Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
Sudden cardiac arrest (SCA), and its common consequence sudden cardiac death (SCD), is the common cardiac pathway for death. There are a diverse array of cardiac and noncardiac causes and mechanisms underlying the development of SCA and SCD. While SCA and SCD are most likely deterministic processes rather than true stochastic processes, the inability to delineate these processes presents a challenge in addressing this major public health problem. Current estimates for out-of-hospital SCDs are still in the range of 380,000/year in the United States alone, , with an additional 200,000 in-hospital cardiac arrests. Generally, its impact is defined by the “rule of 50s”: SCD accounts for 50% of all cardiovascular deaths, approximately 50% of all SCDs are unexpected first expressions of a cardiac disorder, and it often strikes during the victim’s productive years, accounting for up to 50% of years of potential life lost due to heart disease. Despite recognition of an association between forewarning symptoms of syncope and SCD dating to Hippocrates around 400 bc , advances in prediction, prevention, and management of unexpected SCA and SCD did not begin to emerge until approximately 50 years ago. It is anticipated that the major insights into causes, pathophysiology, and preventive and management strategies developed during the past few decades will continue to evolve .
SCD is not a uniform entity resulting from a single precipitating diagnosis. SCD is natural death from cardiac causes heralded by abrupt loss of consciousness within 1 hour of the onset of an acute change in cardiovascular status. As such detailed information is often lacking, various definitions of SCD include up to a 24 hour period and death during sleep. Moreover, at least half of SCDs are unwitnessed, providing substantial uncertainty to the terminal events. Preexisting heart disease may or may not have been known to be present, but the time and mode of death are unexpected. This definition incorporates the key elements of natural, rapid, and most importantly unexpected death by a cardiac cause or mechanism. It consolidates previous definitions that have conflicted, mainly because the most useful operational definition of SCD in the past differed for clinicians, cardiovascular epidemiologists, pathologists, and scientists attempting to define pathophysiologic mechanisms. As the epidemiology, clinical expression, causes, and mechanisms began to be understood, these differences merged.
To satisfy clinical, scientific, legal, and social considerations, four temporal elements must be considered: (1) prodromes, (2) onset, (3) cardiac arrest, and (4) biologic death ( Fig. 70.1 ). Because the proximate cause of SCA is an abrupt disturbance in cardiovascular function resulting in loss of consciousness due to cessation of cerebral blood flow, any definition must recognize the brief time interval between onset of the mechanism directly responsible for cardiac arrest and the consequent loss of blood flow. The 1-hour definition primarily refers to the duration of the “terminal event,” which defines the interval between the onset of symptoms signaling the pathophysiologic disturbance leading to cardiac arrest and the onset of the cardiac arrest itself. Based on human centrifuge studies carried out during the early years of the space program, the time between abrupt cessation of cerebral blood flow and loss of consciousness can be 10 seconds or less.
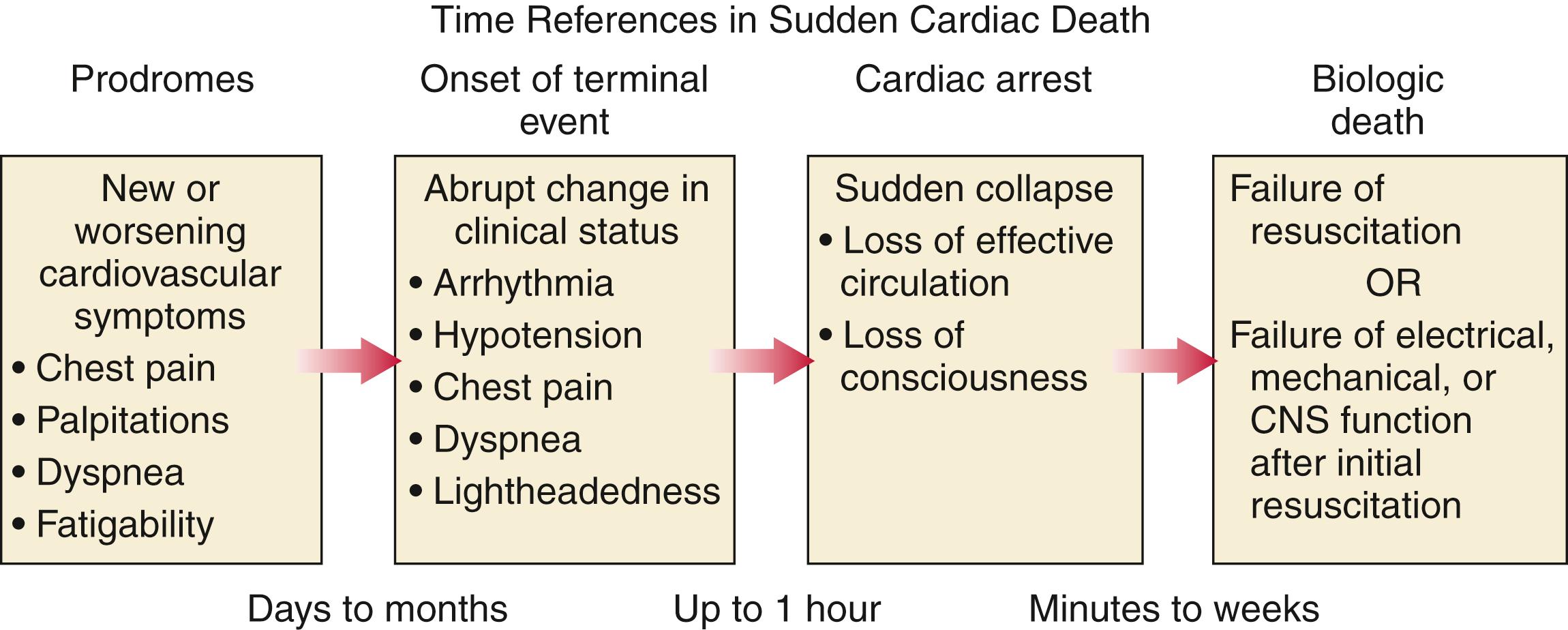
Prodromes, occurring weeks or months before an event, are generally predictors of an impending cardiac event, but not specific for SCA itself. The same premonitory signs and symptoms may be more specific for imminent cardiac arrest when they begin abruptly. Sudden onset of chest pain, dyspnea, or palpitations and other symptoms of arrhythmias often precede the onset of cardiac arrest and define the onset of the 1-hour terminal event period that brackets the cardiac arrest. The fourth element, biologic death, is an immediate consequence of cardiac arrest, unless there is a successful intervention, and usually occurs within minutes. The generally accepted clinical-pathophysiologic definition of up to 1 hour between onset of the terminal event and biologic death requires qualifications for specific circumstances. For example, since the development of community-based interventions and life support systems, patients may now remain biologically alive for a long period after the onset of a pathophysiologic process that has caused irreversible damage and will ultimately lead to death. In this circumstance, the causative pathophysiologic and clinical event is the cardiac arrest itself rather than the factors responsible for the delayed biologic death. Thus death remains defined biologically, legally, and literally as an absolute and irreversible event timed to cessation of all biologic functions, but most studies link the definition of SCD to the cardiac arrest rather than to a biologic death that occurs during hospitalization after cardiac arrest or within 30 days. Finally, forensic pathologists studying unwitnessed deaths continue to use the definition of sudden death for a person known to be alive and functioning normally 24 hours before, and this remains appropriate within obvious limits. Among the precautions is the recognition that not all sudden deaths are cardiac in origin.
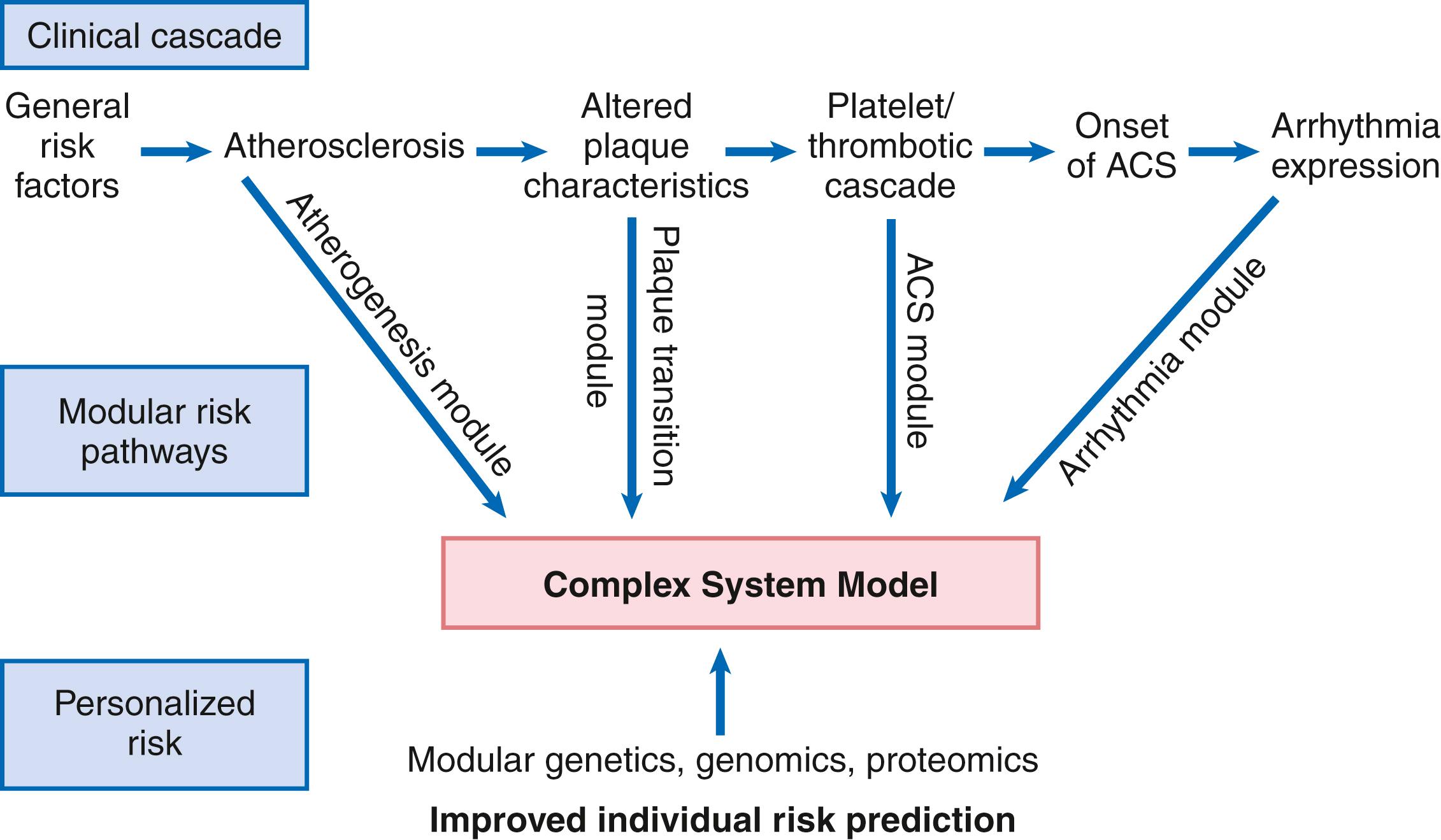
Epidemiologic studies of SCD are difficult to interpret for both theoretical and practical reasons. There are persisting inconsistencies about the definition and challenges in accessing data and adjudicating individual cases in data sets, in determining pathophysiologic mechanisms, and in making distinctions between population risk and individual risk. In addition, the fact that SCA leading to SCD has short-term dynamics superimposed on a long-term static or dynamic substrate introduces unusual epidemiologic complexities, including long-term risk prediction based on the evolution of atherogenesis, myocardial hypertrophy, and ventricular muscle dysfunction over time and modulation by transient (short-term) variables such as ischemia, hemodynamic shifts, atherosclerotic plaque disruption and thrombosis, and autonomic variations. The differences between chronic disease evolution and transient events call for different forms of epidemiologic modeling ( Table 70.1A ). Furthermore, the emerging field of genetic epidemiology adds another dimension for consideration, and there is a need to focus on interventional epidemiology , a term coined to define the population dynamics of therapeutic outcomes.
