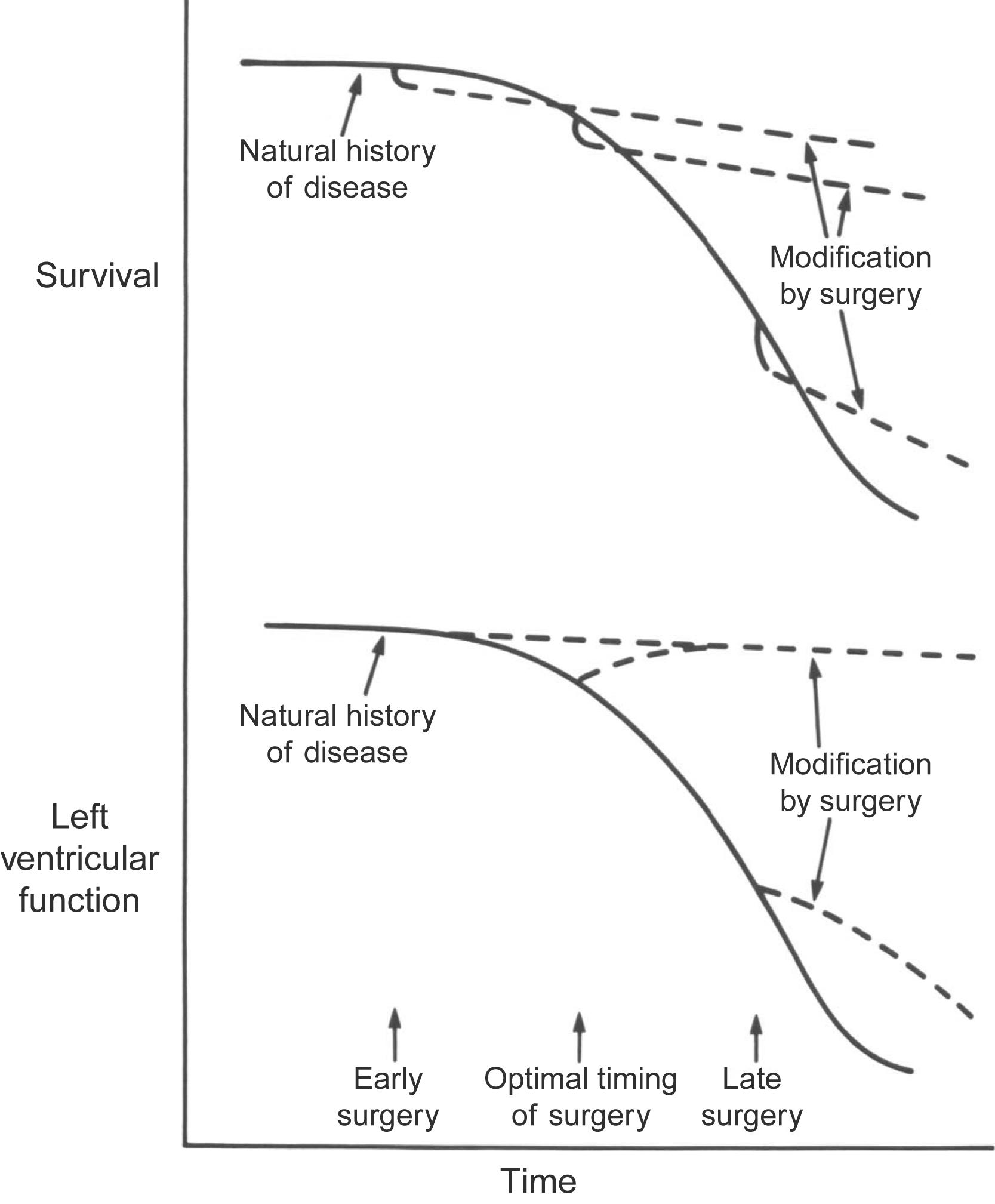
In an individual patient, the results of valve replacement and the anticipated pathology depend on the: (1) details of the procedure, as well as both intraoperative and subsequent postoperative management, (2) extent of intraoperative myocardial injury, (3) overall functional status and irreversible and often chronic, structural alterations in the heart, lungs, and other organs secondary to the original valvular abnormality, (4) coexistent obstructive coronary artery disease (CAD) and associated surgery, such as coronary artery bypass grafting (CABG), and (5) type of valve substitute used and its potential vulnerabilities, largely related to materials reliability and interactions of the device with natural(host) tissues, as well as its relative benefit to cardiac structure and function .
Both early and late mortality and morbidity after cardiac valve replacement have decreased substantially in recent years, owing to improved knowledge of the natural history of disease, better timing of surgery, patient selection, surgical technique and postoperative care, and the development of improved substitute valves. Operative mortality for AVR averages 4% or less for AVR alone and 8% for AVR with CABG . Operative mortality for mitral valve replacement (MVR) averages 5%–9% . Mortality increases with preoperative functional class, age, diabetes mellitus, and preexisting CAD. Patient variables such as gender, original valve pathology, functional defect, urgency of surgery and follow-up care may also influence outcome. In particular, the risk for a repeat valve replacement (reoperation for a failed prosthesis or other problem) is increased to 7% for aortic and 20% for mitral, and even higher when it is prompted by an emergent problem .
Following AVR with either mechanical or tissue valves, the probability of 5-year survival is about 80%, and of 10-year survival is about 70% . Strong patient-related correlates of excessive late statistical mortality are advanced age at implantation, preoperatively impaired left ventricular function, increased left ventricular and left atrial size, and CAD.
Observational clinical investigations of individual valves and randomized studies comparing different valve types consistently show that one or more valve-related problems causes reoperation or death in ~60%–70% of patients with substitute valves within 10–15 years postoperatively . The overall rates of valve-related complications are similar for mechanical prostheses and bioprostheses. However, the frequency and nature of valve-related complications are markedly dependent on the prosthesis type, model, size, site of implantation, and patient characteristics (e.g., ventricular function, arrhythmias).
The overall causes of death following valve replacement vary according to the postoperative interval, as shown in Table 17.1 . Clinically, operative mortality is primarily linked to myocardial and/or respiratory failure, bleeding, infection, stroke, and/or technical problems. Preexisting cardiovascular disease and operative complications are the overwhelming source of mortality in the early postoperative period (generally considered 30 days); in this period, only a small fraction of deaths are attributable to the valve substitute . In contrast, prosthesis-associated complications are common in valve recipients beyond the early postoperative interval . Thromboembolic complications with mechanical valves (MHV) are common in the first year, while limitations to bioprosthesis durability become prevalent only beginning ~4–5 years postoperatively. Prosthesis-associated complications usually develop slowly, as with bioprosthetic valve structural degeneration, and permit reoperation with its attendant risks, however, they can occasionally be catastrophic and fatal, as exemplified by prosthetic valve thrombosis or endocarditis.
| Early deaths (<30 days) | 279 total |
|---|---|
| Cardiovascular and operative | 262 (94%) |
| No anatomic lesion | 108 |
| Myocardial necrosis | 68 |
| Technical | 4 |
| Other cardiovascular | 41 |
| Pulmonary | 19 |
| Other | 23 |
| Prosthesis-associated | 17 (6%) |
| Thromboembolism | 7 |
| Endocarditis | 2 |
| Dehiscence/paravalvular leak | 5 |
| Disproportion | 3 |
| Late deaths (>30 days) | 99 total |
| Cardiovascular and operative | 44 (44%) |
| Congestive heart failure | 14 |
| Myocardial infarction | 8 |
| Sudden death | 6 |
| Other | 16 |
| Prosthesis-associated | 46 (47%) |
| Thrombosis | 13 |
| Thromboembolism | 8 |
| Hemorrhage | 3 |
| Endocarditis | 14 |
| Paravalvular leak | 6 |
| Structural dysfunction | 2 |
| Noncardiac | 9 (9%) |
Cardiac, vascular, and noncardiac complications of operation account for the majority of early deaths . Specifically, the causes include hemorrhage, aortic dissection or disruption associated with cannulation sites or the aortotomy done for suturing a replacement AV, emboli from plaques, calcium masses or thrombus, pulmonary embolism or insufficiency, low cardiac output, arrhythmias stroke, and sudden death (with or without ischemic myocardial necrosis). Deep to the closed sternum and skin incision, the pericardium is usually closed following valve replacement but may be left open owing to ongoing bleeding or edema of thoracic structures. Wound infection, including sternal infection, occurs in up to 5% of patients who have an elective sternotomy. In the excision of the valve, there may be trauma to adjacent structures, including an adjacent valve. In particular, for AVR, the coronary ostia, membranous septum, atrioventricular (A-V) node and adjacent left bundle, for MVR, the interatrial septum, A-V conduction system, and circumflex coronary artery are vulnerable. Occasionally, complications of investigative procedures or treatment undertaken before surgery contribute to the cause of death.
