Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pulmonary artery catheter (PAC) is a physical object creating a conundrum. Since its introduction in the 1970s, the PAC has been simultaneously hailed for its ability to provide physiologic data not easily obtainable by other means and condemned as a useless and potentially harmful invasive monitor. Very little hard data support continued use of the PAC, and some data support avoiding its use altogether. Despite considerable controversy over the clinical use and safety of the PAC, the PAC provides an important means of invasive intensive care unit (ICU) monitoring. This chapter reviews the history of right-sided heart catheterization; examines the basis of insertion, data collection, interpretation, and troubleshooting; and explores the clinical evidence for and against the use of pulmonary artery (PA) catheterization and determination of resuscitation in critically ill surgical patients.
The PAC was introduced in the late 1960s, approved for clinical use in 1970, and quickly incorporated into the armamentarium of the critical care physician. By 1999, 1.5 million catheters were sold, and presumably used, each year in the United States. The PAC is classified by the Food and Drug Administration (FDA) as a class II device and requires general and specific controls to reasonably assure safety and effectiveness. Even as considerable controversy and political debate over its use continues, the catheter has never been considered a lifesaving device and, as a result, is exempt from licensing and required evaluation.
Since its development, the PAC has enjoyed growing use and acceptance as a monitoring device. Indeed, its popularity has followed closely the advent of critical care as a specialty, and thus is considered by many a primary tool of the critical care physician. After years of use, and with little data supporting its benefits, concerns about the overall use and safety of the PAC appeared in several publications beginning in the late 1980s. Gore and colleagues examined 3000 patients with acute myocardial infarction (MI) and the relationship of outcome to PA catheterization. This study of over 3000 patients with acute MI reported a higher mortality rate in patients with hypotension who received a PAC (42% vs. 32%). Higher mortality rate was also reported in the subgroup of patients with congestive heart failure (CHF) who received a PAC (44% vs. 25%). In addition, patients who received a PAC had longer hospital stays. Several observational and retrospective studies quickly followed with similar results. Many in the critical care community discounted these trials, believing that PAC placement was more common in patients with more severe illness, with attributable mortality rates. A 1990 Canadian trial was the first to attempt to prospectively study the use of PAC in critically ill patients. In what was to become a recurring theme, however, the study failed because of a 35% exclusion rate, with many clinicians refusing to randomize their patients. A lack of clinical consensus regarding PAC use followed, and the PAC enjoyed continued widespread use until the debate was reignited in 1996. Connors et al studied PAC use in 5735 critically ill ICU patients, carefully matching illness severity and other confounding variables between the PAC and control groups. Ultimately, this study found that patients treated with PAC had increased 30-day mortality rate, mean cost, and ICU stay. Subset analysis was unable to identify any group of patients who actually benefited from PAC.
As a result of these and similar data, and in the same issue of the Journal of the American Medical Association , Bone and Dalen called for a National Heart, Lung, and Blood Institute (NHLBI) randomized prospective clinical trial to test the efficacy and safety of the PAC. They went on to spark considerable controversy by suggesting that the Food and Drug Administration FDA issue a moratorium on the use of the PAC until such time that the safety and use be measured in an appropriate clinical trial. In response to this call for a moratorium, both NHLBI and Society of Critical Care Medicine (SCCM) consensus conferences were convened in 1997. The Pulmonary Artery Catheter Consensus Conference Consensus Statement was published later that same year. This statement did not support a moratorium on PACs, citing adequate level IV evidence to support the possibility of benefit of PAC in patient groups including MI and trauma patients, but conceded that appropriate clinical trials were needed to measure its use and safety. The NHLBI conference came to similar conclusions. Many trials followed, all with limited numbers of patients or low randomization rates, increasing the likelihood of a type II error and selection bias. Additionally, protocols and therapies backed by quality evidence were rarely used.
In 2003, a Canadian group published the first prospective randomized study with sufficient patient enrollment to have statistical power and authority. In this study, 1994 patients were randomized to surgery without a PAC versus with a PAC. The authors found there were no differences in hospital survival and in 6- and 12-month survival. There was, however, an increase in the number of pulmonary embolism (PE) events, with eight reported in the catheter group versus none in the observation group. The authors concluded that no benefit from PA catheterization could be found in elderly high-risk surgical patients. Although this study was important in that it was the first to randomize a significant cohort of patients, the randomization rate, 52%, remained low. Furthermore, it included a disproportionate number of older, critically ill patients, excluding younger trauma or septic patients. Other trials have followed that also showed mixed results. More recently Shah et al performed a meta-analysis of 13 randomized clinical trials between 1985 and 2005. This study totaling 5051 patients was performed using a random effects model to estimate the odds ratio for death, hospital days, and pressors and found no difference between patients with and without a PAC.