| A.Utility For Risk Prediction | |||
|---|---|---|---|
| Strategy | Examples | Measures | Utility |
| Conventional risk factors | Framingham risk index | Prediction of evolution of disease | High for the population Low for the individual |
| Anatomic disease screening | Coronary calcium score and CT angiography | Identification of abnormal coronary arteries | High for anatomic identification Low for individual event prediction |
| Clinical risk profiling | Ejection fraction, stress testing, imaging techniques | Extent of disease | High for small, high-risk subgroups Low for large, low-risk subgroups |
| Transient risk predictors | Inflammatory markers; thrombotic cascade | Prediction of unstable plaques; acute changes in vascular status | Uncertain feasibility |
| Personalized risk predictors | Familial/genetic profiles | Individual SCD expression | Improved clinical precision |
| B.Pathophysiologic Epidemiology | |||
| Substrate-based risk | Coronary heart disease State of epicardial and intramyocardial vessels Myocardial infarction Myopathy, infiltration, inflammation, valvulopathy Hypertrophy; myocardial fibrosis |
||
| Expression-based risk | Left ventricular dysfunction and heart failure Metabolic abnormalities Autonomic dysfunction |
||
| Mechanism-based causes | VF/pulseless VT PEA Asystole |
||
In reference to risk for SCD from coronary heart disease, clinical categories ranging from general population risk to personalized risk profiling are paralleled by the partition of risk predictors into the pathophysiologic categories of substrate-based risk and expression-based risk ( Table 70.1B ). Substrate-based risk refers to prediction of the evolution or identification of vascular or myocardial substrates that establish risk for SCD (i.e., atherogenesis, scar patterns, remodeling) and to quantification of these risks. It should not be perceived as limited to anatomic features because risk substrates may exist at a molecular level, such as those characterized by the ion channelopathies that are associated with SCD. In contrast, expression-based risk refers to the identification of mechanisms and pathways that contribute to the clinical manifestation of the risk established by the substrate. This category includes plaque transition and acute coronary syndromes (plaque disruption and thrombogenesis) and their potential for specific expression as an arrhythmic event in susceptible individuals.
The worldwide incidence of out of hospital cardiac arrest (OHCA) leading to SCD is variable and difficult to estimate because numbers vary as a function of reporting practices and the prevalence of coronary heart disease in different countries (see Chapter 2 ). The annual number of out-of-hospital SCDs in the United States is derived from multiple sources, such as retrospective death certificate data, American Heart Association (AHA) statistical updates based on data from the National Center for Health Statistics, and extrapolations from population-based surveillance. Data from large surveillance studies, such as the Resuscitation Outcomes Consortium (ROC), have contributed additional insight into the subtleties of data collection and interpretation.
Statistical analyses from the same death certificate data sources have ranged from fewer than 250,000 SCDs annually when the etiologic definition is limited to coronary heart disease (International Classification of Diseases, ninth edition [ICD-9], classifications 410-414) to more than 460,000 SCDs/year when all causes are included. Extrapolations from community-based sources set nationwide figures at fewer than 200,000,000 SCDs annually. Because these broad ranges and the reported regional differences in incidence and outcomes of cardiac arrest suggest that an accurate number can be found only by performing carefully designed prospective epidemiologic surveillance studies, the most widely cited estimates remain in the range of 380,000 SCDs annually, as suggested in the 2020 AHA statistical update. These figures suggest an overall incidence of between one and two deaths/1000 persons in the general population. The annual number of emergency rescue (EMS) assessed OHCAs in people of any age in the United States in 2015 was 356,000.
The temporal definition of sudden death strongly influences epidemiologic data. Retrospective death certificate studies have demonstrated that a temporal definition of sudden death of less than 2 hours after the onset of symptoms results in 12% to 15% of all natural deaths being defined as “sudden” and almost 90% of all natural sudden deaths having cardiac causes. In contrast, application of a 24-hour definition of sudden death increases the fraction of all natural deaths falling into the sudden category to more than 30% but reduces the proportion of all sudden natural deaths resulting from cardiac causes to 75%.