Aortic or other vascular dissection can result if a perfusion cannula enters the media, either at an arterial insertion site or through an atherosclerotic plaque while being passed along the vessel, or at the site of a vascular wound or clamp. Coronary ostial injury can be caused during surgery, by coronary artery cannulas used for myocardial perfusion during bypass; such injury may manifest beyond the postoperative interval when subsequent fibrous proliferation of the intima causes ostial stenosis. Intimal thickening in the ascending aorta due to turbulence distal to a prosthesis can also contribute to ostial stenosis. Sutures may impinge on a vessel and distort or stenose its lumen, or act as a nidus for thrombus, which can occlude the vessel or be a source of distal emboli.
Myocardial ischemia may occur during surgical valve replacement, acute myocardial necrosis may be observed at autopsy in patients who have died early following valve replacement, especially, and not exclusively those with chronic atherosclerotic coronary arterial occlusions. Nevertheless, the extent of myocardial injury in patients with a benign postoperative course is not known. Ischemic myocardial injury is most often related to inadequate myocardial preservation, but iatrogenic damage to either the coronary arteries, direct trauma to the myocardium at the site of operation, or embolic phenomena can play a role. Improved techniques of intraoperative myocardial protection have greatly reduced the incidence of significant perioperative ischemic myocardial injury.
Circumferential hemorrhagic necrosis and the stone heart syndrome comprising complications of reperfusion following global myocardial ischemia during CPB are rare today. The full-blown lesion comprises a hemorrhagic, subendocardial zone of myocardium, often partially transmural and extensive from apex to base, and sometimes affecting the right ventricle. The microscopic lesion is myocardial necrosis with contraction bands, small foci of which may be seen even today in patients who have died following valve surgery.
In the implantation of a mitral prosthesis, valve and papillary muscle excision and suture placement can be associated with important traumatic acute pathology. Rarely, owing to excessively deep excision or suturing along the posterior leaflet, the myocardium is ruptured at the level of either the papillary muscles or the chordae, or in an immediately subannular location, causing massive hemorrhage with death on the operating table or soon after. A partial thickness tear can lead to a false aneurysm, which can subsequently rupture. Moreover, deep dissection or a deep suture placed in the anteromedial mitral annulus can damage the conduction system and cause complete heart block. The left circumflex coronary artery, in the A-V groove is only a few millimeters away from the attachment of the posterior mitral leaflet and can be injured or entrapped by a suture placed to anchor a mitral prosthesis. A deep anterior suture could tear or tether the left or noncoronary cusp of the AV. The incidence of these complications has been virtually eliminated and postoperative cardiac function improved by contemporary MVR techniques which usually do not excise the entire mitral leaflets and preserve some of the submitral apparatus as well as the routine use of intraoperative transeosophageal echocardiography (TEE) .
Late mortality and morbidity following cardiac valve replacement result predominantly from prosthesis-associated complications or cardiac failure. Late cardiac failure can occur despite otherwise successful valve replacement ( Fig. 17.2 ) and patients with valvular heart disease can develop ischemic or other heart disease postoperatively . Autopsy studies, clinical analyses, and greater accessibility to noninvasive investigations such as CT and MR show complications (premortem) and that deaths are device-related in 25%–61% of patients . Moreover, valve-related complications frequently necessitate reoperation or reintervention. Late outcome following cardiac valve replacement is also critically dependent on both irreversible cardiac pathology secondary to the original valvular disease (especially myocardial hypertrophy, and degeneration caused by chronic pressure or volume overload, as well as pulmonary vascular disease) and superimposed CAD, whether or not valve replacement is combined with aortocoronary bypass graft surgery. Nonvalve-related cardiac conditions collectively account for nearly one-half of late deaths. Both valve-related and nonvalve-related deaths following valve replacement may often occur suddenly. Specific valve-related complications will be discussed below following a description of the devices used as cardiac valve substitutes.
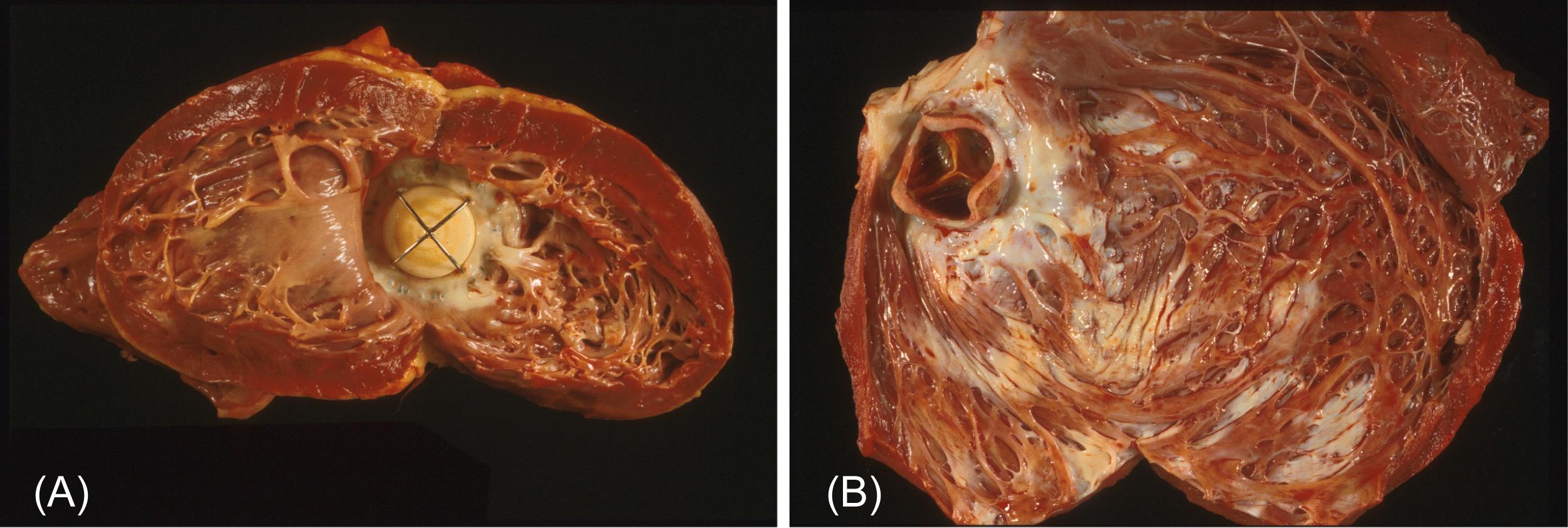
Replacement of a diseased heart valve by a substitute PHV relieves much of the pressure and/or volume overload induced by the original disease. Left ventricular hypertrophy is reversible in many cases, though the extent to which hypertrophy associated with valvular disease is capable of regression and the factors regulating the extent of resolution are uncertain . Hemodynamic adjustment is not always accompanied by a reversal of all deleterious myocardial changes, indeed, many patients with adequately functional heart valve prostheses eventually die of or require transplantation for congestive heart failure due to myocardial decompensation (see Fig. 17.2 ).