A 2006 Cochrane Collaboration meta-analysis of all randomized controlled trials in adults comparing management with and without a PAC was published. Importantly, of the 12 trials identified, only one reported any systematic differences in the care provided to the patients other than the presence of a PAC. However, the authors described possible sources of bias for each of the studies: allocation bias, performance bias, attrition bias, and detection bias. The majority of patients in the surgical trials underwent routine major surgery and most of the trials were small and single center; only one study was adequately powered. The authors ultimately concluded there was no evidence of benefit or harm from a PAC.
In the same year, the Fluid and Catheter Treatment Trial (FACTT) was published in which the ARDSnet trialists randomized 1000 patients with acute lung injury/acute respiratory distress syndrome (ALI/ARDS) to management using the PAC or the less invasive alternative, the central venous catheter. Additionally, patients were randomized to receive a conservative versus a liberal fluid management strategy. Trauma patients composed less than 10% of all patients. One of the strengths of this trial was the detailed, explicit management protocol, ensuring standardization of treatment across 36 centers. Additionally, all study personnel underwent extensive training in measurement and interpretation of PAC-derived data. Pressure tracings also underwent centralized review. Similar to other studies, this trial was characterized by a low enrollment rate: 91% of screened patients were excluded. In this study, the PAC-guided therapy did not improve survival and was associated with twice as many catheter-related complications (predominantly arrhythmias). Serious complications were rare and there were no deaths related to insertion.
No randomized prospective trial to date, however, has been able to demonstrate an overall benefit to PA catheterization in critically injured patients. In data limited to trauma patients, there is some recent evidence to show a benefit for PAC use in the injured. A retrospective database study of over 53,000 patients drawn from the National Trauma Data Bank showed a reduction in mortality rate in older