Prospective studies have demonstrated that approximately 50% of all deaths caused by coronary heart disease are sudden and unexpected and occur shortly (instantaneous to 1 hour) after the onset of symptoms. Because coronary heart disease is the dominant cause of both sudden and non-SCDs in the United States, the fraction of total cardiac deaths that are sudden is similar to the fraction of deaths from coronary heart disease that are sudden, although there does appear to be geographic variation in the fraction of coronary deaths that are sudden. , It is also of interest that the age-adjusted decline in mortality from coronary heart disease in the United States during the past half-century has not changed the fraction of coronary deaths that are sudden and unexpected. , Furthermore, the decreasing age-adjusted mortality does not imply a decrease in absolute numbers of cardiac or sudden deaths because of the growth and aging of the U.S. population and the increasing prevalence of chronic heart disease, including heart failure. Yet a substantial 44% decline in the SCD rate in patients with heart failure and reduced ejection fraction enrolled in clinical trials from 1995 to 2014 has been observed. It does not appear that the cumulative SCD burden in absolute numbers is tracking the age-adjusted decrease in cardiac deaths that has been evolving during the past 40 to 50 years. In a prospective study of SCD victims in Finland undergoing autopsy between 1998 and 2012, while the proportion of SCDs resulting from coronary heart disease decreased, the concomitant increase in SCDs attributable to nonischemic causes maintained a relatively stable rate of SCD. The landscape of SCD over time is further complicated by the suggested shift of mechanisms of out-of-hospital SCA from ventricular tachyarrhythmias to pulseless electrical activity/asystole, in part as a consequence of ICD efficacy.
Three factors are of primary importance for identification of populations at risk and consideration of strategies for prevention of SCD: (1) the absolute numbers and event rates (incidence) among population subgroups ( Fig. 70.2A ), (2) the clinical subgroups in which SCDs occur ( Fig. 70.2B ), (3) competing risks, and (4) the time dependence of risk.
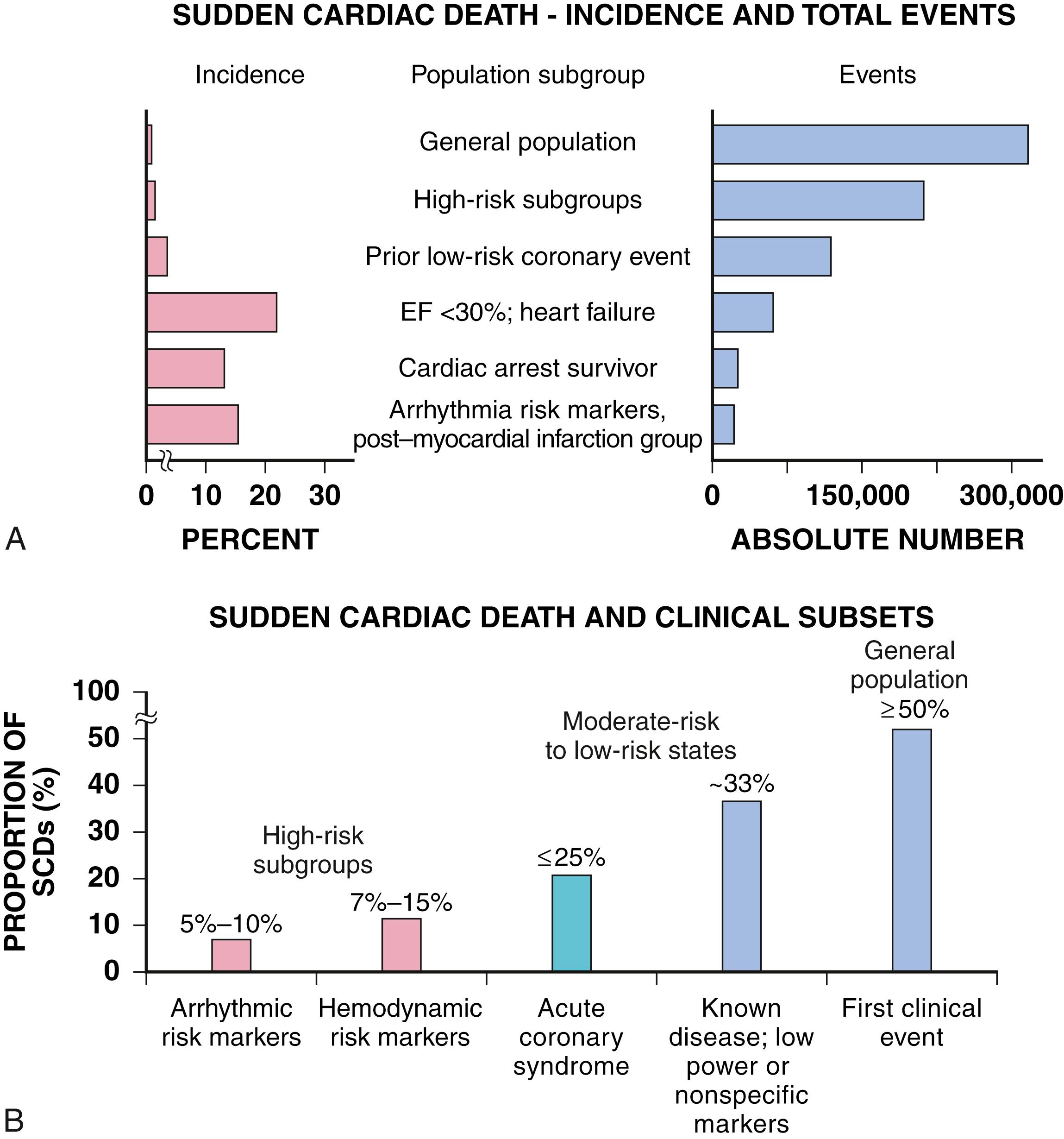
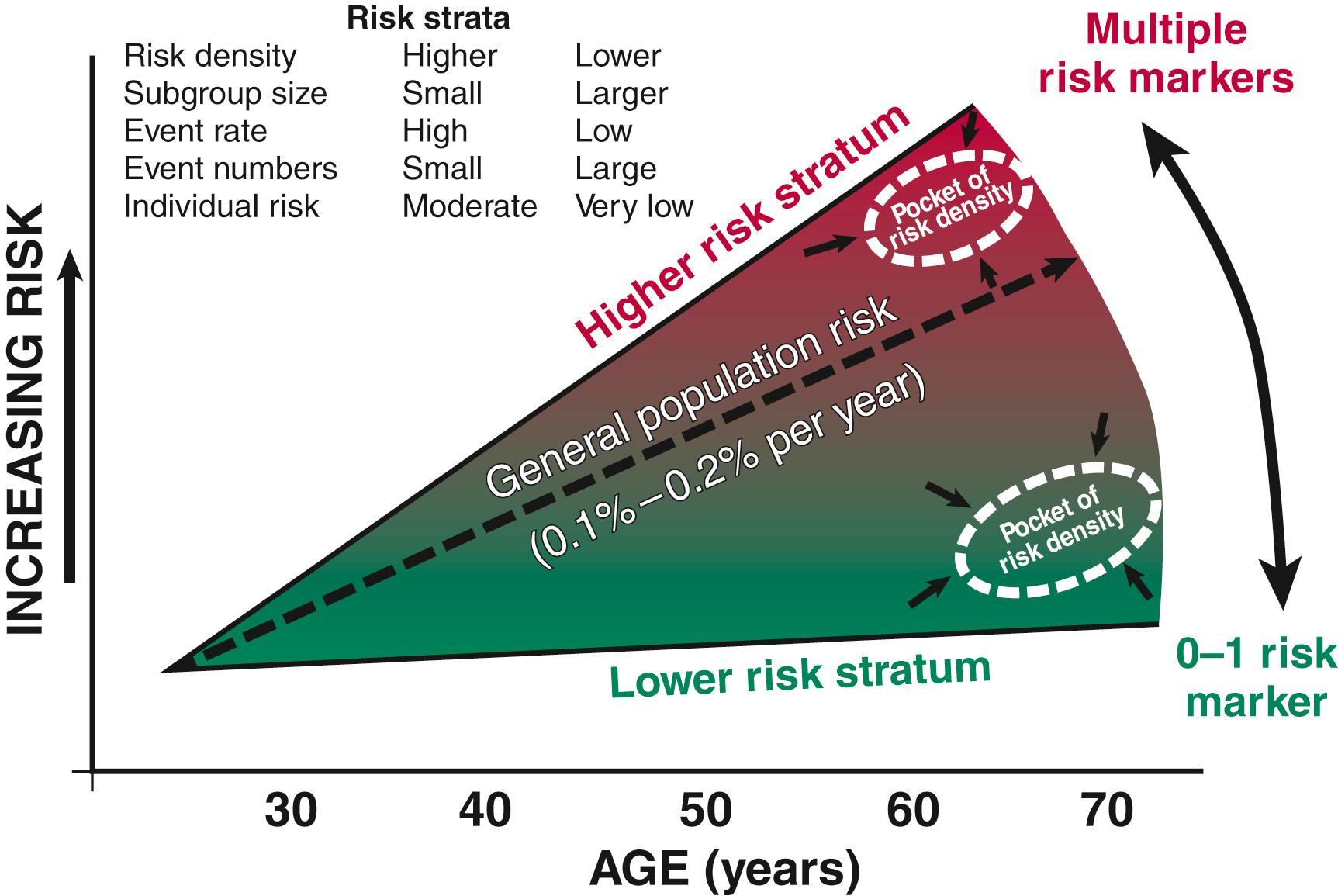
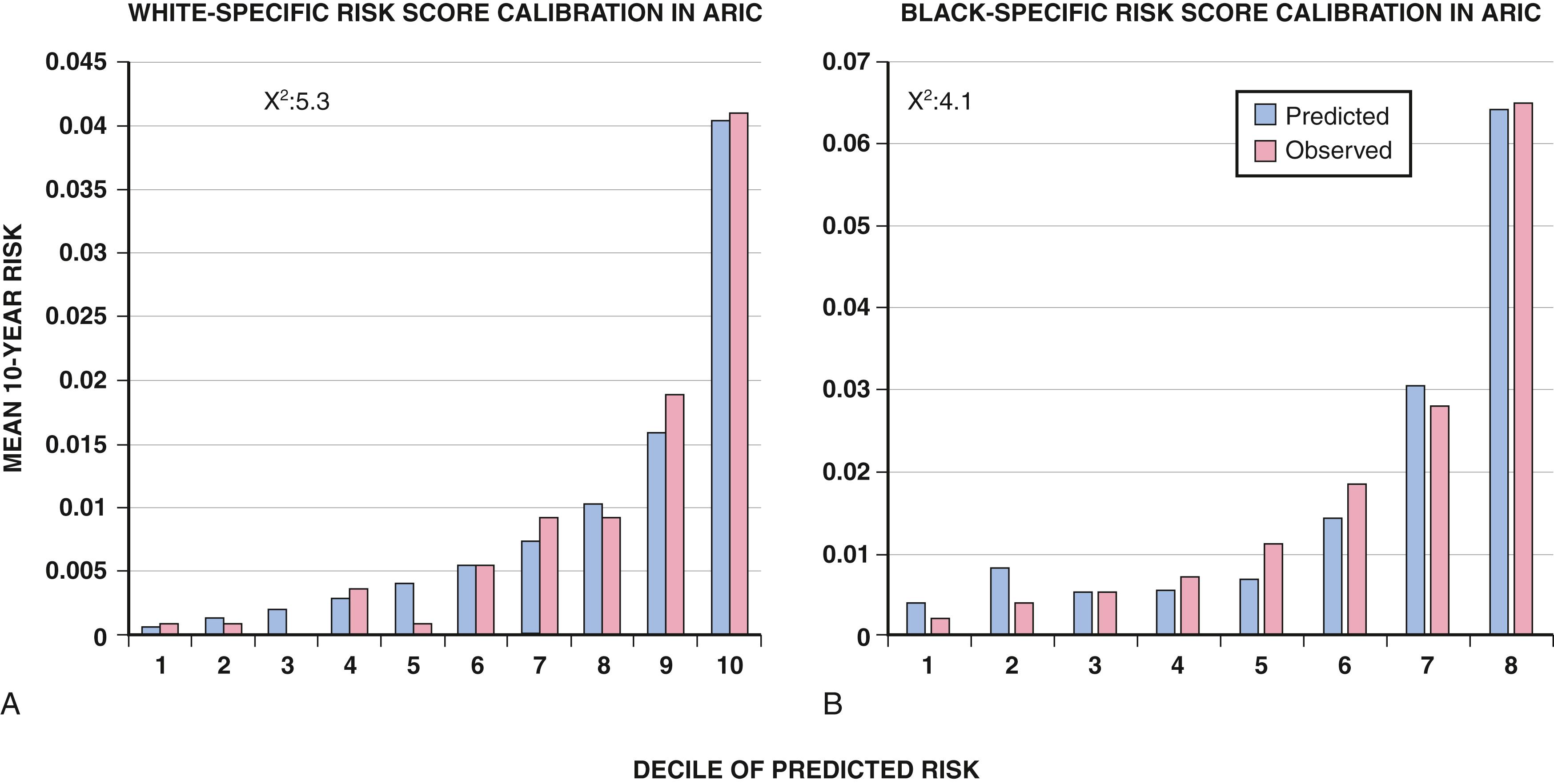
When the estimated 380,000 SCDs that occur annually in the United States are viewed as a global figure for an unselected adult population 35 years and older, the overall incidence is calculated to be in the range of 0.1% to 0.2%/year (1 to 2/1000 population; Fig. 70.2A ). This general population includes the large proportion of SCDs that occur as a first clinical manifestation of previously unrecognized heart disease, as well as SCDs that can be predicted with somewhat greater accuracy in higher risk subgroups ( Fig. 70.2B ), as it is impractical to plan an intervention designed for the general population that would be applied to the 999/1000 who do not have an event to reach and possibly influence the 1/1000 who will experience an event. Figure 70.2A highlights this problem by expressing the incidence (percent per year) of SCD among various subgroups and comparing the incidence figures with the total number of events that occur annually in each subgroup. Thus, despite the large absolute number at risk in the general population and the impact of preventive interventions on population risk for coronary artery disease, the precise ability to identify these individuals for targeted therapy for SCD prevention is an unmet challenge. The cost and risk-to-benefit uncertainties limit the nature of such broad-based interventions and demand a higher resolution of risk identification. Two fundamental approaches for attacking this challenge can be followed: a general population strategy targeting prevention of acquired risk factors such as obesity (primordial prevention) and primary prevention by control of manifest risk factors and a more focused individual risk strategy based on identification and intervention in small subsets of the general population with a high density of risk ( Fig. 70.3 ). Several population-based risk scores have been reported that provide an initial attempt to identify subsets within the general population who have heightened risk of SCD. , Based on data from the Atherosclerosis Risk in Communities (ARIC) Study, a race-adjusted risk score incorporating readily available variables from the medical record—age, sex, total cholesterol, lipid-lowering and hypertension medication use, blood pressure, smoking status, diabetes, and body mass index—provided good internal discrimination, which held up with external validation in the Framingham study.
On moving from the total adult population to a subgroup at higher risk because of the presence of selected coronary risk factors, there may be a 10-fold or greater increase in the annual incidence of events, with the magnitude being dependent on the number and types of risk factors operating in specific subgroups. Higher resolution is desirable and can be achieved by identification of more specific subgroups. However, the corresponding absolute number of deaths may become progressively smaller as the subgroups become more focused ( Fig. 70.2A ), unless the gradients of risk are steep. Applying the ARIC-based risk score to the U.S. National Health and Nutrition Examination Survey, it was noted that there is an exponential rise in calculated risk by decile with a 10-fold gradient in risk between the highest and lowest decile. Up to half of all SCDs attributable to coronary heart disease are first clinical events, and another 20% to 30% occur in subgroups of patients with known coronary heart disease who are profiled to be at relatively low risk for SCD on the basis of current clinically available markers ( Fig. 70.2B ). It is notable that when the ARIC-based risk score was applied to a general population, those in the highest risk deciles had a very high burden of risk factors. Moreover, these patients may have occult heart disease, providing an opportunity for early detection and treatment. The principle of a high proportion of SCDs occurring as first events or in previously asymptomatic individuals with a high burden of risk factors also applies to SCD in the young (under age 35 years).