The first prosthesis used for treating aortic valve disease was invented by Dr Charles Hufnagel and implanted in the descending thoracic aorta (i.e., not in the AV position). Used to treat aortic regurgitation, the Hufnagel ball-in-cage valve had both components fabricated from plexiglass . Subsequently, enabled by the development of CPB, orthotopic (i.e., in the site of the diseased valve) cardiac valve replacement was first performed successfully in 1960 .
The characteristics of an ideal heart valve replacement device were originally enumerated by Harken in 1962 , and have subsequently been modified ( Table 17.2 ). Despite development and investigation of over 80 models of substitute valves and progressive improvement in the technology of their design, materials, and manufacture over the past half-century , only a small number of heart valve models have achieved wide clinical use and no heart valve type available today perfectly satisfies the criteria described in Table 17.2 .
| Offers no resistance to physiologic blood flow |
| Has central blood flow and no transvalvular gradient |
| Closes rapidly and in the appropriate phase of the cardiac cycle, with no incompetence |
| Is nonthrombogenic and requires no anticoagulation |
| Creates no thromboemboli |
| Dopes not support the growth of infectious organisms |
| Is durable |
| Is technically simple to implant and maintain fixed at the appropriate anatomic site |
| Is chemically inert and nontoxic |
| Does not physically damage blood elements |
| Is not noisy or otherwise annoying |
| Cost is reasonable |
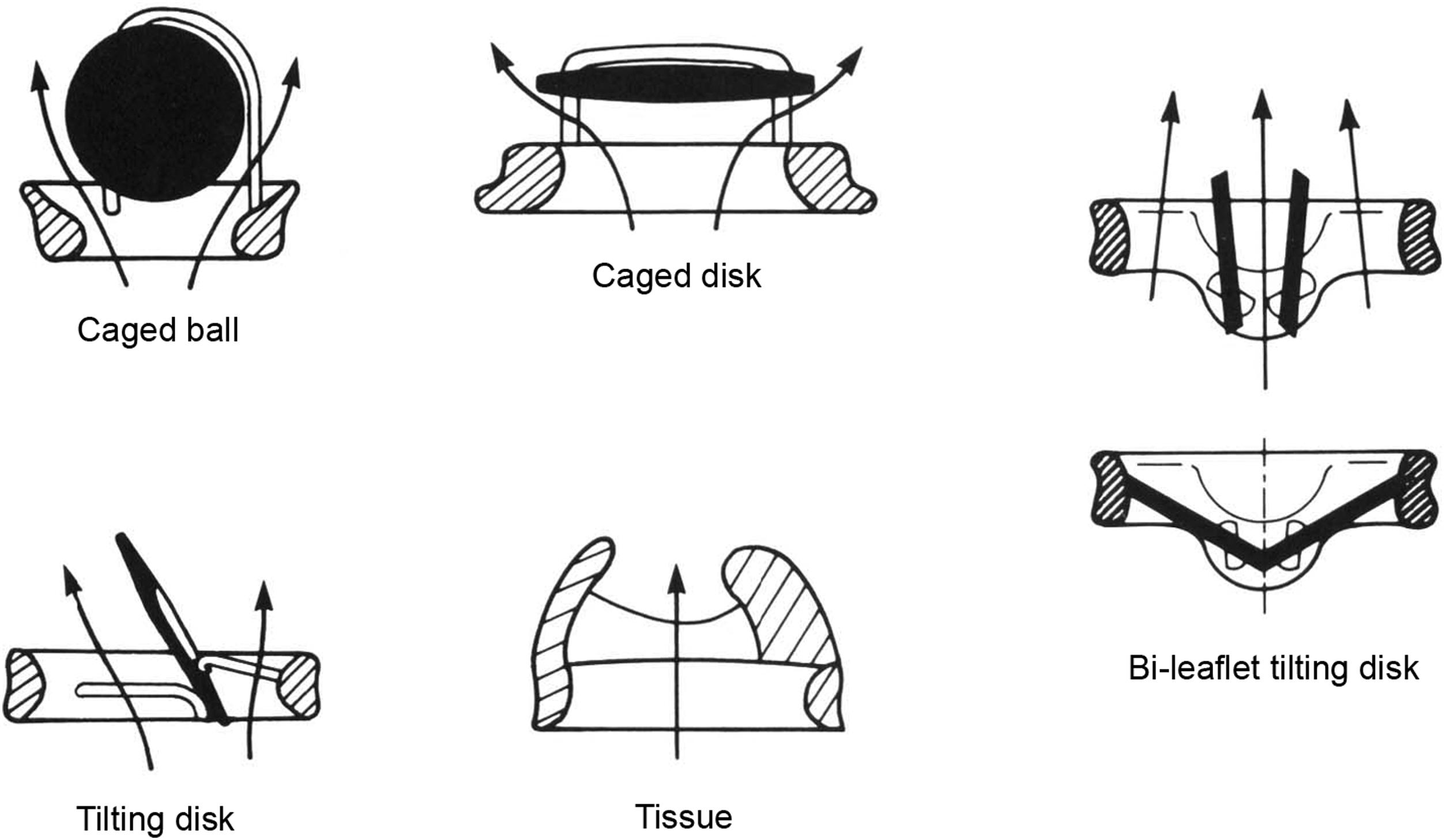
Most available and previously used PHV fall primarily into several general categories defined by design and materials of construction, and are illustrated schematically in Fig. 17.3 . PHV in use today either have all components manufactured of synthetic, nonbiologic material ( MHV ) or are constructed, at least in part, of either human or animal tissue ( tissue valves ). Tissue valves composed of chemically processed animal tissues, called bioprosthetic heart valves (BHV) or bioprostheses , are most frequently used.
