Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The past decade has seen rapid development in cardiovascular imaging technologies coupled with novel clinical applications. Noninvasive cardiac imaging technologies currently allow for assessment of cardiac morphology, function, perfusion, and metabolism. Both cardiac computed tomography (CCT) and cardiac magnetic resonance (CMR) imaging have interesting and unique advantages compared with alternate imaging modalities. Understanding the applications and limitations of these modalities will permit their optimal and efficient use in the future.
For decades, investigators sought to develop new technologies that would allow rapid noninvasive imaging of the heart. One such technology that has evolved rapidly in the past several decades has been CCT. CCT now permits visualization of the coronary arteries and lumen, as well as providing assessment of cardiac function, valvular structures, prosthetic materials, the pericardium, left atrial anatomy, congenital heart disease, pulmonary arterial and venous anatomies, and diseases of the aorta.
Imaging the heart and coronary arteries with CT is an extremely challenging undertaking for several reasons, and requires more sophisticated hardware and software analysis tools than are required for imaging other body regions. Major difficulties arise because coronary arteries are relatively small moving structures with branches of interest in the range of 2 to 4 mm in diameter. The coronary arteries show rapid cyclic motion throughout the cardiac cycle—essentially moving in three dimensions with each heartbeat. Furthermore, when the subject breathes, the heart and vessels move within the chest due to diaphragmatic motion.
Several major advances in recent years have dramatically improved the resolution of CCT images. Improvement in scanner technology in the past 5 years includes increased Z-axis coverage, now up to 16 cm with some vendors, which means that the entire heart can be imaged in a single gantry rotation. In addition, gantry rotation speeds have also continued to improve; they currently approach 0.2 seconds per 360-degree rotation, which results in improved temporal resolution. ECG gating has also improved. These improvements have significantly decreased overall patient radiation exposure, which is now substantially less than traditional SPECT and is often less than 2 mSv. As the United States continues to deal with the obesity epidemic, vendors have started manufacturing CT scanners with increasingly larger bore diameters and table weight limits to better accommodate these patients.
Over its relatively short history, several different CT technologies have been used for cardiac imaging. Electron beam CT (EBCT), which was initially introduced in the mid-1970s, uses an electron source reflected onto a stationary tungsten target to generate x-rays, which allows for rapid scan times. EBCT is well suited for cardiac imaging because of its high temporal resolution (50–100 ms), with an estimated slice thickness of 1.5 to 3 mm and the ability to scan the heart in a single breath hold. The primary use of this technique was for coronary arterial vessel wall calcium volume and density, which generated a patient-specific score. Coronary calcium scores are independent of other traditional cardiac risk factors in the prediction of cardiac events, and as such, can be considered an excellent biomarker for the presence of coronary artery disease (CAD) and the risk of future cardiac events.
EBCT has since been largely supplanted by multidetector CT (MDCT) technology, which involves a mechanically rotated x-ray source within a cylindrical gantry with a collimated detector located 180 degrees opposite that permits the simultaneous acquisition of more data (“slices”). Collimator rows that measure 0.625 mm provide for markedly increased spatial resolution and for complete acquisition of data during one breath hold. However, what MDCT offers is improved spatial resolution to make coronary CT angiography (CTA) feasible.
Coronary CTA was initially performed using MDCT machines capable of obtaining only four to eight slices per scan. As technology has advanced, 256-slice (and higher) scanners are now available that allow acquisition of higher resolution images without the requirement for long breath holds or extremely slow heart rates. It is currently recommended that CTA be performed using a minimum 64-slice scanner. Newer technology allows up to 320 to 512 anatomic slices to be simultaneously acquired. With a minimal slice thickness of 0.6 mm, an entire heart can be imaged in a single heartbeat. Even with newer generation scanners, temporal resolution at its best now approaches 140 ms, and cannot reach what can be obtained routinely in a cardiac catheterization laboratory, which is closer to 33 ms. To overcome the necessity of a slow heart rate, one vendor has placed two x-ray sources in the scanner (termed dual-source imaging) at 90-degree angles to one another. This technology offers an improved temporal resolution even with heart rates approaching ≥100 beats/min.