Temporal elements in risk for SCD have been analyzed in the context of both biologic and clinical chronology. In the former, epidemiologic analyses of risk for SCD in populations have identified three patterns: diurnal, day of the week, and seasonal. General patterns of heightened risk during the morning hours, on Mondays, and during the winter months have been described. , An exception to the diurnal risk pattern is SCD in sleep apnea, in which the risk tends to be nocturnal.
Ambient temperature is an environmental factor associated with risk for SCD. , Both excessive cold and excessive heat have been linked to risk for cardiac arrest, although the studies did not determine whether temperature extremes are associated with ventricular tachyarrhythmias versus other mechanisms of cardiac arrest. However, significant cooling of the core temperature can lengthen the time course of repolarization of ventricular myocardium and prolong the QT interval, while sweating associated with increases in core temperature can alter electrolyte balance. Elevated temperature is a risk for SCA in patients with Brugada syndrome (see Chapter 63, Chapter 67 ). Another environmental variable, short-term ambient air pollution conditions, has been associated with increased risk of OHCA, but not consistently. , , In patients with an implantable defibrillator, exposure to fine particulate material correlated significantly to episodes of ventricular tachycardia and fibrillation. A sympathetically mediated mechanism has been postulated.
In the longer term, risk for SCD is not linear as a function of time after changes in cardiovascular status. Survival curves after major cardiovascular events, which identify risk for both SCD and total cardiac death, usually demonstrate that the most rapid rate of attrition occurs during the first 6 to 18 months after an index event. Thus, there is a time dependence of risk that focuses the potential opportunity for maximum efficacy of an intervention during the early period after a cardiovascular event. Even though the rate of attrition decreases after the early spike in mortality, a secondary delayed increase in risk occurs in post–myocardial infarction (MI) patients 2 to 5 years after an index event, probably related to ventricular remodeling and heart failure.
The incidence of sudden death has two peak ages: within the first year of life (including sudden infant death syndrome [SIDS]; see Chapter 82 ) and between 45 and 75 years of age. Among the general populations of infants younger than 1 year and middle-aged or older adults, the incidence is surprisingly similar. In adults older than 35 years, the incidence of SCD is in the range of 1/1000 persons/year ( Fig. 70.4A ), with an age-related increase in risk over time as the prevalence of coronary heart disease increases in parallel with advancing age.
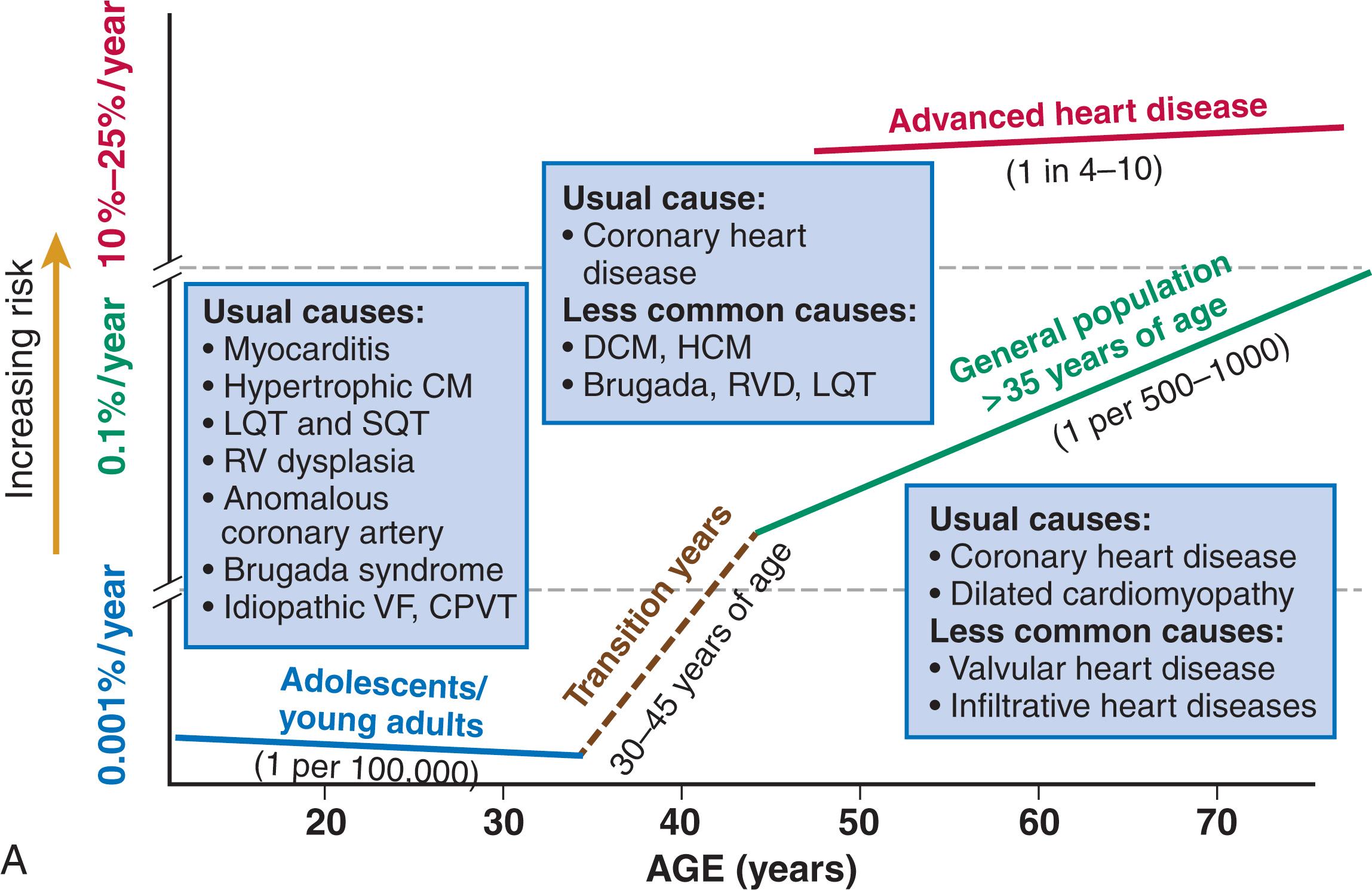
The incidence in infants is 73/100,000 person-years and is most commonly associated with complex congenital heart disease, and the incidence in children and adolescents is approximately 4 to 6/100,000 person-years versus 125/100,000 person-years in adults ( Fig. 70.4A ). One study demonstrated that approximately 40% of SCDs in this age category were unexplained, based on the absence of an autopsy or premortem clinical diagnosis, but postmortem genetic studies identified a likely cause in 27% of such cases that underwent studies.
In contrast to incidence, however, the proportion of deaths caused by coronary heart diseases that are sudden and unexpected decreases with advancing age. In the 20- to 39-year age group, approximately 75% of the deaths attributable to coronary heart disease in men are sudden and unexpected, with the proportion falling to approximately 60% in the 45- to 54-year age group and hovering close to 50% thereafter. Age also influences the proportion of any cardiovascular cause among all causes of natural sudden death in that the proportion of coronary deaths and of all cardiac causes of death that are sudden is highest in the younger age groups whereas the fraction of total sudden natural deaths that result from any cardiovascular cause is higher in the older age groups. At the other end of the age range, only 19% of sudden natural deaths in children between 1 and 13 years of age have cardiac causes; the proportion increases to 30% in the 14- to 21-year age group.
In the transition age range between adolescence and young adulthood (to the age of 25 years) and in the middle and older ages (beginning at 35 years of age), coronary heart disease emerges to its position as the dominant cause of SCD. However, rare disorders, such as hypertrophic cardiomyopathy (HCM), Brugada syndrome, long-QT syndrome, right ventricular dysplasia, and idiopathic myocardial fibrosis, are significant contributors to the distribution of causes of SCD in this age group.
A number of studies comparing racial differences in the relative risk for SCD in white and Black with coronary heart disease in the United States had yielded conflicting and inconclusive data. More recent studies demonstrate a higher risk for cardiac arrest and SCD in Blacks than in whites. Data from the ARIC and REGARD studies show that Blacks had an adjusted hazard ratio for SCD of 1.38 to 1.97 compared with whites. , SCD rates in Hispanic populations have been less well studied but do not appear to show increased risk (see Chapter 93 ).
SCD syndrome has a large preponderance in men relative to women during the young adult and early middle-age years because of the protection that women enjoy from coronary atherosclerosis before menopause ( Fig. 70.4B ). There is at least a threefold increase lifetime risk for SCD among men aged 45 to 65 years compared with women, with only mild attenuation of this accentuated risk at age 75 years. Even though the overall risk for SCD is much lower in younger women, coronary artery disease is the most common cause of SCD in women older than 40 years, and the classic coronary risk factors, including cigarette smoking, diabetes, use of oral contraceptives, and hyperlipidemia, all influence risk in women (see Chapter 91 ). In an autopsy study, the profile of women experiencing SCD differed significantly from men; women were older (70 versus 64 years), more commonly had nonischemic causes (28% versus 24%) and primary myocardial fibrosis (5.2% versus 2.6%), and more commonly had ECG evidence of left ventricular (LV) hypertrophy with or without repolarization abnormalities.
Familial patterns of risk for SCD, which result from known or suspected genetic variations, are emerging as important factors for risk profiling. This concept is generally applicable to both disease development and SCD expression in the common acquired disorders and in a specific sense to inherited arrhythmogenic conditions associated with SCD. The various genetic associations can be separated into four categories ( Table 70.2 ): uncommon inherited primary arrhythmic syndromes (e.g., long-QT syndromes, Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia or fibrillation; see Chapter 63 ), uncommon inherited structural diseases associated with risk for SCD (e.g., HCM, right ventricular dysplasia; see Chapter 52, Chapter 54 ), “acquired” or induced risk for arrhythmias (e.g., drug-induced long-QT interval or proarrhythmia, electrolyte disturbances), and common acquired diseases associated with risk for SCD (e.g., coronary heart disease, nonischemic cardiomyopathies; see Chapter 38, Chapter 39, Chapter 50 ). Genetic variants mapped to loci on many chromosomes are being defined as the molecular bases for these entities and associations.
| Genetically Based Primary Arrhythmia Disorders |
| Congenital long-QT syndrome, short-QT syndrome Brugada syndrome Catecholaminergic polymorphic VT/VF J wave syndromes Nonsyndromic VT/VF |
| Inherited Structural Disorders with Risk for Arrhythmic SCD |
| Hypertrophic cardiomyopathy Right ventricular dysplasia/cardiomyopathy |
| Genetic Predisposition to Induced Arrhythmias and SCD |
| Drug-induced “acquired” long-QT syndrome (drugs, electrolytes) Electrolyte and metabolic arrhythmogenic effects |
| Genetic Modulation of Complex Acquired Diseases |
| Coronary artery disease, acute coronary syndromes Congestive heart failure, dilated cardiomyopathies |
The multiple specific mutations at gene loci-encoding ion channel proteins associated with the various inherited arrhythmia syndromes (see Chapter 63 ) represent a major advance in the understanding of a genetic and pathophysiologic basis for these causes of sudden death. In addition, the role of modifier genes and mutation specificity in the severity of clinical phenotypes in long–QT interval syndromes (LQTS) and structural diseases such as HCM is of increasing interest. These observations may provide screening tools for individuals at risk, as well as the potential to devise specific therapeutic strategies. In a study of screening ECGs for long-QT in children entering first grade and those in the seventh grade, and subsequent genetic testing in those children who were positive, there was a suggestion that the incidence of inherited LQT was considerably higher (∼1/1000) at the age of 12 years (seventh graders) than earlier estimates from studies based on diagnoses from general clinical expressions. Moreover, the cumulative risk of SCA among those with unrecognized or untreated LQTS was reported to be 13% before the age of 40 years. In addition, gene loci identified by genome-wide association studies may also serve as candidates for investigation of the role of low-penetrance mutations or polymorphisms in SCD caused by more common conditions, such as coronary heart disease.