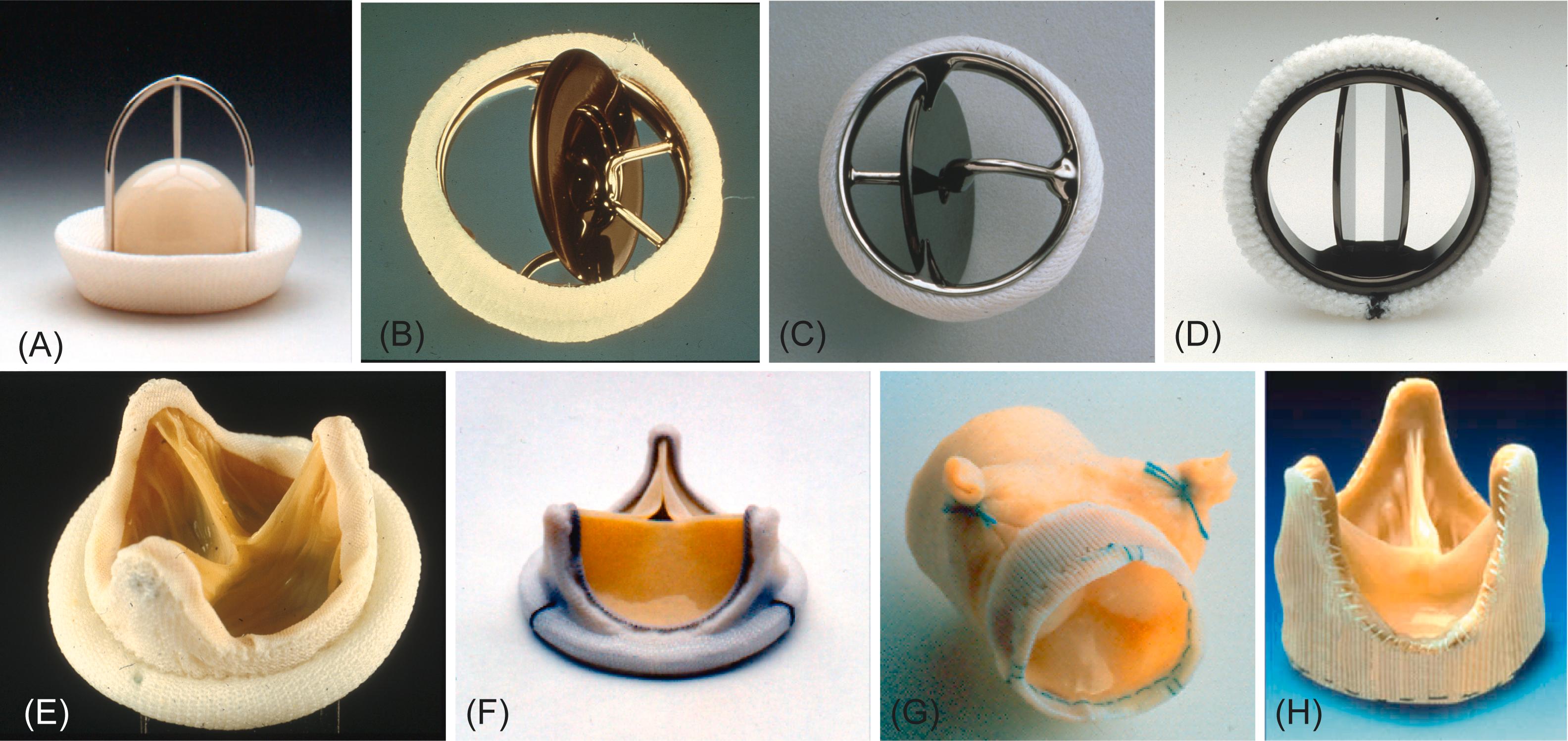
Some bioprostheses have stents; others are nonstented. The valves that may be encountered by pathologists are listed in Table 17.3 . Representative configurations are illustrated in Fig. 17.4 . The terminology used to describe such devices is summarized in Table 17.4 . A more complete listing of FDA approved valves, their manufacturer, and their current status is in the Appendix .
| Type | Model |
|---|---|
| Mechanical | |
| Caged ball | Starr-Edwards |
| Single tilting disk | Bjork-Shiley |
| Medtronic Hall | |
| Bileaflet tilting-disk | St Jude Medial (Standard, Regent, Silzone) |
| Carbomedics (now Sorin) | |
| On-X | |
| Tissue (xenograft/heterograft bioprosthesis) | |
| Porcine aortic valve | Carpentier-Edwards Porcine aortic valve |
| Hancock Porcine (Standard, MO) | |
| Hancock II aortic valve | |
| St Jude Medical—EPIC porcine aortic prostheses | |
| Bovine pericardial | Carpentier-Edwards bovine pericardial valves |
| Mitroflow bovine pericardial prosthesis | |
|
|
| Stentless porcine aortic valve | Toronto SPV |
| Medtronic Freestyle SPV | |
| Tissue (allograft/homograft/autograft) | |
| Term | Meaning |
|---|---|
| Related to biomaterial/source/treatment/support | |
| Mechanical prosthesis | Valve in which the functional components are all synthetic and composed of metals, polymers, ceramics, or carbon |
| Tissue (tissue-derived) valve | Any valve in which the prime functional components are derived from animal or human tissues, valvular or otherwise (see below) |
| Bioprosthesis | Valve composed of tissue, often chemically treated, mounted on a prosthetic stent (e.g., porcine aortic valve or bovine pericardial bioprostheses, autologous fascia lata valve) with a sewing cuff |
| Heterograft/xenograft | Valve or tissue transplanted from an individual of one species to that of another (e.g., porcine aortic valve or bovine pericardium implanted into a patient) |
| Homograft/allograft | Valve or tissue implanted in individual of the same species (e.g., a valve from a human cadaver implanted into a patient) |
| Autograft | Valve or tissue removed from one site to another in the same individual (e.g., a patient’s own pericardium or fascia lata fashioned into valve cusps and mounted on a stent, or a pulmonic valve transplanted to the aortic root) |
| Stentless bioprosthesis | Tissue valve without prosthetic frame or sewing ring |
| TAVI valve | Tissue valve mounted in a metallic expandible cage, to be catheter deployed for replacing the diseased aortic valve by minimally invasive procedure. |
| Related to valve structure | |
| Occluder/poppet | Moving part(s) of a mechanical prosthesis that block(s) reverse flow when valve is closed and partially move(s) out of the flow stream when open |
| Cusps | Flexible, moving tissue components |
| Stent/struts | Prosthetic support structures of a mechanical or tissue valve |
| Sewing ring | Cloth that surrounds the base of a valve, through which sutures are placed to anchor it in the annulus |
| Skirt | Synthetic fabric wrapped around the outside of a metallic stent (in someTAVI valves) |
Published numbers suggest that approximately 150,000 substitute aortic valves were implanted in the United States and about 250,000 worldwide in 2019 .
Substitute heart valves respond passively to hemodynamic stimuli manifested as pressure gradients and flow changes within the heart. Competency (i.e., ability to prevent back-flow when closed) of a valve substitute generally derives from its intrinsic structure [either seating of its mobile occluder(s) or apposition of tissue flaps]. Owing to the combination of passive function, rigid frame, and sewing ring, most MHV and BHV types can, theoretically, be used to replace a diseased valve at any site. Heart valve substitutes are manufactured in a broad range of sizes to match the size of the orifice remaining after the patient’s diseased valve is excised. The sizes are expressed as a diameter, typically 19–25 mm for AVs and 25–35 mm for MVs.
Except for a subgroup of valves implanted through a catheter (TAVI) and stentless and sutureless valves (to be discussed later), contemporary mechanical and tissue valves have a fabric (usually Dacron mesh) sewing cuff that surrounds the base of the prosthesis. Sutures placed into this cuff by the surgeon are intended to anchor the device into the surgically prepared annulus. Nevertheless, sewing rings have different configurations in semilunar (i.e., mitral and tricuspid) compared to A-V (i.e., aortic and pulmonary) prostheses, owing to the need to conform to differences in annular anatomy, and more subtle differences to accommodate technical details of implantation within an anatomic region (e.g., aortic annular vs supraannular). With its inflow and outflow surfaces exposed to blood and its outer rim forming the peripheral margin of the prosthesis, the sewing ring is an important site of interaction between a prosthesis and surrounding tissue . In most cases, organized thrombus and/or fibrous tissue (pannus) derived from and contiguous with the adjacent endo -myocardium or aortic wall will ultimately cover the rough cloth surface of a valve sewing cuff. Uncomplicated organization of thrombus and tissue incorporation into the sewing cuff optimally yields a thin layer of largely endothelialized, mature collagen associated with minimal mononuclear inflammatory cells or giant cells. The gradual covering of HVP surfaces by endothelium may reduce thromboembolic episodes. However, too little or too much tissue growth can lead to complications, namely paravalvular leak or to stenosis of the orifice, respectively (see below). A disadvantage of the sewing cuff is that it occupies space, thereby reducing the size of the available orifice for blood to flow through the valve and usually leaves a persisting residual gradient across the valve (see below).
