Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Infections can involve all three layers of the heart, and virtually all classes of organisms infect cardiac tissues. Because the entire cardiac output is delivered to the lungs and other vital organs by the cardiac ventricles with each cardiac cycle, the propensity for blood-borne infections to be carried to and from the heart is substantial.
This chapter is divided according to the layers involved, in the order of frequency seen by the surgical pathologist: infective endocarditis (IE) involving the valves (and, rarely, the endocardium lining the chambers); myocarditis involving the myocardium; and pericarditis involving the pericardium.
The true incidence of IE is uncertain, but it has been estimated to account for 1 of every 1000 hospital admissions. The morbidity of this disorder is substantial because it can cause cardiac valvular insufficiency, congestive heart failure, and cardiac conduction system abnormalities. Embolization of infected vegetations can damage vital organs, including brain, kidney, and lung. Circulating immune complexes that develop in response to endovascular infection can result in microvascular injury, arthritis, and renal failure.
Revised diagnostic criteria for IE have improved diagnosis, although cases of IE continue to be discovered at the time of valve surgery and at autopsy. The emergence of fastidious microorganisms has led to increased recognition of the microbiologic spectrum of this disease. The widespread use of endovascular prosthetic devices and bioprosthetic grafts has expanded the scope of IE and complicated its management. Extracorporeal membrane oxygenation is currently being used in major medical centers for the cardiorespiratory support of critically ill patients, and it has been associated with increased risk of endovascular infection, especially from Enterobacteriaceae and Candida spp. New left ventricular assist devices have also been associated with bloodstream infections. Finally, research into bacterial interactions with vascular endothelium and recognition of the importance of bacterial biofilms promise to change how IE is treated in the future.
The pathology of IE is complex; it reflects the virulence of the organism, host immunity, the biology of the endocardial surface, and the topography of infection. For example, infection of a cardiac valve by Staphylococcus aureus in a patient with acquired immunodeficiency syndrome (AIDS) is more likely to produce a rapidly progressive syndrome of valvular incompetence and acute heart failure than is endocarditis due to an organism of low virulence in a normal host. Whereas IE can affect an ostensibly normal endocardial surface, it far more commonly targets anatomically distorted valves ( Box 9.1 ). The site of infection is critical with respect to the spectrum of possible complications; for example, IE on the left side of the heart is more likely to result in acutely life-threatening embolic events, including cerebral and myocardial emboli, than is right-heart endocarditis. Extension of aortic valve infection into the heart can produce myocardial abscess and complete heart block. Infected fistulous tracts forming between cardiac chambers can lead to intracardiac shunting of blood, depending on the anatomy of the involved valve. For these reasons, IE must always be approached nongenerically because the specific pathology determines both potential complications and the optimal therapeutic approach in each case.
Vegetation or intracardiac abscess confirmed histologically
OR
Demonstrated by culture or histology in a vegetation or in an embolic vegetation or intracranial abscess
Positive blood culture for typical IE organisms from two separate blood cultures
Persistently positive for typical IE organisms blood culture drawn >12 hours apart
Persistently positive blood culture for typical IE organisms from all three or a majority of four blood cultures or a majority of four blood cultures
Single positive blood culture for C. burnetii or phase I antibody titer >1:800
Echocardiogram supportive of endocarditis
Type of Study
TEE recommended as first test in patients with (a) prosthetic valve, (b) possible endocarditis by clinical criteria, and (c) those suspected of paravalvular abscess
Positive findings: oscillating intracardiac mass on valve or supporting structure, or in the path of regurgitant jets, or on implanted material in the absence of alternative explanation, abscess, or dehiscence of prosthetic valve or new valvular regurgitation
Predisposing heart condition or intravenous drug use
Temperature > 38.0°C
Vascular phenomena: arterial emboli, pulmonary infarcts, mycotic aneurysms, intracranial bleed, conjunctival hemorrhages, Janeway lesions
Glomerulonephritis, Osler nodes, Roth spots, positive rheumatoid factor
Positive blood culture that does not meet major criterion
Major criteria: 2
Minor criteria: 5
Major and minor one major + three minor
IE, Infective endocarditis; TEE, transesophageal echocardiography.