patients and patients with higher injury severity scores. Overall, PAC use was shown to be beneficial in patients with severe shock with a base deficit of 11 or more, injury severity scores higher than 25, and age over 61. This large nonrandomized cohort database study is the first and only study to show a clear benefit from PAC use in the severely injured patient.
One bias confounding PAC use and study are disparate factors that affect which patients are treated with the PAC. In 2000, Rapoport et al reported a comprehensive look at the characteristics of PAC use. This group retrospectively examined 10,217 patients in 34 ICUs in the United States and showed that full-time ICU staffing was associated with a decreased likelihood of PAC use. Catheter use was associated with white race/ethnicity and private insurance. Patients admitted to a surgical ICU were two times more likely to have a PAC. This study is revealing in that it is indicative of the lack of an established protocol and the presence of an established bias in PAC placement.
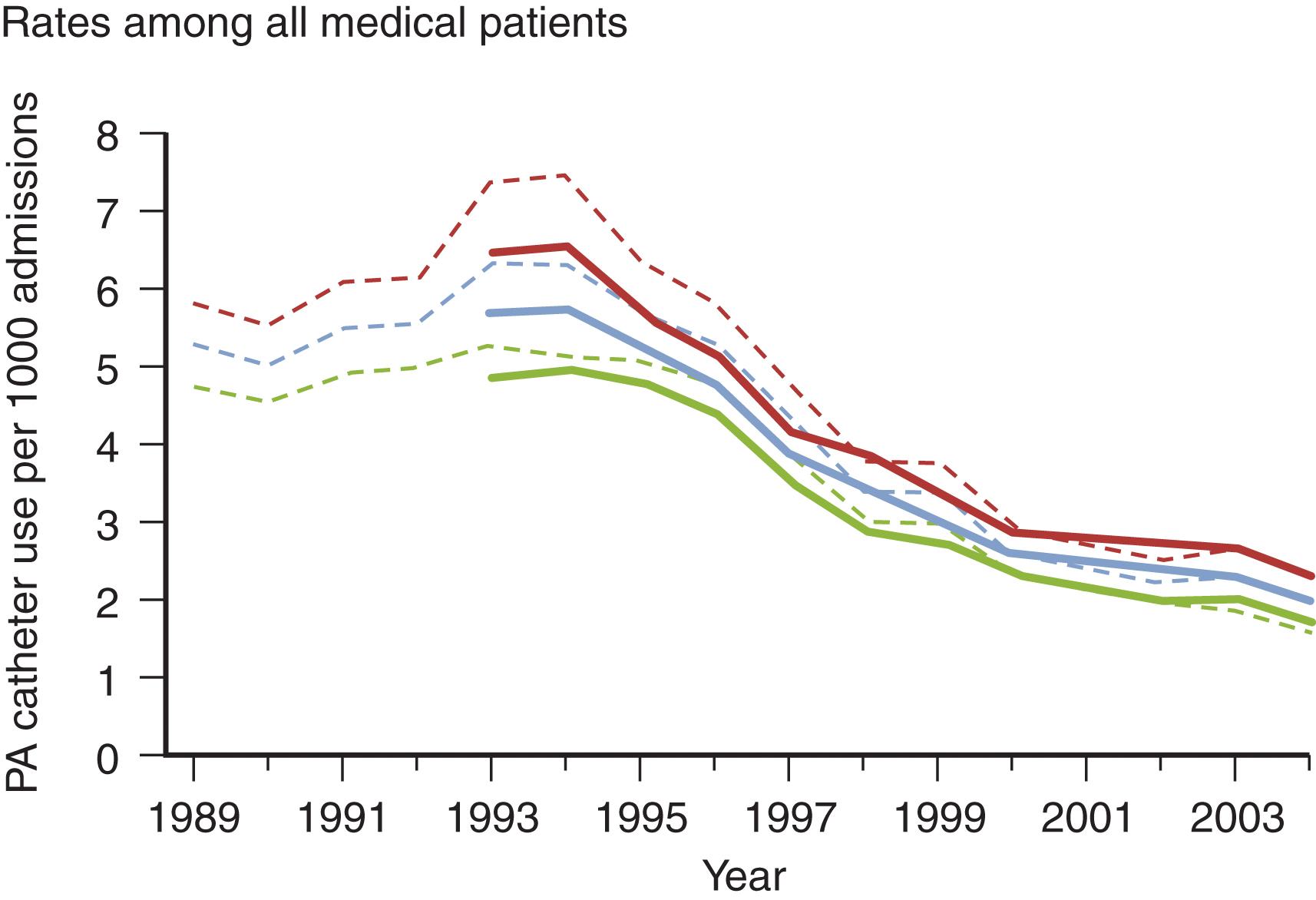
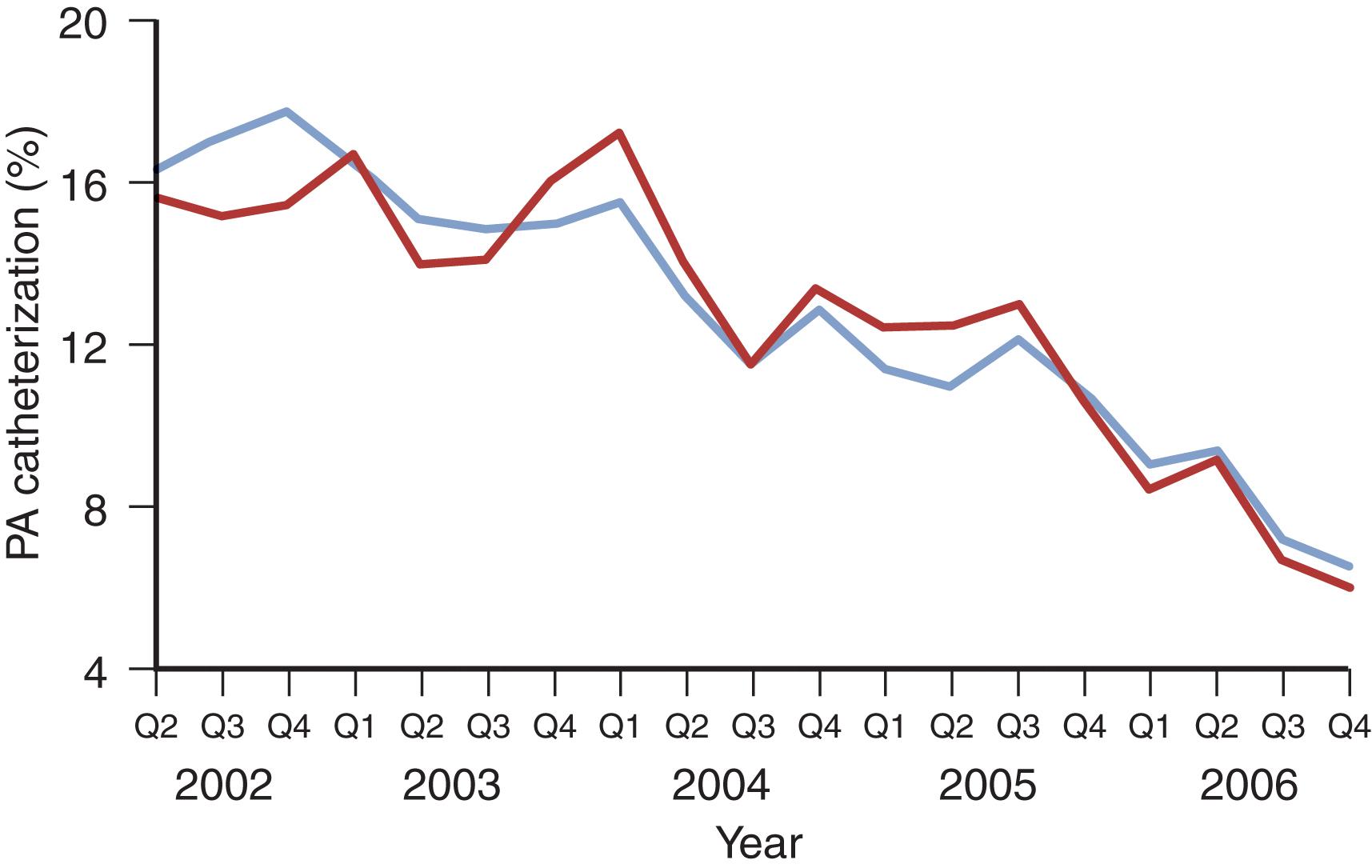
Another factor confounding many PAC studies is the absence of quality-control measures to ensure accurate interpretation of PAC-derived data. A survey of critical care practitioners revealed that 47% of physicians were unable to correctly determine the pulmonary artery occlusion pressure (PAOP) from a pressure trace with a clearly identified end-expiration. Even more concerning, 61% did not recognize an unambiguous indication that the introducer had been placed in a systemic artery. Another study reported that “experienced physicians’ accuracy in predicting confirmed-valid PAOP tracings using waveform analysis was no better than a coin toss.” Similarly, a survey of critical care nurses revealed that only 57.7% could correctly read a PAOP tracing on a clear pressure trace. Considering the mounting concerns regarding inaccurate measurement, incorrect interpretation of data, absence of protocols to guide action based on PAC-derived data, and scant evidence of patients benefiting from those actions, it is not surprising that enthusiasm for PAC use has waned. PAC use decreased by 65% from 1993 to 2004 and decreased more than 50% by 2006 ( Figs. 1 and 2 ). This declining experience has further skewed the risk-benefit ratio as the current and future generations of ICU physicians will be more prone to error in insertion, interpretation, and appropriate application of PAC-related data.