In the past, for coronary CTA using a single x-ray source scanner, it was typically necessary to obtain images with heart rates at <65 beats/min. An oral or intravenous β-blocker usually was given to patients to slow the heart rate. Newer generation scanners have somewhat lessened these rigid low heart rate requirements, although the adage still holds when imaging the heart that slower tends to be better. Coronary CTA requires intravenous administration of a contrast agent to opacify the lumen of the coronary arteries. The intravenous contrast agents used for CTA carry the same dose-dependent risks in patients with renal dysfunction as contrast agents used for cardiac catheterization, as well as the risk of an allergic reaction to iodine. Respiratory motion is minimized by patient breath holds up to 10 seconds, depending on scanner generation and patient body size. Data acquisition varies somewhat based on scanner type. The most common data acquisition protocol uses a spiral mode involving continuous data acquisition during constant rotation of the x-ray tube while the patient is simultaneously and continually advanced on the table through the x-ray gantry. To minimize radiation exposure, data acquisitions can be performed in sequential mode (step and shoot). This involves acquisition of single transaxial slices sequentially as a patient is advanced stepwise through the scanner.
Excessive cardiac motion can lead to blurring of the contours of the coronary vessels. For this reason, a regular heart rate is necessary for optimal imaging of the coronary arteries. Relative contraindications to performing CTA include the presence of frequent ectopic beats or atrial fibrillation. Coordinating data acquisition and analysis to the cardiac cycle involves either prospective triggering or retrospective gating. In prospective triggering, data are acquired in late diastole, based on simultaneous ECG recordings. In retrospective gating, data are collected during the entire cardiac cycle. Postprocessing then allows only data from specific periods of the cardiac cycle to be used for image reconstruction.
Coronary artery calcium (CAC) has emerged as a robust marker of subclinical atherosclerosis. CAC scoring uses no contrast and readily detects calcium because of its high x-ray attenuation coefficient (or CT number) measured in Hounsfield units (HUs) ( Fig. 11.1 ). The Agatston scoring system assigns a calcium score based on the maximal CT number and the area of calcium deposits. Recently, analysis of several large clinical data sets confirmed that the CAC score is a robust predictor of coronary events, particularly in the asymptomatic patient population, independent of traditional risk factors. In at least one study, the calcium score was more predictive than C-reactive protein and standard risk factors for predicting CAD events.
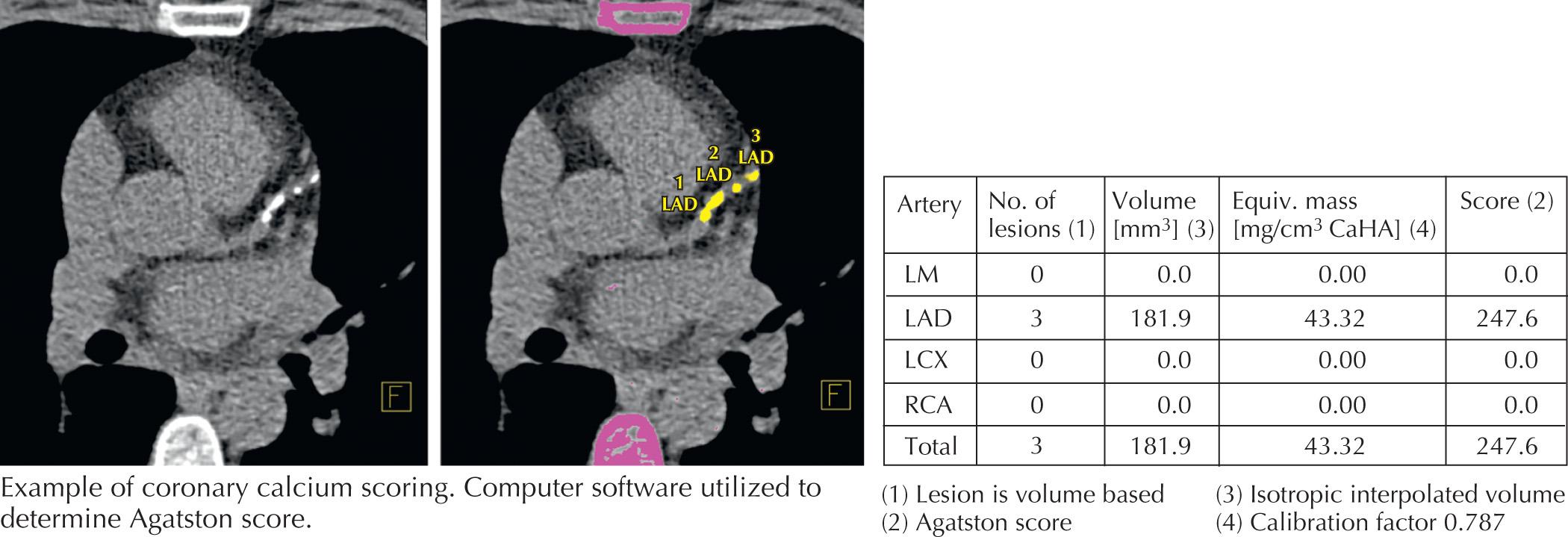