To the extent that SCD is an expression of underlying coronary heart disease, hereditary factors that contribute to risk for coronary heart disease operate nonspecifically for the SCD syndrome. Various studies have identified mutations and relevant polymorphisms along multiple steps of the cascade, from atherogenesis to plaque destabilization, thrombosis, and arrhythmogenesis, each of which is associated with increased risk for a coronary event ( Fig. 70.5 ). Several studies have suggested that SCD as the initial expression of coronary heart disease demonstrates familial clustering, including general population surveillance studies, family histories of cardiac arrest survivors in the community, studies of ventricular fibrillation (VF) during acute MI, and postmortem evaluation of SCD cases ( eTable 70.1 ).
| Study Site | Cohort | Controls | Family History Measure | Outcome |
|---|---|---|---|---|
| Seattle ∗ 1988-1994 |
EMS SCA subjects | Population matched | Hx of MI or PCA in first-degree relatives | 2.85 versus 1.96/1000/year RR = 1.57 (95% CI, 1.27-1.95) |
| Paris † 1967-1994 |
Population surveillance | Retrospective analysis | Hx of PCA in first-degree relatives | 18.6% versus 9.9% OR = 1.80 (95% CI, 1.11-2.88) |
| Netherlands ‡ 2001-2005 |
STEMI with VF | STEMI without VF | Hx of SCD in first-degree relatives) | 43.1% versus 25.1% OR = 2.72 (95% CI, 1.84-4.03) |
| Finland § 2000-2003 |
SCD with AMI AMI survivors |
Population controls | SCD or AMI in first-degree relatives without ASHD | SCD = 5.2%; AMI = 3.3% OR for SCD/AMI = 1.6 (95% CI, 1.2-2.2; P = 0.01) |
| SCD = 5.2%; controls = 2.3% OR for SCD/controls = 2.2 (95% CI, 1.6-3.0; P = 0.001) |
∗ Friedlander Y, et al. Family history as a risk factor for primary cardiac arrest. Circulation 1998;97:155.
† Jouven X, et al. Predicting sudden death in the population: the Paris Prospective Study I. Circulation 1999:99:1978.
‡ Dekker LR, et al. Familial sudden death is an important risk factor for primary ventricular fibrillation: a case controlled study in acute myocardial infarction patients. Circulation 2006;114:1140.
§ Kaikkonen KS, et al. Family history and the risk of sudden death as a manifestation of an acute coronary event. Circulation 2006;114:1162.
Risk prediction for SCD is far more challenging than simply profiling risk for coronary artery disease by means of the conventional risk factors for coronary atherogenesis (see Chapter 33, Chapter 34 ). While the latter is useful for identifying levels of population risk and some aspects of individual risk, it is not sufficient for distinguishing individual patients at risk for SCD from those at risk for other manifestations of coronary heart disease (see Chapter 35, Chapter 36, Chapter 37, Chapter 38, Chapter 39, Chapter 40 ).
Multivariate analyses of selected risk factors (e.g., age, sex, diabetes mellitus, blood pressure/hypertension, current smoking, body mass index, lipid lowering medication use) have demonstrated that the majority of all SCDs occur in the upper deciles of risk ( Fig. 70.6 ). It is likely that the interactions of multiple risk factors potentiates the sum of the individual risks. Comparison of risk factors in victims of SCD with those in people with any manifestation of coronary artery disease does not provide useful patterns to distinguish victims of SCD from the overall pool. However, a history of diabetes mellitus and a tendency to longer QTc intervals on random ECGs are suggested as potential markers of interest for prediction of SCD. ECG analyses by artificial intelligence merits investigation for SCD risk stratification (see Chapter 11 ). Familial clustering of SCD as a specific manifestation of the disease may lead to the identification of specific genetic abnormalities that predispose to SCD.
Hypertension is a clearly established risk factor for coronary heart disease and also emerges as a highly significant risk factor in the incidence of SCD (see Chapter 26 ). However, there is no influence of increasing systolic blood pressure levels on the ratio of sudden deaths to total coronary heart disease deaths. No relationship has been observed between cholesterol concentration and the proportion of coronary deaths that were sudden. Neither the electrocardiographic pattern of LV hypertrophy nor nonspecific ST-T wave abnormalities influence the proportion of total coronary deaths that are sudden and unexpected; only intraventricular conduction abnormalities are suggestive of a disproportionate number of SCDs, an old observation reinforced by data from some device trials that suggest the importance of QRS duration as a risk marker, but one with low individual predictive ability.
The conventional risk factors used in early studies of SCD are risk factors for the evolution of coronary artery disease. The rationale is based on two facts: (1) Coronary disease has been considered the structural basis for 80% of SCDs in the United States, and (2) coronary risk factors are easy to identify because they tend to be present continuously over time (see Fig. 70.5 ). However, there is evolving evidence that the anatomic consequences of coronary artery disease may not account for as large a proportion of SCDs in adults as previously estimated, with hypertension, LV hypertrophy, and myocardial fibrosis being identified as dominant anatomic/pathophysiologic factors. In a Finnish autopsy study of SCD victims, there was a decline in coronary artery disease with an increase in hypertensive heart disease with LV hypertrophy (and no coronary artery disease). A 10-year longitudinal study of clinical associations identified in hospitalized OHCA victims demonstrated a trend toward decreasing structural heart disease associations, paralleled by an increasing dominance of dynamic, transient pathophysiologic events. Transient pathophysiologic events are being modeled epidemiologically in an attempt to express and use them as clinical risk factors for both profiling and intervention. Nonetheless, data suggest that longitudinal and transient risk predictors may have their power blunted by clinical interventions, such as percutaneous coronary intervention during acute coronary syndromes and post-MI beta blocker therapy.
Identification of specific clinical markers of risk for SCD as a specific expression of both coronary heart disease and other cardiovascular disorders has been a goal for many years. LV ejection fraction has been the most popular of such markers for clinical trials but with limited sensitivity, encouraging investigators to seek additional markers. These additional markers are quite variable, depend on the underlying structural heart disease, and likely reflect the multifactorial basis for SCD. Functional metrics such as LV size, strain, and functional status all have demonstrated prognostic significance. Electrical abnormalities in depolarization (QRS duration, fragmented QRS, late potentials) and repolarization, including QT interval, dispersion, and dynamicity, also have prognostic significance. Noninvasive measures of autonomic abnormalities have also demonstrated prognostic significance. Finally, imaging evidence of fibrosis/scarring and autonomic denervation have also demonstrated prognostic significance. Emerging data on the role of biomarkers and genetics may also provide important prognostication. It is notable that no single parameter has adequate predictive power to impact clinical decisions on an individual level.
The general framework for considering SCD risk is to consider the underlying substrate, potential triggers, and other modulating factors. The substrate may represent an anatomical or molecular (e.g., ion channel) abnormality. Common triggers include ventricular ectopic activity and exercise or other conditions of sympathoexcitation. While ventricular ectopic activity was once thought to represent a treatment target for prevention of SCD, the Cardiac Arrhythmia Suppression Trial (CAST) dispelled this notion. Exercise is a well known trigger for SCD, but it must be emphasized that habitual exercise dramatically lowers the risk. Nevertheless, many markers related to exercise have been found to be related to risk of SCD including the acceleration and deceleration of heart rate with and after exercise and exercise repolarization dynamics.
The Framingham Study demonstrated a striking relationship between functional classification and death during a 2-year follow-up period. However, the proportion of deaths that were sudden did not vary with the functional classification, including those free of clinical heart disease and those in functional class IV. Generally, it has been shown that mortality increases with functional class. Competing risk of death from heart failure and other causes does diminish the proportional risk of SCD.
A strong association has been found between cigarette smoking and all manifestations of coronary heart disease (see Chapter 28 ). The Framingham Study demonstrated that cigarette smokers have a two- to threefold increase in risk for sudden death in each decade of life at entry between 30 and 59 years and that this is one of the few risk factors in which the proportion of deaths attributable to coronary heart disease that are sudden increases in association with the risk factor. Importantly, smoking cessation can reverse the excess risk associated with smoking. In support of a direct effect of smoking on arrhythmogenesis, survivors of out-of-hospital cardiac arrest who continued to smoke had a higher recurrence of cardiac arrest than those who stopped.
Alcohol has complex effects on the heart. Light to moderate alcohol consumption was associated with a reduced risk for SCD in the Physicians’ Health Study, but there was no reduction in those consuming two or more drinks per day. Alcohol abuse is associated with increased risk of atrial fibrillation, MI, and congestive heart failure, conditions associated with SCD. It is likely that there is a U-shaped relationship between alcohol consumption and SCD (see Chapter 84 ).
Obesity also influences the proportion of coronary deaths that occur suddenly. With increasing relative weight, the percentage of coronary heart disease deaths that were sudden and total coronary heart disease mortality in the Framingham Study increased linearly. In the ARIC study, obesity was associated with increased risk of SCD but only in nonsmokers (see Chapter 30 ).
Associations between levels of physical activity and SCD have been studied with variable results. Epidemiologic observations have suggested a relationship between low levels of physical activity and increased risk for death from coronary heart disease. The Framingham Study, however, showed an insignificant relationship between low levels of physical activity and the incidence of sudden death but a high proportion of sudden to total cardiac deaths with higher levels of physical activity. An association between acute physical exertion and the onset of MI and SCD has been suggested, particularly in individuals who are habitually physically inactive. Analysis from the Physicians’ Health Study demonstrated a 17-fold relative increase in SCD associated with vigorous exertion and the 30 minute postexertion period compared with periods of lower level activity or inactive states. However, the absolute risk for events was very low (one event/1.5 million exercise sessions). Habitual vigorous exercise markedly attenuated risk. A clue that intensity of exercise may play a role in SCA risk comes from an observation in college athletes, suggesting that division 1 basketball players are at higher risk than division 2 and division 3 athletes. Information about physical activity relationships in various clinical settings, such as overt and silent disease states, is still lacking (see Chapter 32 ).