Several factors are considered by a surgeon or interventional cardiologist in choosing a PHV for (and in consultation with) an individual patient. These include patient age, comorbid cardiac and noncardiac conditions, potential compliance with an anticoagulation regimen required following MHV implantation owing to the risk of thrombosis with these devices, the patient’s wishes and anticipated life span. The importance of these factors relative to outcomes and expected pathology will be discussed below. The most important determining factor between the selection of a mechanical or biological valve for a particular patient is related to the relative risks of thrombosis, thromboembolism, and anticoagulant therapy with MHV, or structural valve deterioration, degeneration and/or dysfunction (SVD) with BHV (discussed in detail below).
In addition to the sewing cuff described above, MHV generally have three essential components: (1) one or more rigid but mobile part(s), each of which is called an occluder , usually a ball (also called a poppet ) or a hinged leaflet of circular or semicircular profile, (2) a cage-like superstructure or housing that guides and restricts occluder motion, and (3) a valve body or base which provides support for the housing or cage and provides the seat for the occluder(s) . The internal orifice of the valve housing defines the effective area (often termed effective orifice area , or EOA) through which blood can flow. In the closed phase, an occluder contacts the ring or seat and fills or overlies the lumen of the prosthesis. MHV prostheses can be separated into three major types according to their flow characteristics (recall Figs. 17.2 and 17.3 ): (1) those in which blood passing through the valve orifice impinges on a centrally placed occluder and must swirl around it (lateral flow prostheses) exemplified by caged-ball and caged-disk valves (seldom used now); (2) those in which a single tilting occluder divides the flow into two stream (infrequently used now); and (3) those of hinged, bileaflet tilting-disk construction, in which two tilting hemidisks divide the flow into three compartments.
MHVs that have been used most widely and likely to be encountered by pathologists include the Starr-Edwards (Edwards Life Sciences, Santa Ana, CA, United States) caged-ball valve ; the Bjork-Shiley (previously manufactured by Shiley Co., Irvine, CA, United States) and Medtronic-Hall (Medtronic, Inc, Minneapolis, MN, United States) tilting-disk valves; and the St Jude Medical (St Jude Medical, Inc., St Paul, MN, United States) , and On-X (On-X Life Technologies, Inc., Austin, TX, United States) bileaflet tilting-disk valve prostheses. Although slightly modified from its original form, the Starr-Edwards caged-ball valve had a longer history of use than any other prosthesis type . Thus results using this prosthesis serve as a benchmark against which those of other valves can be measured. Owing to durability problems which became apparent only following extensive clinical use (discussed later), the previously popular Bjork-Shiley tilting-disk valve with distinct inflow and outflow struts was discontinued, though a more robust monostrut version became available . Moreover, several types of caged-disk valves and other tilting-disk valves were used in the early decades of valve replacement, but intrinsic obstruction, poor durability, and vulnerability to thromboembolism prompted removal from the market . Presently, bileaflet tilting-disk MHVs are the most frequently implanted type of MHV worldwide.
Initially, MHV superstructures and rings were composed of metal—either cobalt–chromium alloy (e.g., Starr-Edwards and Bjork-Shiley valves) or titanium (e.g., Medtronic-Hall valve), and the occluders were originally plastic (e.g., the Starr-Edwards valve had a silicone poppet) and later pyrolytic carbon. Originally developed as a coating for nuclear fuel particles in the 1960s, pyrolytic carbon is rigid, strong and highly resistant to wear, fatigue, and thrombus formation . Most contemporary commercially available MHVs have all parts of the valve composed of pyrolytic carbon (other than the sewing cuff).
Since the stream of blood flow through a MHV prosthesis must separate, course around the occluder(s), and reconstitute distally, MHVs usually have areas of stasis, predominantly distal to the orifice and occluder(s) and in the low flow hinge areas. The combination of stasis and nonphysiological surfaces potentiates thrombus formation . Thus at this time, virtually all patients with any MHV receive lifetime anticoagulation therapy to lessen the risk of thrombosis and thromboembolic complications with an INR suggested by the manufacturer . As discussed below, anticoagulant therapy carries a significant risk of hemorrhage.
BHV mimic natural semilunar valve anatomy and have cusps composed of animal or human tissue. Tissue valves comprise: (1) xenografts (also called heterografts and including animal tissue-derived valves, most notably porcine AV or bovine pericardial bioprostheses), (2) allografts [also called homografts and including aortic (or pulmonic) valves primarily from human cadavers, usually with an associated aortic (or pulmonary) arterial sleeve as a conduit], or (3) autografts (e.g., composed of patients own pericardium, or the patient’s own pulmonary valve transplanted to the aortic root). Fascia lata autografts (and dura mater for a brief period) were previously used . Xenograft bioprostheses, first introduced clinically in the 1970s, and now widely used, are preserved by chemical crosslinking of collagen with aldehydes. Allografts and autografts have been used either fresh, often treated with an antibiotic, or otherwise preserved (see later).
The advantages of tissue valves include a central pattern of flow resembling that of native cardiac valves and low thrombogenicity (low vulnerability to thrombosis). Consequently, most patients with bioprosthetic valves and virtually all of those with allografts or autografts do not require long-term anticoagulation. Thus unless there is another indication for anticoagulation (e.g., atrial fibrillation, prior thromboembolism, severe left ventricular dysfunction, or hypercoagulable state), tissue valves can be an attractive choice for patients in whom anticoagulation is either contraindicated or undesirable. Nevertheless, most patients with bioprostheses will be prescribed aspirin or warfarin anticoagulation for the first 3 months following implantation while the potentially thrombogenic sewing cuff is being covered with host tissue. Durability limitations constitute the major impediment to the long-term success of commercially available bioprostheses and most other tissue valve substitutes.
The concept and later reality of a stent-mounted, chemically treated, nonthrombogenic and nonantigenic tissue valve replacement was pioneered by Dr Alain Carpentier . Chemical preservation allows a stable “shelf-life” of several years, enhances material stability, permits a surgeon to have valves of various sizes readily available for implantation, and minimizes the potential of immunologic rejection of the tissue component, while maintaining both the thromboresistance and antimicrobial sterility characteristic of the natural tissue. The most frequently used chemical preservative for bioprosthetic tissue is glutaraldehyde (1,5-pentanedialdehyde; CHO[CH 2 ] 3 CHO), used historically for tanning leather and, for several decades, as a fixative for electron microscopy . This dialdehyde forms complex, degradation-resistant, cross-links between protein molecules, especially collagen (but not elastin), the most abundant structural protein of natural valves and other tissue substitutes (such as pericardium). In contrast, since formaldehyde-induced cross-links are unstable, formaldehyde-pretreated bioprostheses used in the early days of tissue valve replacement frequently developed premature materials failure . The antibacterial and antifungal efficacy of low concentrations of glutaraldehyde is poor (especially against spores, as exemplified by a cohort of tissue valves several decades ago contaminated with Mycobacterium chelonae ), adjunctive solution sterilization procedures are necessary (e.g., transient treatment with alcoholic glutaraldehyde solutions).
Most commercially produced bioprostheses are mounted on and supported by a metal or plastic stent , usually with three posts (called struts ) to support their three cusps (tissue histology shown in Fig. 17.5 ). Stent-mounting facilitates surgical implantation in the semilunar or atrioventricular valve sites. Covered by Dacron or animal tissue (usually pericardium), bioprosthetic tissue valve stents vary in structural design, flexibility, and composition between manufacturers and models. In contrast, nonstented porcine valves (shown in Fig. 17.5G and H ) , semilunar valve allografts , and pulmonary autografts (see later) are usually sewn directly into the surgically prepared valve site; and their configuration does not permit atrioventricular valve replacement. The widely used stented bioprostheses are therefore hybrid structures, partly biologic (i.e., cuspal tissue) and partly synthetic (i.e., stent supports, fabric covering, and sewing ring).