The coronary calcium score is derived by identifying coronary arterial tree segments that have attenuation characteristics (HU) greater than a certain value (100–130 depending on software and patient size) that correlate with the attenuation due to calcium. These calcified lesions are scored by size and density, with a weighting factor for increasing density. Discrete lesions are scored separately, and the density of calcium within each lesion is graded from 1 to 4 according to the HU. The sums of all the lesions are totaled to arrive at a single coronary calcium score. In general, the higher the score, the greater the amount of calcified plaque within the arterial tree. There is a positive correlation of cardiac events with this score. However, it is important to remember that exclusively noncalcified coronary artery plaques have been reported in up to 4% of asymptomatic patients.
The Multiethnic Study of Atherosclerosis (MESA) Group published a series of articles that suggests that the calcium score is an independent risk factor for cardiac events. Also, MESA's website ( https://www.mesa-nhlbi.org/calcium/input.aspx ) has the capacity to allow comparison of the calcium score of an individual patient against their large database. This score takes into account age, sex, and race, and generates a percentile compared with the database studies. The presence of a high calcium score may prompt clinicians to use more aggressive therapy as if they were reclassified into a higher risk group or to convince patients who are reluctant to take drugs (e.g., statins) to take their disease more seriously. Recent studies have shown that a zero CAC score demonstrated an annual event rate in asymptomatic subjects of only 0.11% (10-year risk of only 1.1%). Among asymptomatic patients, the incidence of abnormal nuclear stress testing is 1.3%, 11.3%, and 35.2% for calcium scores <100, 101 to 400, and >400, respectively. Studies that have looked at serial calcium scanning have noted that calcified plaque progression is significantly and independently associated with a worse overall prognosis.
Chest pain is a common clinical problem and one of the most common complaints of individuals presenting for urgent medical evaluation. One of the most important, life-threatening causes of chest pain is CAD. Although cardiac catheterization is the best method to assess for the presence of hemodynamically significant obstructive CAD, it is impractical as a screening test. It is invasive and costly, can be especially dangerous in some patients, and when used broadly as a screening tool, it is performed on a substantial number of patients who have no significant CAD and/or whose chest pain is unrelated to cardiac causes. Approximately 10% to 25% of the patients who are referred for invasive coronary angiography are found to have normal coronary arteries or nonobstructive CAD. Furthermore, several meta-analyses have demonstrated poorer prognosis and increased numbers of hard cardiac endpoints with nonobstructive CAD compared with normal coronary arteries.