The role of social determinants of health on cardiac risk factors and mortality are well recognized, but no data specifically links this to SCD. There is an association with significant elevations in life change scores during the 6 months before a coronary event, and the association is particularly striking in victims of SCD. In women, it has been reported that those who die suddenly were less often married, had fewer children, and had greater educational discrepancies with their spouses than did age-related control subjects living in the same neighborhood. A history of psychiatric treatment, including phobic anxieties, cigarette smoking, and greater quantities of alcohol consumption than in control subjects also characterized the sudden death group. Behavioral changes (e.g., inactivity) secondary to depression appeared to relate more closely to event rates than did depression itself. Acute psychosocial stressors have been associated with a higher risk for cardiovascular events, including SCD. The risk appears to cluster around the time of the stress in victims with preexisting risk, with the stressor simply advancing the time of an impending event. Natural disasters, such as earthquakes and tsunamis, may be associated with a transient increase in SCD, though specific aggravation of ventricular tachyarrhythmias in patients with implantable defibrillators may not be noted. The possibility of physical stress–induced coronary plaque disruption has been suggested.
A marked reduction in the LV ejection fraction is the most powerful of the known predictors of total mortality and SCD in patients with chronic ischemic heart disease, as well as in those at risk for SCD from other causes (see later). Increased mortality, independent of other risk factors, is measurable with ejection fractions higher than 40%, but the greatest rate of change in mortality occurs at levels between 30% and 40%. An ejection fraction of 30% or lower is the single most powerful independent predictor of SCD but has low sensitivity and specificity. Notably, relying on a low ejection fraction as the sole risk-stratifier misses a large number of SCDs that occur at lower incidence rates among the very large subset of patients with normal or moderately reduced ejection fractions and unrecognized disease. Generally, ejection fraction measurements are highly variable, among imaging techniques and even in a given patient depending on loading conditions. Contractility is a major factor determining the ejection fraction and its role in SCD risk may be related to quantifying the extent of scar or myopathy. There are emerging data that LV fibrosis may be a better predictor of cardiac events than ejection fraction alone.
Most forms of ambient ventricular ectopic activity (premature ventricular complexes [PVCs] and short runs of nonsustained ventricular tachycardia [VT]) have a benign prognosis in the absence of structural heart disease (see Chapter 40, Chapter 67 ). An exception is the polymorphic forms of nonsustained VT that occur in patients without structural heart disease but can have a molecular, functional, drug-related, or electrolyte-related basis for high-risk arrhythmias. When present in coronary disease-prone age groups, PVCs select a subgroup with a higher probability of coronary artery disease and SCD. Exercise-induced PVCs and short runs of nonsustained VT indicate some level of risk for SCD, even in the absence of recognizable structural heart disease. However, the data available to support this hypothesis are conflicting, with the possible exception of polymorphic runs of nonsustained VT. Additional data suggest that PVCs and nonsustained VT during both the exercise and recovery phases of a stress test predict increased risk. Arrhythmias in the recovery phase, previously thought to be benign, appear to predict higher risk than do arrhythmias in the exercise phase, and there is a gradient of risk with increasing severity of arrhythmias.
The occurrence of PVCs in survivors of MI, particularly if frequent and having complex forms such as repetitive PVCs, predicts an increased risk for SCD and total mortality during long-term follow-up. Data are conflicting on the role of measures of frequency and forms of ventricular ectopic activity as discriminators of risk, but most studies have cited a frequency cutoff of 10 PVCs/hour as a threshold level for increased risk. Several investigators have emphasized that the most powerful predictors among the various forms of PVCs are runs of nonsustained VT, although this relationship is now questioned. It is important to note that much of the foundational data that demonstrated the important prognostic role of ejection fraction after MI also demonstrated the similarly important role of PVC frequency.
While both ejection fraction and PVC frequency were shown to be important prognostic markers after MI, only PVCs were considered to be modifiable with antiarrhythmic drugs. The results of CAST (Cardiac Arrhythmia Suppression Trial; see Chapter 64 ), which was designed to test the hypothesis that suppression of PVCs by antiarrhythmic drugs alters the risk for SCD after MI, were surprising for two reasons. First, the death rate in the randomized placebo group was lower than expected, and second, the death rate among patients in the encainide and flecainide arms exceeded control rates by more than threefold. The excess death rates may be accounted for by drug-induced proarrhythmia during ischemic events. The SWORD (Survival with Oral d -Sotalol) study, a comparison of d -sotalol with placebo in a post-MI population with a low mortality rate, also demonstrated excess risk in the drug treated group. Whether the conclusions from CAST, CAST II, and SWORD extend beyond the drug studies or to other diseases remains to be learned. What is clear is that markers of risk for SCD do not necessarily represent appropriate treatment targets.
LV dysfunction is the major modulator of risk associated with chronic PVCs after MI. The risk for death predicted by post-MI PVCs is enhanced by the presence of LV dysfunction, which appears to exert its influence most strongly in the first 6 months after infarction. Delayed deterioration of LV function, probably because of remodeling after MI, may increase the risk further.
Decades of evaluations of ECG-based testing for abnormalities in depolarization, repolarization, and cardiac autonomic function provide consistent data linking these markers to mortality and/or SCD risk in populations but with little utility for individual risk prediction and clinical decision making. Emerging tests such as contrast-enhanced magnetic resonance imaging of the infarction, as well as noninfarct patterns of fibrosis seen on MRI delayed hyperenhancement, and sympathetic imaging with 11C-hydroxyephedrine or I- m -iodobenzylguanidine (MIBG) require further evaluation to assess utility for individual risk prediction (see Chapter 18 ). The potential of genetic risk profiling based on studies of familial clustering of SCD also requires further evaluation.
Diseases of the coronary arteries and their consequences have been estimated to account for at least 80% of SCDs in Western countries, but recent observations suggest that the magnitude of this excess burden may be decreasing. Coronary artery disease is also the most common cause in many areas of the world in which the prevalence of atherosclerosis is lower. As developing nations improve access to health care for communicable disease in the earlier years of life, coronary atherosclerosis and its consequences may emerge as a larger problem. Continued focus on control of underlying risk factors will be key to prevention of SCD.
Despite the established dominant relationship between coronary atherosclerosis and SCD, complete understanding of SCD requires recognition that less common and often rare coronary vascular disorders ( Table 70.3 ) may be identifiable before death and have therapeutic implications. Many of these entities are relatively more common causes of SCD in adolescents and young adults, in whom the prevalence of coronary heart disease-related SCDs is much lower before the age of 30 years (see Fig. 70.4A ).
|
|
The structural and functional abnormalities of the coronary vasculature as a result of coronary atherosclerosis interact with the electrophysiologic alterations that result from the myocardial impact of an ischemic burden (see Chapter 37, Chapter 38, Chapter 39, Chapter 40 ). The relationship between the vascular and myocardial components of this pathophysiologic model, as well as its modulation by hemodynamic, autonomic, genetic, and other influences, establishes multiple patterns of risk derived from the fundamental disease state ( Fig. 70.7 ). Risk is modulated by multiple factors that can be either transient or persistent, and transient modulations may interact with persistent changes. The myocardial component of this pathophysiologic model is not static over time, and the term persistent must be viewed with caution because of the gradual effects of remodeling after an initial ischemic event and the effects of recurrent ischemic episodes. SCA and SCD resulting from transient ischemia or acute MI differ in physiology and prognosis from the risk for SCA implied by a previous MI with or without subsequent ischemic cardiomyopathy. In general, the short-term risk for life-threatening events is associated more closely with acute ischemia or the acute phase of MI, and longer-term risk is associated more with transient ischemia, myocardial scarring, remodeling, ischemic cardiomyopathy, alterations in autonomic modulation, and heart failure.
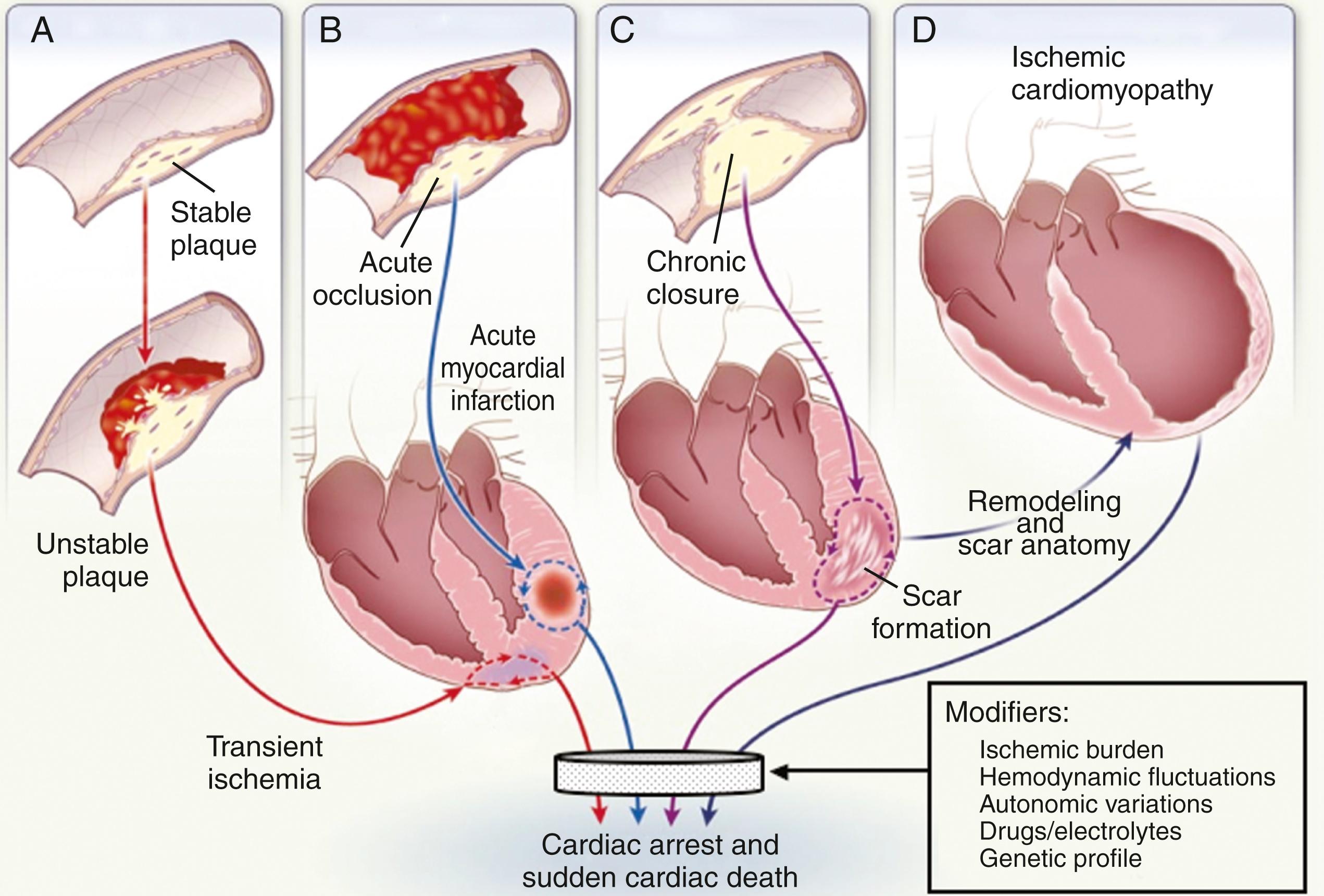
Nonatherosclerotic coronary artery abnormalities include congenital lesions, coronary artery embolism, coronary arteritis, and mechanical abnormalities of the coronary arteries. Among the congenital lesions, anomalous origin of a left coronary artery from the pulmonary artery (see Chapter 21, Chapter 82 ) is relatively common and associated with an increased death rate in infancy and early childhood without surgical treatment. The early risk for SCD is not excessively high, but patients who survive to adolescence and young adulthood without surgical intervention are at risk for SCD. Other forms of coronary arteriovenous fistulas are much less frequent and associated with a low incidence of SCD.