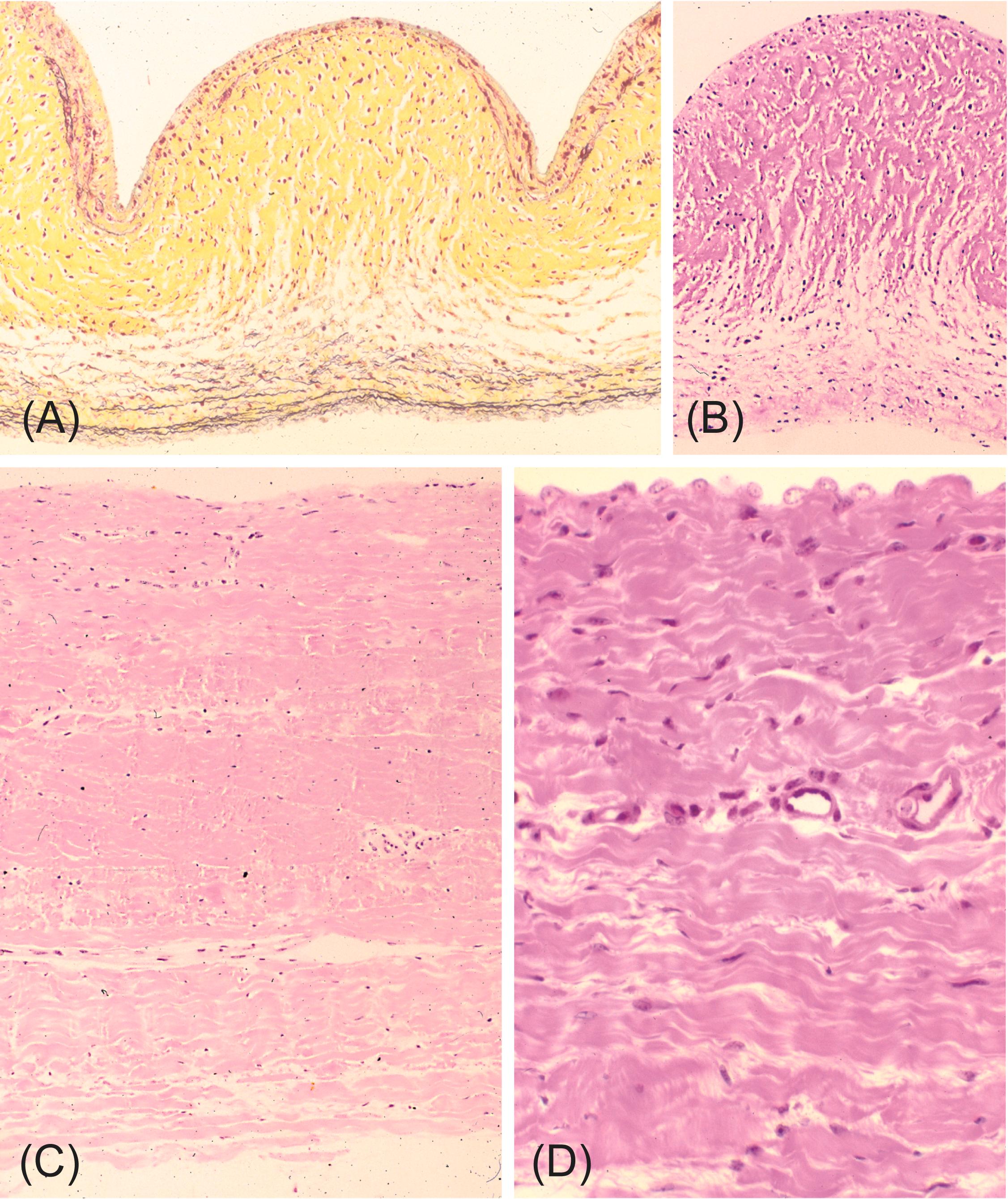
Several decades ago, the most frequent type of tissue valve substitute used clinically was a glutaraldehyde-preserved pig AV mounted on a cloth-covered stent (also known as a porcine aortic BHV ). Although much of the aortic wall is trimmed away in their fabrication, aorta adjacent to the valve cusps and the intervening natural cuspal attachments is incorporated into each valve. Two commercially available products dominated porcine valve use: Carpentier-Edwards valve (Edwards LifeSciences, Santa Ana, CA, United States) and Hancock valves (Medtronic, Anaheim, CA, United States). These two commercial variants differed slightly in configuration, in the thickness of the porcine aortic tissue, in the concentration of glutaraldehyde used in pretreatment (0.2% for the Hancock valve and 0.6% for the Carpentier-Edwards valve), and stent composition [polyacetal (Delrin) in the Hancock, and Elgiloy metal alloy in the Carpentier-Edwards valve].
Today, bioprostheses are used far more frequently than MHV and pericardial BHV are the most widely used type of bioprosthesis . They have cusps composed of glutaraldehyde-treated parietal pericardium (usually of bovine origin) attached to a frame. First-generation bovine pericardial valves included the Ionescu-Shiley (Dacron-covered titanium frame) and the Mitroflow (Dacron-coated Delrin frame) had pericardium wrapped around the outside of the frame, but owing to problems associated with structural dysfunction, clinical enthusiasm for early pericardial valves was dampened following early failures of these original designs . In these BHV, the externally mounted tissue had an alignment stitch (Ionescu-Shiley) and/or cross-stitches at the stent post (Mitroflow), both of which could lead to damage to cusps and early tissue failure. Newer designs (with tissue mounted on the inside of the stent) and durability-enhancing tissue treatments have enhanced the clinical success and broad acceptance of pericardial BHV. Today the Carpentier-Edwards Perimount (Edwards LifeSciences, Santa Ana, CA, United States) is the most frequently used BHV, though other pericardial valves are available .
The natural AV and its cuspal tissue are remarkably well adapted to its function . AV cusps have three layers with different composition and properties which make them uniquely adapted to open and close cyclically. AV cusps open against the aortic wall and close completely under minimal reverse pressure, maintaining full competency throughout diastole. Although the pressure differential across the closed valve (approximately 80 mmHg) induces a large load on the cusps, cuspal prolapse does not normally occur because the cusp’s fibrous network effectively transfers the resultant stresses to the aortic valve annulus and the aorta.
The structural details and functional motion of bioprosthetic valves depend on tissue source, fixation, stent configuration, and tissue attachment (see later). For example, bovine pericardial bioprostheses and porcine AV bioprostheses differ in several important respects ( Fig. 17.5 ). Although porcine valves contains a mixture of type I and type III collagens, bovine parietal pericardial collagen is almost entirely type I . Parietal pericardium is composed of (1) a serosal layer, originally covered by mesothelial cells; (2) fibrosa (accounting for almost the entire thickness), which contains laminated collagen, scattered elastic fibers, nerves, blood vessels, and lymphatics; and (3) superficial connective tissue, with loosely arranged collagen and elastic fibers . Thus the inner surface of parietal pericardium (facing the pericardial space) is smooth, while the external surface from which blood vessels and fat are dissected away, is relatively rough (see Fig. 17.5C ). Most pericardial bioprostheses are fabricated with the smooth surface as the outflow aspect of the cusps; and the inflow surface is the rough side. Pericardial BHV cusps are thicker than those of AVs, ~0.3–0.5 mm compared to 0.2–0.3 mm, respectively, have a nearly uniform thickness throughout the cusp, a flat “knife-cut” edge and this pericardium lacks the functional adaptations of the natural tri-layered aortic valves described earlier.