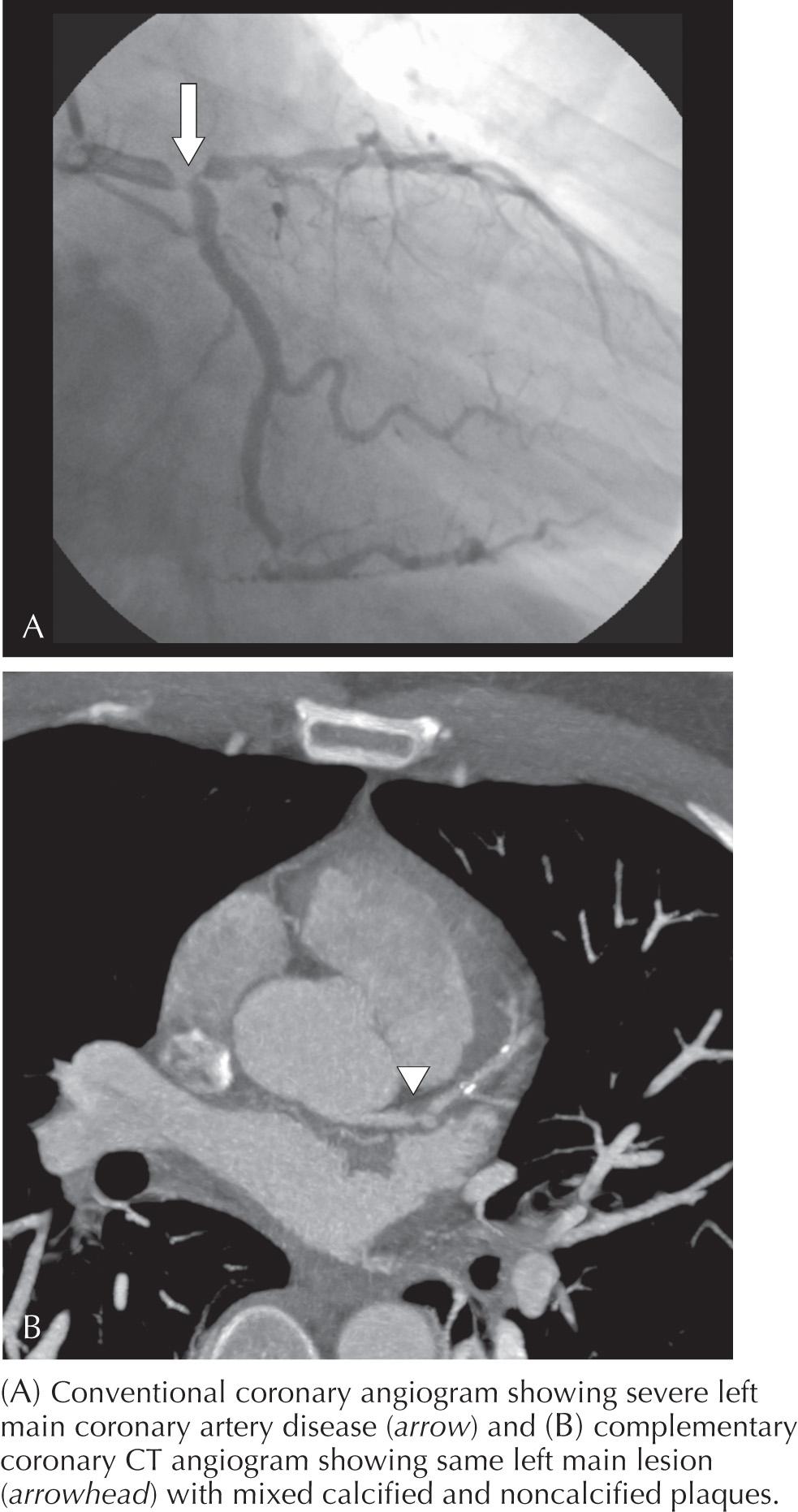
CT angiography (CTA) uses intravenous contrast to differentiate the vessel lumen from the vessel wall. In 2010, the American College of Cardiology and many other societies with interests in cardiac imaging put together recommendations of appropriateness criteria for the use of cardiac CTA (CCTA) that include appropriate ( Box 11.1 ) and inappropriate uses of this technology. The most common appropriate use is diagnostic study of patients presenting with chest pain who do not have significant ECG changes or elevated cardiac biomarkers, but who do have an intermediate probability of CAD ( Fig. 11.2 ). At experienced centers with careful data acquisition, sensitivities range from 83% to 99% and specificities range from 93% to 98%, with remarkably high estimated negative predictive values (95%–100%), indicating that CCTA may be used to reliably rule out the presence of significant flow-limiting coronary atherosclerotic disease. It should be pointed out that CCTA would be inappropriate for patients at high risk for or with other indications of cardiac ischemia, such as elevated biomarkers or significant ECG changes. Those patients should be referred immediately for invasive imaging. Currently, there is no indication for performing CCTA in asymptomatic patients. The appropriateness criteria definitively recommend against the use of CCTA in the asymptomatic population until further evidence suggests that it would positively affect outcomes.
Low/intermediate pretest probability of CAD
Uninterpretable ECG or unable to exercise
Evaluation of suspected coronary artery anomalies
Uninterpretable or equivocal stress test (exercise, perfusion, or stress echo)