These anatomic variants are associated with an increased risk for SCD, particularly during exercise. When the anomalous artery passes between the aortic and the pulmonary artery root, the takeoff angle of the anomalous ostium creates a slitlike opening of the vessel that reduces the effective cross-sectional area for blood flow. The less common origin of the left coronary artery from the right sinus of Valsalva is a higher risk variant, but the origin of the right coronary artery from the left sinus of Valsalva, while lower risk, accounts for a proportion of SCDs that should not be ignored, based on the incidence of this anomaly. Congenitally hypoplastic, stenotic, or atretic left coronary arteries are uncommon abnormalities associated with a risk for MI in the young, but not for SCD.
Coronary artery emboli occur most commonly in aortic valve endocarditis and from thrombotic material on diseased or prosthetic aortic or mitral valves. Emboli can also originate from LV mural thrombi, the left atrium in patients with atrial fibrillation, or as a consequence of surgery or cardiac catheterization. Symptoms and signs of myocardial ischemia or infarction are the most common manifestations. In each of these categories, SCD results from the electrophysiologic consequences of embolic ischemia.
Mucocutaneous lymph node syndrome (Kawasaki disease; see Chapter 97 ) carries a risk for SCD in association with coronary arteritis. Polyarteritis nodosa and related vasculitis syndromes can cause SCD, presumably because of coronary arteritis, as can coronary ostial stenosis in syphilitic aortitis. The latter has become a rare manifestation of syphilis.
Several types of mechanical abnormalities are listed among the causes of SCD. Coronary artery dissection, with or without dissection of the aorta, occurs in Marfan syndrome (see Chapter 42, Chapter 82 ) and has also been reported after trauma and in the peripartum period of pregnancy. Among the rare mechanical causes of SCD is prolapse of myxomatous polyps from the aortic valve into the coronary ostia, as well as dissection or rupture of a sinus of Valsalva aneurysm with involvement of the coronary ostia and proximal coronary arteries. Finally, deep myocardial bridges over coronary arteries (see Chapter 21 ) have been reported in association with SCD occurring during strenuous exercise, possibly caused by dynamic mechanical obstruction. Scattered fibrosis in the distribution of the affected vessel is commonly seen at postmortem examination and suggests a chronic or intermittent ischemic burden over time. Deep bridging seems to be more common in association with HCM. However, the more common superficial bridges in the absence of other disorders are of less concern, and SCD associated with this anatomy is uncommon.
Coronary artery spasm may cause serious arrhythmias and SCD (see Chapter 36, Chapter 37 ). It is usually associated with some degree of concomitant coronary atherosclerotic disease. Painless myocardial ischemia, associated with either spasm or fixed lesions, is recognized as a mechanism of previously unexplained sudden death. It has been suggested that based on absence of markers of risk for a high rate of recurrence, patients with documented life-threatening arrhythmias associated with vasospastic angina receive either medical therapy and ICDs or both. Different patterns of silent ischemia (e.g., totally asymptomatic, post-MI, and mixed silent/anginal patterns) may have different prognostic implications. In post-MI patients, silent ischemia correlates with an increased risk for SCD.
LV hypertrophy, an independent risk factor for SCD, is associated with many causes of SCD and may be a physiologic contributor to mechanisms of potentially lethal arrhythmias. Underlying states resulting in LV hypertrophy include hypertensive heart disease with or without atherosclerosis, valvular heart disease, obstructive and nonobstructive HCM (see Chapter 54 ), primary pulmonary hypertension with right ventricular hypertrophy, and advanced right ventricular overload secondary to congenital heart disease. Each of these conditions is associated with risk for SCD, and it has been suggested that patients with severely hypertrophic ventricles are particularly susceptible to arrhythmic death.
Risk for SCD in patients with obstructive and nonobstructive HCM was identified in the early clinical and hemodynamic descriptions of this entity. , In patients who have the obstructive form, a majority of all deaths are sudden. However, survivors of cardiac arrest in this group may have a better long-term outcome than might survivors with other causes, and reports have suggested that the risk for primary cardiac arrest and SCD in those with HCM is lower than previously thought. It is suggested that it is now less than 1%/year, perhaps related to risk stratification based therapies such as the implantable cardioverter defibrillator. Yet this is a significant cause for SCD in the young. ,
A substantial proportion of patients with obstructive and nonobstructive HCM have a family history of affected relatives with premature SCDs of unknown cause. Genetic studies have confirmed autosomal dominant inheritance patterns, but with significant allelic and phenotypic heterogeneity. Most of the mutations are at loci that encode elements in the contractile protein complex, the most common being myosin binding protein C and beta-myosin heavy chain, which together account for more than half of identified abnormalities. The genetics of HCM is characterized by a large number of private mutations with variable expression. Possible interaction with modifier genes may account for variable expression among carriers of a specific variant, but there is also a possibility that a number of HCM variants thought to be disease-causing in the Black population are actually benign variants unique to the Black population that are missed because of underrepresentation in control populations.
Specific clinical markers have not been especially predictive of SCD in individual patients, although young age at onset, a strong family history of SCD in a first-degree relative, magnitude of the LV mass, wall thickness >3 cm, unexplained syncope, nonsustained VT, LV apical aneurysm, and LV systolic dysfunction appear to indicate higher risk. Both a substantial provocable gradient, regardless of the resting gradient, and a high resting gradient alone identify high risk for SCD. The mechanism of SCD in patients with HCM was initially thought to involve outflow tract obstruction, possibly as a consequence of catecholamine stimulation, but later data have focused on lethal arrhythmias as the common mechanism of sudden death in this disease.
The pathogenesis of the arrhythmias in HCM is discussed in Chapter 54 . The observation that patients with nonobstructive HCM, such as the diffuse, midcavitary, and to a lesser extent the apical variety, are also at risk for SCD suggests that an electrophysiologic mechanism secondary to the hypertrophied muscle itself plays a major role. In athletes younger than 35 years, HCM is the most common cause of SCD, in contrast to athletes older than 35 years, in whom coronary heart disease is the most common cause.
The advent of therapeutic interventions that provide better control of congestive heart failure has improved the long-term survival of these patients (see Chapter 50, Chapter 51, Chapter 52 ). However, the proportion of patients with heart failure who die suddenly is substantial, especially among those who appear clinically stable (i.e., functional class I or II). The mechanism of SCD may be tachyarrhythmic (VT or VF) or nonshockable bradyarrhythmias or asystole. The absolute risk for SCD increases with deteriorating LV function, but the ratio of sudden to nonsudden deaths is inversely related to the extent of functional impairment. In patients with cardiomyopathy who have good functional capacity (classes I and II), total mortality risk is considerably lower than in those with functional classes III and IV, but the probability that a death will be sudden is higher ( Fig. 70.8 ). Unexplained syncope has been observed to be a powerful predictor of SCD in patients who have functional class III or IV symptoms, regardless of the cause of cardiomyopathy.
Heart failure with preserved ejection fraction (HFpEF) has a risk for mortality over time similar to that of heart failure with reduced ejection fraction (see Chapter 51 ). In a summary of the cause specific mortality in HFpEF from the I-Preserve, CHARM-Preserved, and TOPCAT trials, 68% were cardiovascular. SCD accounted for approximately 40% of the cardiovascular deaths (27% of all deaths). The precise etiology remains unknown with both VT and bradyarrhythmias recorded in these patients. To date, there have been no targeted interventions that have successfully reduced the risk of SCD in this population.
Ischemic cardiomyopathy provides the strongest association between chronic heart failure and SCD. The prevalence of ischemic cardiomyopathy had been increasing because of better acute MI survival statistics coupled with late remodeling. Other causes include “idiopathic” fibrosis, alcoholic and postmyocarditis cardiomyopathies, peripartum cardiomyopathy (see Chapter 92 ), and the familial pattern of dilated cardiomyopathy, many of the latter being associated with lamin A/C mutations. Other gene loci have also been implicated. A residual group of undefined causes have been classified as idiopathic cardiomyopathy.
All causes of acute cardiac failure (see Chapter 47, Chapter 48 ), in the absence of prompt interventions, can result in SCD as a result of the circulatory failure itself or secondary arrhythmias. The electrophysiologic mechanisms involved have been proposed to be caused by acute stretching of ventricular myocardial fibers or the His-Purkinje system on the basis of its experimentally demonstrated arrhythmogenic effects. However, the roles of neurohumoral mechanisms and acute electrolyte shifts have not been fully evaluated. Among the causes of acute cardiac failure associated with SCD are massive acute MI, acute myocarditis, acute alcoholic cardiac dysfunction, acute pulmonary edema in any form of advanced heart disease, and a number of mechanical causes of heart failure, such as massive pulmonary embolism, mechanical disruption of intracardiac structures secondary to infarction or infection, and ball valve embolism in aortic or mitral stenosis (see Table 70.3 ).