AV cusps have their natural attachments to the supporting aortic wall preserved in a porcine bioprosthesis, however, in pericardial BHV, the valve cusps are composed of individually obtained pieces of tissue that are artificially attached to stent supports, either inside or outside the frame, depending on the model. Cuspal motion induces stresses in pericardial valves directed toward “commissural” attachments, leading to stress concentrations in the tissue at these sites. In addition, the cloth-covered inner support frame of some pericardial valves, alignment sutures, and cross-stitches can cause cuspal progressive abrasion damage and cusp tearing . It is not surprising then that cusp tearing often begins near the top of the stent post or along cuspal attachments and is a common mode of pericardial valve failure (see later). Finally, as previously mentioned, surfaces and edges generated during pericardial tissue procurement, dissection, and valve fabrication, are not natural, as in a porcine valve; collagen bundles at these surfaces tend to splay during function, allowing host cells and fluid to enter the tissue, and lead to structural weakness. Since clinical studies comparing valves fabricated from these different materials have in general suggested modest superiority of pericardial BPV (over porcine aortic valve BPV) with respect to outcomes, hemodynamics, and complications, including durability, there has evolved virtually exclusive availability and use of pericardial (over porcine aortic) surgical valves in all anatomic sites. Recent data show that (at least some) modern pericardial BHV exceed current standards for stented and stentless models in in vitro accelerated durability testing. The Edwards Perimount Magna Ease retains its hemodynamics even at 1 billion cycles (in vitro), roughly equivalent to 25 years of function, in at least some cases.
Changes induced in bioprosthetic tissue during various steps in the process of valve fabrication are summarized in Table 17.5 . Most importantly, owing to tissue handling and aldehyde pretreatment of bioprosthetic tissue, surface cell layers are denuded, deep cells are rendered nonviable, and extracellular matrix is altered ( Fig. 17.6 ) . Thus preexisting cells in either porcine valve or bovine pericardial prostheses are incapable of repairing progressive damage during function, and the cuspal tissue is “mechanically locked.” The AV spongiosa layer has valvular interstitial cells, essential to maintain the interstitium and repair damage to the rest of the valve tissue. In the aldehyde-treated valves, these cells are dead and there is no possibility of repair. Red blood cells, fibrin, and inflammatory cells can adhere to and get “entangled” in the superficial (“naked”) collagen bundles of BH. Occasionally, small, single, textile fibers derived from materials used to pack the valves are noted on the surfaces of cusps (usually the sinus’ surface).
| Crosslinking of collagen |
| Loss of cell viability |
| Loss of surface endothelium |
| Loss of surface mesothelial cells |
| Disruption and fragmentation of cell membranes |
| Flattening of cusp and stretching/loosening of collagen bundles |
| Loss of amorphous extracellular matrix |
| Stress aggregation/concentration by mounting on stent |
| Adherence of textile fibers to surface (from packaging) |
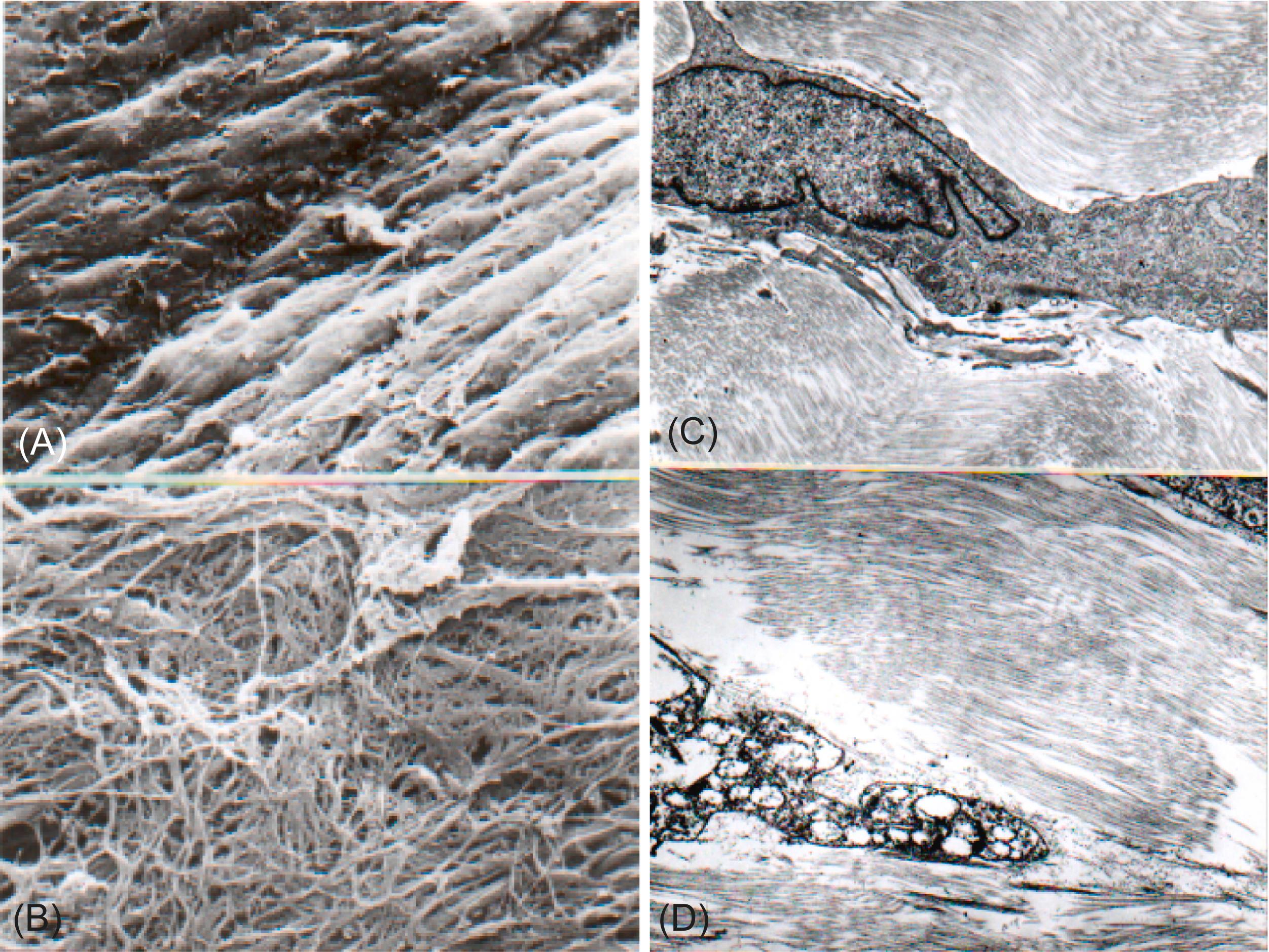
Specific changes may be dependent on the particular fixation techniques employed. For example, in first-generation porcine valves, fixation was at a back pressure of 80 mmHg, imparting a permanent diastolic configuration. Porcine valve cusps fixed under such a load and bovine pericardial cusps become relatively stiff and inflexible; they do not open smoothly, but rather bend in a series of kinks, thereby inducing buckling and substantial, localized repetitive mechanical stresses, accentuating tissue fatigue .