PACs are commonly 100 cm long with an exterior French diameter of 7.5. Available with or without a heparin or antibiotic coating, they commonly contain latex rubber, an important consideration in latex-allergic patients. The 7.5-F diameter is further separated into three lumens. At the far distal end of the catheter is the PA port, which is used to transduce PA pressure and draw mixed venous blood. Just proximal to this port is a 1.5-mL balloon, which facilitates both the “floating” of the catheter and is also used to distally occlude the PA to measure PAOP. Another side infusion port, used for instillation of fluid, vasoactive agents, and medications, is located 15 cm proximal to the end of the catheter. Proximal to the side infusion port is the right atrium (RA)/central venous pressure (CVP) port, which is designed to be positioned at the vena cava/RA junction. This RA/CVP port is transduced to measure the CVP. Like the proximal infusion port, it can also be used as an infusion port for medication and fluids.
Along with ports that allow for pressure transduction and fluid infusion, the PAC also incorporates a thermal coil and proximal and distal thermistors for the measurement of cardiac output . Cardiac output is calculated by measurement of the change in temperature of blood between a proximal thermistor and a more distally placed thermistor. Traditionally, a cooled fluid bolus was injected proximally and the temperature of this bolus (now slightly warmed by blood flow) was measured by the thermistor at the tip of the catheter. Using formulas discussed later in this chapter, the cardiac output could be calculated. Most modern catheters now use a proximally placed thermal coil incorporated into the catheter which gently warms blood proximally and extrapolates from the temperature difference proximally and distally to give a continuous calculation of cardiac output. (A more detailed discussion of cardiac output monitoring appears later in the chapter.)