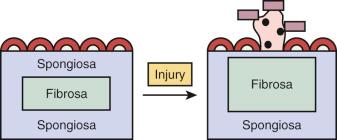
Most cases of IE involve the cardiac valves. The normal atrioventricular mitral and tricuspid valves of the cardiac inflow tracts and the semilunar aortic and pulmonary valves of the outflow tracts share a common structure. They consist of a dense, avascular, collagenous core, termed the valvular fibrosa, which is contiguous with the fibrous skeleton of the heart, surrounded by a spongiosa that consists of a loose matrix of collagen, elastic fibers, and glycosaminoglycans. The valve surfaces are lined by endothelium. This topographic arrangement is critical to the normal function of the valves during the cardiac cycle. The cardiac ventricles fill with blood that passes through the open leaflets of atrioventricular valves during diastole, and the valves must remain tightly apposed during systole. Conversely, the semilunar valves must open during systole and remain competently closed during diastole. This is achieved by the elastic and deformable properties of the valves. Because the normal cardiac valve is normally avascular, stromal cells that contribute to the valvular fibrosa and spongiosa must dynamically maintain valvular structure.
With the mechanical wear and tear that accompanies age—valves must open and close almost 3 million times in the course of a 75-year lifetime—the normal configuration of the valve changes. Fibrosis is the most common complication of aging, whereas myxomatous change can result from altered hemodynamics (e.g., the functional insufficiency that follows dilatation of a valve ring) or genetically encoded defects (e.g., Marfan syndrome).
Mechanical degeneration leads to increased collagen deposition by valvular mesenchymal cells and to a relative decrease in the size of the spongiosa. In addition, shear forces produce focal endothelial denudation of the valvular surface, leading to the deposition of a platelet-fibrin coagulum that signals subendothelial fibrogenesis.
Valvular calcifications complicate both age-related fibrosis and postinflammatory valvulitis. The structural distortions produced by scarring are further enhanced by calcifications that grow by accretion to be nodular and bulky, leading to valvular stenosis and insufficiency. In some cases, concomitant foci of ossification also develop.
Rheumatic fever, an immunologic complication of group A Streptococcus infection, is the most common cause of valvulitis. Rheumatic fever causes a pancarditis that affects the cardiac valves, myocardium, and pericardium. Histiocytic inflammation is accompanied by neovascularization and fibrosis. In the case of the atrioventricular valves the chordae tendineae become scarred and foreshortened, promoting the late hemodynamic consequences of valvular distortion. The end result is a scarred valve that is predisposed to further deformation by dystrophic calcification and functionally to both stenosis and insufficiency.
IE results from the growth of microorganisms on the endocardial surfaces of the cardiac valves or vascular endothelium. Most infections are caused by bacteria that have been entrapped in a mesh of fibrin and platelets previously deposited along an injured endocardial surface. These deposits are termed vegetations. They are friable and grow by accretion to be potentially bulky, depending on the cause, location, and duration of the infection. In the absence of antimicrobial treatment, effective healing of vegetations does not occur, and the risk of infected platelet–thrombi dislodging and traveling into the circulation is substantial.
In the past, IE was termed “bacterial endocarditis,” but with increased recognition that nonbacterial species, including fungi and rickettsia, can also cause endocarditis, the term IE is currently preferred. Modifiers, such as acute, subacute, or chronic, refer to the clinical course of the disease; however, they are imprecise and do not correlate well with the underlying pathology. Instead, IE should be considered a spectral disorder that can exhibit either an aggressive or an indolent course, depending on the circumstances of infection.
The once-universal mortality associated with IE has been substantially reduced by improved diagnosis, antimicrobial treatment, and aggressive surgical intervention. The current mortality rate ranges from 10% to 30%. In the first half of the 20th century, most cases of IE were complications of rheumatic mitral valvular disease. However, with decreased prevalence of rheumatic valvular disease and increased aging of the population, the senile fibrocalcific aortic valve has become an increasingly common presentation. New sources of infection include intravenous drug use and the widespread iatrogenic use of intravenous catheterization. Host immunosuppression in patients receiving corticosteroids and other immunosuppressant agents, human immunodeficiency virus (HIV)-1 infection, diabetes, renal failure, alcoholism, and cirrhosis all substantially increase the risk for development of IE.
Cardiac and vascular prostheses are potential nidi for infection and present a continued risk for IE after implantation. Right-heart pacemaker implantation can lead to infection along the pacemaker leads and the tricuspid valve. S. aureus and Staphylococcus epidermidis are the most common pathogens in the early (first 60 days) and late periods, respectively, after implantation. Left-ventricular assist devices are currently used in the treatment of intractable left ventricular failure and as a bridge to cardiac transplantation. The prevalence of IE associated with these devices ranges from 15% to 44%, and the diagnosis can be difficult to establish noninvasively. Enterococcus and Staphylococcus spp. are the most common infective agents, but fungi and low-virulence organisms also cause disease.