a Preoperative planning for transcatheter structural heart interventions.
Assessment of complex congenital heart disease, including anomalies of coronary circulation, great vessels, and cardiac chambers and valves
Evaluation of coronary arteries in patients with new-onset heart failure to assess etiology
Evaluation of cardiac masses
Patients with technically limited images from transthoracic echocardiogram, MRI, or transesophageal echocardiogram
Evaluation of pericardial conditions
Evaluation of pulmonary vein anatomy before invasive radiofrequency ablation for atrial fibrillation
Noninvasive coronary vein mapping before placement of biventricular pacemaker
Noninvasive coronary arterial mapping, including internal mammary artery before repeat cardiac surgical revascularization
Evaluation of suspected aortic dissection or thoracic aortic aneurysm
Evaluation for suspected pulmonary embolism
CAD , Coronary artery disease.
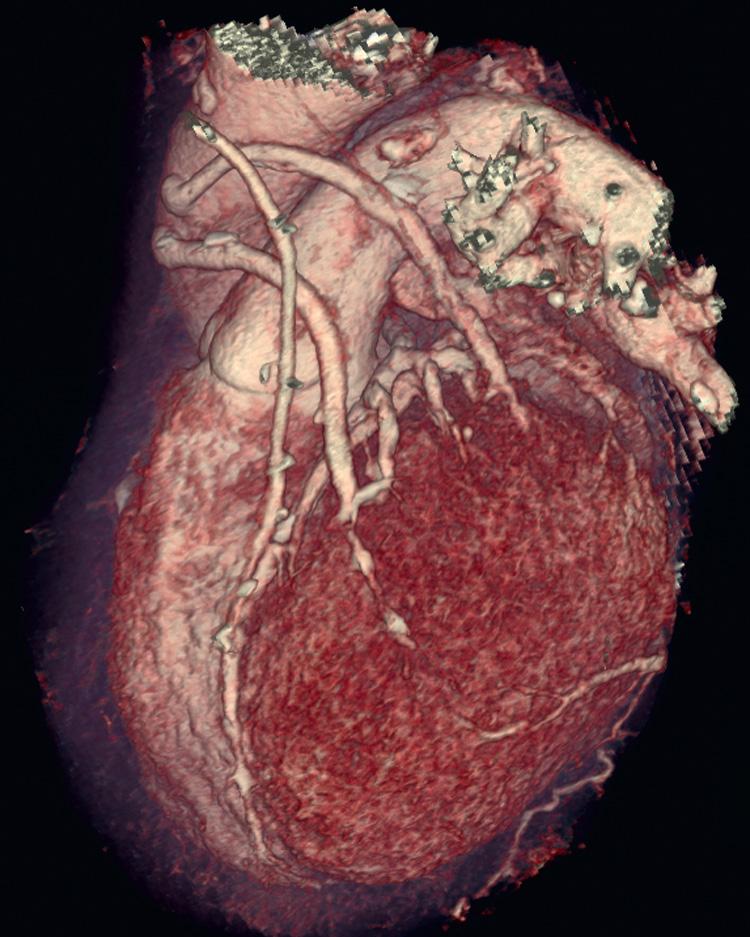
Bypass graft imaging is more easily accomplished than coronary artery imaging because of the larger size of bypass grafts (particularly saphenous vein grafts) and less rapid movement of bypass grafts compared with native coronary arteries. The patency or occlusion of grafts can be determined by the presence or absence of distal target vessel contrast enhancement ( Fig. 11.3 ). Imaging internal mammary grafts are often more difficult because of artifacts caused by metallic clips near the grafts. Imaging of coronary artery stents is challenging because of artifacts caused by metal that can obscure visualization of the coronary artery lumen. Studies that evaluated CCTA to assess in-stent