Following implantation, additional and characteristic pathologic changes occur in all types of BHV (summarized in Table 17.6 ) . Shortly following implantation, BHV cuspal surfaces become variably covered with fibrin, platelets, and inflammatory cells, usually predominantly monocytes/macrophages, but neutrophils and/or multinucleated giant cells may be present. The presence of more than scattered inflammatory cells deep within the cusp substance is unusual and should raise suspicion of infection. Clusters of lymphocytes, macrophages, and a few plasma cells of host origin, and foreign-body giant cells are sometimes found in and on the connective tissue at the junction between hosts valve tissue and sewing ring, or sutures. A narrow rim of thrombus is also frequently seen at this site.
| Superficial deposition of platelets and fibrin |
| Surface, superficial, and/or deep mononuclear inflammatory cells |
| Abrasion |
| Endothelialization (focal and variable) |
| Fluid (plasma) insudation |
| Cusp sagging |
| Fragmentation of superficial collagen |
| Homogenization of architecture and loss of cell staining |
| Calcification |
| Intracusp hematoma |
|
| Infection colonization by microorganisms |
| Cusp tear, destruction, and loss of tissue |
The growth of host tissue (pannus) and endothelialization of bioprosthetic valves is slow, usually limited to the basal attachments and often remains incomplete, even years after implantation . Although it is reasonable to hypothesize that endothelialization of a bioprosthetic valve might decrease its propensity to thrombosis, infection, insudation of fluid or blood, or calcific or noncalcific degeneration, evidence is lacking to support a beneficial effect of pre- or postimplantation endothelialization of a bioprosthesis. Pannus when excessive can lead to progressive cusp stiffening, decreased mobility, immobilization and even orifice obstruction (see later). Focal superficial microthrombi are frequently noted along the surfaces of cusps. Owing to the absence of either a functional endothelium or other impermeable barrier, or to sutures used in manufacture piercing cuspal tissue, proteins, and other constituents of plasma, penetrate into the cusps ( fluid insudation ). Fluid insudation and erythrocytes are commonly noted deep in the cuspal tissue of removed bioprostheses. Larger accumulations of blood may yield grossly visible hematomas ( cusp hematoma ) that stiffen cusps or possibly provide sites for mineralization .
Valve function in vivo also gradually results in generalized architectural homogenization with connective tissue disruption, mineralization (discussed below), and, in some cases, lipid accumulation (sometimes with cholesterol crystals) and/or amyloid deposition . With all types of bioprostheses, the intrinsic cuspal architecture is often disrupted with large collagen bundles appearing separated, distorted, indistinct, and hyalinized (appearance akin to a eosinophilic smudge). After several months of function, there is loss of definition of virtually all basophilic (hematoxylinophilic staining components), including cell nuclei, and the cusps are homogeneously eosinophilic.
Bioprostheses can also be affected by systemic diseases. While rare, this has been reported with Whipple’s disease , and carcinoid heart disease occurring in the right atrium and on surfaces of bioprosthetic tricuspid valves .
The clinically significant categories of valve-related complications are: (1) thromboembolic problems and those resulting from the anticoagulant therapy used to prevent them, (2) infection, (3) structural deterioration (i.e., failure or degeneration of the biomaterials comprising a mechanical or tissue prosthesis), and (4) nonstructural dysfunction (a heterogeneous group of complications and modes of failure not encompassed in the previous groups) . The cumulative incidence of failure of mechanical and bioprosthetic valve replacements at 10 years postoperatively is similar, but the nature, time dependence, and consequences of major modes of failure differ . Representative data from one study that randomized patients to a mechanical or a bioprosthetic valve are shown in Table 17.7 . Contemporary MHV have the advantage of good durability, but the disadvantage of a high risk of thrombosis, thromboembolism, and anticoagulation-related hemorrhage. These complications can be catastrophic and fatal. In contrast, bioprostheses have the advantage of relative low rates of thromboembolic complications at least as low as those of mechanical prostheses, despite an absence of chronic anticoagulation therapy in most patients. BHV have the disadvantage of limited durability due to structural valve degeneration (SVD), often requiring late redo surgery. Since patient characteristics may play a role in PHV failure, replacement valve selection is best individualized and jointly decided through a discussion between patient and provider ( Table 17.8 ) .
| Aortic valve replacement | Mitral valve replacement | |||||
|---|---|---|---|---|---|---|
| Mechanical ( n =198) | Bioprosthetic ( n =196) | P value a | Mechanical ( n =88) | Bioprosthetic ( n =93) | P value a | |
| Death from any cause | 66%±3% | 79%±3% | .02 | 81%±4% | 79%±4% | .30 |
| Any valve-related complication | 65%±4% | 66%±5% | .26 | 73%±6% | 81%±5% | .56 |
| Systemic embolism | 18%±4% | 18%±4% | .66 | 18%±5% | 22%±5% | .96 |
| Bleeding | 51%±4% | 30%±4% | .0001 | 53%±7% | 31%±6% | .01 |
| Endocarditis | 7%±2% | 15%±5% | .45 | 11%±4% | 17%±5% | .37 |
| Valve thrombosis | 2%±1% | 1%±1% | .33 | 1%±1% | 1%±1% | .95 |
| Perivalvular regurgitation | 8%±2% | 2%±1% | .09 | 17%±5% | 7%±4% | .05 |
| Reoperation | 10%±3% | 29%±5% | .004 | 25%±6% | 50%±8% | .15 |
| Primary valve failure | 0%±0% | 23%±5% | .0001 | 5%±4% | 44%±8% | .0002 |
| Favoring a mechanical prosthesis |
| Long life span expected |
| Mechanical prosthesis existing in a different position than the valve to be replaced |
| Renal failure, on hemodialysis or with hypercalcemia |
| Warfarin therapy required because of other risk factors for thromboembolism a |
| Age ≤55–65 years for AVR and ≤70 years for MVR |
| Previous thrombosis of a biological valve |
| Child or adolescent with growth potential |
| Favoring a bioprosthesis |
| Contraindication or expected noncompliance with warfarin therapy |
| Age ≥55–65 years; needs AVR and does not have risk factors for thromboembolism a |
| Age ≥70 years; needs MVR and does not have risk factors for thromboembolism a |
| Previous thrombosis of mechanical valve |
a Atrial fibrillation, severe left ventricle dysfunction, and previous thromboembolism.
The causes of prosthetic valve failure are summarized in Tables 17.1 and 17.7 and define indications for reoperation, and encompass the potential diagnoses rendered on recovered surgical pathology specimens and modes of valve-related death discovered at autopsy. In our autopsy study largely comprising MHV recipients from the 1960s and 1970s, late death resulted from valve-related complications in 46% (of 99 patients) (recall Table 17.1 ) , while thrombosis, thromboembolism, and anticoagulant-associated hemorrhage caused 28%, 17%, and 7%, respectively. Thus collectively, more than half (52%) of all valve-related deaths (and nearly one-quarter of all postoperative valve replacement deaths) were related to thrombogenicity, the key limitation of MHV substitutes and one which continues today.
Our subsequent surgical pathology study investigated the etiologies of dysfunction from 112 consecutive porcine bioprostheses and 45 MHVs surgically removed during 1980 through 1985 . The causes of failure overall included degenerative dysfunction (53%), endocarditis (16%), paravalvular leak (11%), thrombosis (9%), and tissue overgrowth (5%). Age is an important factor in the selection of a prosthesis for a particular patient; younger patients have accelerated BHV degeneration. Thus the choice of valve types in a population of patients 50–69 years of age is controversial . Sterile paravalvular leak and PHV infective endocarditis were encountered with approximately equal frequency in mechanical and bioprosthetic valves. Thromboembolic complications were also a frequent cause of MHV dysfunction requiring reoperation, but infrequent with bioprostheses. Materials degradation causing dysfunction of MHV was seen entirely with older models that are no longer in use. In contrast, primary tissue deterioration causing structural valve dysfunction was overwhelmingly the most frequent cause of BHV failure (74% of removals at reoperation). This study confirmed that tissue degradation leading to stenosis or regurgitation, or both, is the predominant late valve-related complication of bioprostheses; calcification is contributory to most cases (see later). Bioprosthetic structural dysfunction generally occurred 5 years or later postoperatively and generally induced slow patient deterioration; only ~5% of such failures require urgent reoperation. Today, structural dysfunction of bioprostheses remains the most critical limitation of this valve type. While there are few morphological reports of series of failed TAVI valves, clinical investigations suggest that early models were prone to early failure (see later in TAVI valve section). However, much of the long-term data come from early generations of TAVI valves and with higher rates of intraprocedural complications due to lesser technical proficiency in the early days of TAVI use. Recent data suggest improved durability of TAVI valves on par with surgical BPVs .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here