Insertion of the catheter is done through the gasketed introducer port of a Cordis catheter. Full sterile technique is paramount in order to reduce infection rates. We commonly place the introducer catheter and the PAC sequentially with one sterile preparation and setup, which prevents contamination at the introducer gasket as well as the necessity of repreparing of a previously placed introducer. At times, however, this cannot be avoided, or a PAC will be placed through a previously located introducer line. In this instance, the introducer catheter and surrounding skin should be prepared widely with chlorhexidine preparation. In all cases, wide sterile preparation with chlorhexidine and full sterile precautions including gown, hat, mask, and sterile gloves are essential. Any break in sterile technique has been shown in many studies to significantly increase the infection rate, and implementation of the preceding precautions has been reported to reduce the infection rate to near zero. Another tip is to prepare widely. We cannot stress this seemingly trivial point too strongly. Opening, preparing, and inserting a PAC results in an often-unwieldy octopus of catheter, tubing, and transducers that have to cross through a sterile to unsterile transition zone. Wide preparation of all or most of the bed with a sterile half-sheet facilitates easy handling and reduced risk of contamination. Once an introducer catheter is in place, the PAC is removed from the packaging and the proximal end is passed to a nurse or assistant who will connect and flush the catheter ports. At this time, the transducer is connected, zeroed, and tested. The balloon is also tested. Placement of the catheter sheath allows future adjustments without additional sterile preparation.
Constant verbal communication between the floater and the assistant is essential. Once the tip of the catheter is passed through the introducer and into the blood vessel, it must be advanced only when the balloon is inflated (up), and conversely it must never be withdrawn unless the balloon is deflated (down). The slang term “floating a swan” refers to the fact that the catheter advances by floating along with the blood flow. Direct advancement can cause vascular injury or perforation. A constant communicative banter—”Balloon up?” “Balloon up”; “Balloon down?” “Balloon down”—can prevent injuries such as arterial, cardiac, and PA rupture.
Initially the catheter is inserted to the 10- to 15-cm mark, allowing the balloon to pass through the introducer. Once the catheter tip is safely past the introducer, the balloon is inflated and the catheter slowly advanced by feeding slack catheter and allowing the catheter to be pulled downstream by the blood flow. A combination of clinical experience, length of catheter inserted, and careful attention to the monitored transducer tracing allows successful placement. As the catheter tip enters the vessel, the initial pressure reading will be the CVP, which first transduced at less than 15 to 25 cm depending on patient size and insertion site. With another 5 to 10 cm of advancement, the catheter passes into the right atrium and the transducer will register a distinct right arterial waveform. Another few centimeters of advancement passes the catheter tip through the tricuspid valve (around 30 cm) and the easily recognizable spike of right ventricle (RV) pressure will register on the monitor. Slow careful advancement of another 5 to 10 cm will pass the catheter into the PA (tracing) and will soon wedge the catheter to produce a flattening of the waveform and characteristic wedge tracing.
Often several attempts to ensure proper wedging are required. When an RV waveform is not evident after 35 to 45 cm or no wedge tracing occurs after the catheter has advanced 10 to 15 cm past the RV tracing, the balloon should be deflated, and the catheter pulled back to the RA or RV. Once a distinct tracing identifies the catheter location, another careful attempt at reinsertion and wedging can begin. When the catheter is inserted and wedged, the pulmonary capillary wedge pressure (PCWP) is noted and the balloon deflated. The catheter is locked into place and the sterile covering advanced to cover the full length of the catheter. Placement of the catheter is confirmed by a chest radiograph.
Serious complications can occur during central venous access, the PA catheterization process, balloon inflation, and subsequent care. Complications during central venous access (including arterial puncture and pneumothorax) are common to all access procedures and are not unique to PAC insertion. The most common complication, arrhythmia, is often temporary and minor and generally occurs during balloon flotation through the right ventricle. Other more serious complications such as cardiac perforation, pulmonary artery rupture, pericardial tamponade, sustained right bundle branch block, and catheter knotting are extremely rare (less than 1%) in experienced hands with modern, softer catheters. Thromboembolic events and catheter infection have become increasingly uncommon with the introduction of heparin-bonded catheters and prompt removal of the PAC once it is no longer indicated.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here