restenosis have been somewhat disappointing, yielding sensitivities of 54% to 83%. Stents <3.0 mm in diameter are much more likely to be difficult to evaluate. An additional important application of CCTA is in patients with congenital abnormalities of their coronary arteries, including anomalous coronary artery origins and the presence of intramyocardial bridges (coronary arteries that, for a portion of their course, are not epicardial but rather covered by a layer of myocardial tissue).
The past several years have seen several new developments in CCTA technology that have focused not just on the coronary artery anatomy, but have also evaluated functional data simultaneously on the presence or absence of myocardial ischemia. There have been several publications in the past few years that have focused on the use of CCT for myocardial perfusion imaging, transluminal attenuation gradients, and corrected coronary opacification indexes and fractional flow reserve calculated from resting CCTA data.
Through appropriate timing of intravenous chamber contrast enhancement, extensive cardiac morphological and functional information can be obtained by CCT. Myocardial mass and ventricular function can be estimated with a high level of accuracy. CCT can also provide a detailed morphological picture of the left atrium and left atrial appendage anatomy, which is information that can be useful before planned catheter ablation for atrial fibrillation or device implantation in the left atrial appendage. Three-dimensional anatomic data obtained by CCT can be fused with electrical mapping data acquired in the electrophysiology laboratory, which greatly facilitates the procedure.