Experimental models have demonstrated that a catheter introduced into the right side of the heart of a rabbit or rat can cause endothelial injury to the tricuspid valve. Endocardial injury greatly increases the risk of developing IE when coupled with subsequent exposure to circulating bacteria ( Fig. 9.1 ). Endothelial injury exposes basement membrane proteins, including laminin, fibronectin, and vitronectin, which serve as adhesion molecules for bacteria. Activation of platelets and thrombus formation further promote bacterial adhesion. Bacterial binding to thrombus is followed by a lag period of several hours before bacterial proliferation is detected.
The risk of developing experimental disease is a function of the virulence and size of the bacterial inoculum and whether the injurious catheter is left in place or removed. For bacterial inocula introduced after catheter removal, the risk of developing IE decreases progressively with time. The histopathology of the valve shows healing of the catheter-induced lesion by reendothelialization, which appears to protect against subsequent bacterial colonization.
Other animal models of IE that do not require an indwelling catheter have been developed. In the guinea pig, electrocoagulation of the aortic valve followed by inoculation with S. aureus or Coxiella burnetii yields IE. In addition, electrical stimulation of the cervical vagus nerve can result in injury of the mitral valve and predisposes to IE with subsequent bacterial challenge.
Specific microbial factors have been examined in the pathogenesis of IE. Resistance by an S. aureus to a thrombin-induced microbicidal protein promotes IE. Fibronectin-binding proteins expressed by S. aureus facilitate binding to fibronectin and act as invasins. Organisms that do not bind fibronectin show decreased propensity to cause IE. Gelatinase/type IV collagenase enhances the virulence of Streptococcus gordonii , and an aggregation substance expressed by Enterococcus (formerly Streptococcus ) faecalis increases virulence in the catheter injury model.
Biofilm formation plays a critical role in the pathogenesis of IE and has important consequences with respect to its treatment. Bacteria can survive as isolated, free-living planktonic organisms or as stationary colonies associated with a substratum. Four criteria have been proposed for the biofilm origin of infection: adherence of pathogenetic bacteria to a substratum; presence of bacteria in clusters or colonies associated with either an endogenous or a host-derived matrix; localized infection; and resistance to antibiotic therapy despite sensitivity of the planktonic organism. Most, if not all, cases of IE associated with prosthetic surfaces meet these criteria. Bacteria within biofilms produce extracellular polymeric substances (EPSs)—slime-producing glycocalices that limit the accessibility of host humoral and cellular defenses, as well as antibiotics, to the organisms that are embedded in the matrix. Biofilms confer other advantages to the bacterial colonies as well. If the availability of growth requirements is limited, biofilm bacteria can convert to a slow-growing, stationary state, so-called persisters. Water channels form within the biomass and serve as a circulatory system through which nutrients are shared and waste products released.
The activities of the biofilm are coordinated by redundant interbacterial genetic signals. The properties of the biofilm make it virtually impossible to eradicate infection as long as the stationary phase persists. Organisms can spread along surfaces by means of a ripple effect or, alternatively, as detached clumps of organisms that can break free of the substratum matrix and travel to distant sites in the circulating blood. These mechanisms explain the local and distant spread of infection in IE.
Structural deformation of a cardiac valve can lead to local shear stresses and injury. Jet lesions produced by critically stenotic valves or shunts from the systemic to the pulmonary circulation increase the risk of IE. Bicuspid aortic valves are at risk because of their propensity to develop both stenosis and insufficiency. Insufficiency of the aortic valve also increases the risk of mitral valve endocarditis because regurgitant flow through an incompetent aortic valve causes fluttering and superficial injury to the anterior mitral leaflet. A comparable mechanism accounts for mural endocarditis along the ventricular septum in aortic insufficiency. In mitral insufficiency, vegetations may develop along jet lesions formed in the left atrium.
In the absence of primary involvement of the cardiac valve, other conditions, including mitral annular calcification and mural thrombi, become important sites of infection in IE. Left-to-right shunts due to congenital heart disease show a propensity to cause endocarditis at the maximum site of the jet stream. Ventricular septal defects are at high risk, whereas secundum atrial septal defects rarely develop IE. However, endocardial cushion or septum primum defects that involve the mitral apparatus and patent ductus lesions are at risk. Approximately 5% of patients with hypertrophic cardiomyopathy and asymmetric septal thickening develop IE.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here