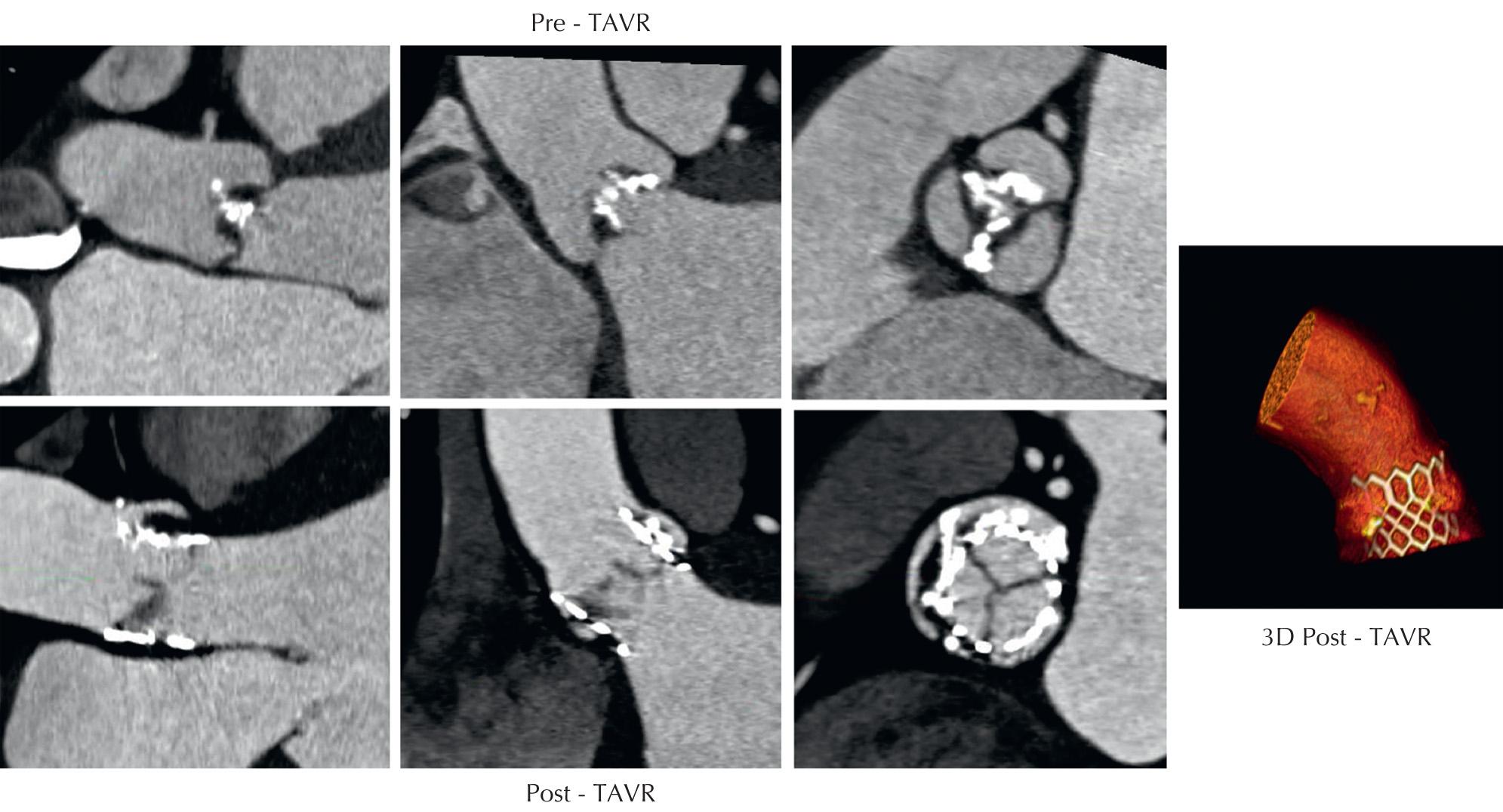
The past several years has seen an explosive growth in the use of transcatheter therapies for the treatment of valvular and structural heart disease. Advancement in transcatheter aortic valve replacement ( Fig. 11.4 ) technology has relied heavily on appropriate aortic annular sizing data obtained with CCT. Studies have demonstrated that accurate three-dimensional aortic annular sizing assessment with CCT results in reduced paravalvular regurgitation, which has been associated with increased mortality following these procedures. In 2012, the Society of Cardiovascular Computed Tomography released guidelines for the use of CCT before TAVR. Furthermore, several new therapies on the horizon for mitral and tricuspid valve repair will also rely heavily on preprocedural CCT for accurate sizing and morphological assessment.
Assessment of complex congenital heart disease, including anomalous coronary artery circulation, great vessels, cardiac chambers, and valves are all appropriate indications for CCT. Specific indications include shunt assessment, aortic geometry in coarctation or Marfan syndrome, partial or total anomalous pulmonary venous return, and pulmonary artery visualization in patients with cyanotic heart disease.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here