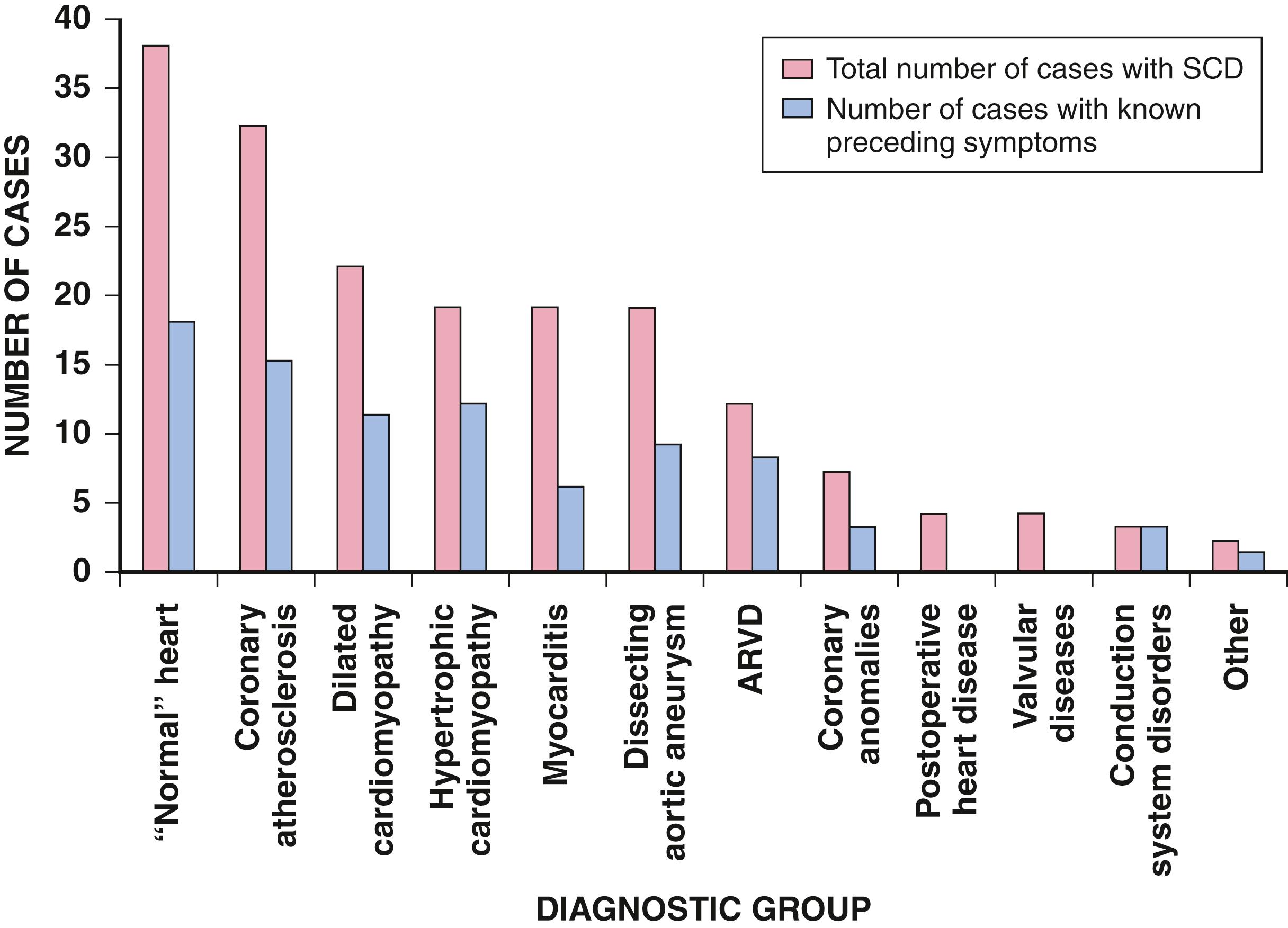
Almost all diseases in this category have been associated with SCD, with or without concomitant cardiac failure. Acute viral myocarditis with LV dysfunction (see Chapter 52, Chapter 55 ) is commonly associated with cardiac arrhythmias, including potentially lethal arrhythmias. Serious ventricular arrhythmias or SCD can occur in patients with myocarditis, even in the absence of clinical evidence of LV dysfunction. This has most recently been reported following SARS-CoV-2 infection during the COVID-19 pandemic. In autopsy series of young people presenting with SCD, myocarditis may be found in up to 10%. As noted during the COVID-19 pandemic, many individuals with myocarditis have no to minimal symptoms ( Fig. 70.8 ). Most data available suggest a bias toward victims younger than 35 years. Focal myocarditis can be associated with SCD and may be missed on autopsy depending on the extent of the cardiac evaluation. Giant cell myocarditis and acute necrotizing eosinophilic myocarditis are particularly virulent for both myocardial damage and arrhythmias. Viral myocarditis can also cause damage isolated to the specialized conducting system and result in a propensity to arrhythmias; the rare association of this process with SCD has been reported. Varicella in adults is a rare cause of striking conduction system disorders, largely involving the intraventricular specialized conducting tissue with very prolonged QRS complexes. LV function is usually preserved, and its relationship to SCD is unclear.
Myocardial involvement in collagen-vascular disorders, tumors, chronic granulomatous diseases, infiltrative disorders, and protozoan infestations varies widely, but SCD can be the initial or terminal manifestation of the disease process in all cases. Among the granulomatous diseases, cardiac sarcoidosis stands out because of the frequency of associated SCD. Clinical cardiac involvement in patients with sarcoidosis is approximately 5% with another 20% to 25% having asymptomatic involvement. The risk for SCD has been related to the extent of cardiac involvement, but ambient arrhythmias, such as nonsustained VT, may indicate risk in such patients with lesser degrees of cardiac involvement. In a report of the pathologic findings in nine patients who died of progressive systemic sclerosis, eight who died suddenly had evidence of transient ischemia and reperfusion histologically, thus suggesting that this might represent spasm of the coronary vessels. Amyloidosis of the heart (see Chapter 53 ) can also cause sudden death. An incidence of 30% has been reported, and diffuse involvement of ventricular muscle or the specialized conducting system may be associated with SCD. Cardiac involvement can occur in both light chain (AL) and transthyretin (ATTR) amyloid. TTR-associated cardiac amyloid tends to express later in life, almost always after the age of 50 years, and observed in up to 4% of the Black population, with or without the disease. Despite a high incidence of treatment for VT/fibrillation, the role of the implantable defibrillator in improving survival has not been established.
This condition with a prevalence ranging between 1/1000 and 1/5000 is associated with a high incidence of ventricular arrhythmias, including polymorphic nonsustained VT and VF and recurrent sustained monomorphic VT (see Chapter 63, Chapter 67 ). Importantly, this is a significant underlying etiology for SCD in young people, likely accounting for 10% to 20% of cases. Importantly, in a high proportion of victims, perhaps as many as 80%, the first manifestation of arrhythmogenic right ventricular dysplasia or right ventricular cardiomyopathy (ARVD/C) is “unexplained” syncope or SCD. With more widespread screening and evaluation for ARVD/C, this is being identified among family members of probands who also have substantial risk for VT, but the clinical characteristics suggest a more aggressive course in the subset who present with SCD—occurring at a younger age, in a higher percent of males, and during high levels of exertion.
SCD is often exercise related, and in some areas of the world where screening for HCM has excluded affected athletes from competition, ARVD/C has emerged as the most common cause of sports-related SCD. High-level competitive sports participation is not recommended for those with a confirmed diagnosis. Although it is generally considered a right ventricular abnormality, with possible late involvement of the left ventricle in advanced cases, a left ventricle–dominant pattern has also been described.
ARVD/C is predominantly an inherited disorder in which the variants cause or predispose to the disease, interacting with high right ventricular strain during exercise. In addition, there is some basis for considering that RV arrhythmogenic responses may be caused by very high-intensity athletic activity as a result of repeated RV strain exposures. The inheritance pattern is autosomal dominant, except in one geographically isolated cluster in which it is autosomal recessive (Naxos disease, plakoglobin locus on chromosome 17). Four loci-encoding components of the desmosome (plakoglobin, desmoplakin, plakophilin 2, and desmoglein 2) are collectively the most common known mutations associated with right ventricular dysplasia. Autosomal dominant mutations have also been identified in the ryanodine receptor locus on chromosome 1 (1q42) (see Chapter 63 ).
Before the advent of surgery for valvular heart disease, severe aortic stenosis was associated with high risk for mortality (see Chapter 72 ). Approximately 70% of deaths were sudden and accounted for an absolute SCD mortality rate of 15% to 20% among all affected patients. In the Contemporary Outcomes After Surgery and Medical Treatment in Patients With Severe Aortic Stenosis (CURRENT AS) registry, the annual rate of SCD was 1.6%/year and 1.1%/year with and without censoring for surgical or transcatheter valve replacement. Even asymptomatic patients experienced a 1.4%/year rate of SCD. In patients with mild to moderate AS, a 0.39%/year rate of SCD has been reported.
The advent of aortic valve replacement has reduced the incidence of SCD, but patients with prosthetic or heterograft aortic valve replacements remain at some risk for SCD caused by arrhythmias, prosthetic valve dysfunction, or coexistent coronary heart disease. The incidence peaks 3 weeks after surgery and then levels off after 8 months. A high incidence of ventricular arrhythmia has been observed during the follow-up of patients with valve replacement, especially those who had aortic stenosis, multiple valve surgery, or cardiomegaly. Among 3726 patients who underwent transcatheter aortic valve replacement, 5.6% were reported to have SCD at average 22 month follow-up. New-onset left bundle branch block was a significant predictor of SCD, but not in those who received a pacemaker, suggesting that AV block may be a significant etiology for SCD in these patients. Similar observations have been made following surgical aortic valve replacement. An association between stenotic lesions of other valves and SCD has not been demonstrated.
Mitral valve prolapse has been associated with SCD. While mitral valve prolapse is common, the risk for SCD is extremely low. There may be a subset of patients with mitral valve prolapse that are particularly susceptible to ventricular arrhythmias and SCD. The estimated incidence of SCD is 0.2% to 0.4% per year. Fibrosis of the papillary muscles and inferobasal left ventricle is noted in these patients. Mapping studies show a papillary muscle origin for ventricular ectopy in patients with mitral valve prolapse and evidence of late gadolinium enhancement in the papillary muscle, though other LV origins for ventricular ectopy have also been noted. SCD is associated with marked redundancy of mitral leaflets, in conjunction with nonspecific ST-T wave changes in inferior leads.
Regurgitant lesions, particularly chronic aortic regurgitation and acute mitral regurgitation, may cause SCD, but the risk is lower than with aortic stenosis. Limited data suggest that inducibility of VT with programmed ventricular stimulation is a significant predictor of arrhythmic events.
This condition may be associated with rapid death resulting from acute disruption of the valvular apparatus (see Chapter 80 ), coronary embolism, or abscesses of valvular rings or the septum; however, such deaths are rarely true sudden deaths because conventionally defined tachyarrhythmic mechanisms are uncommon. Coronary embolism from valvular vegetations can trigger fatal ischemic arrhythmia on rare occasion.
The congenital lesions most commonly associated with SCD are aortic stenosis (see Chapter 82 ) and communications between the left and right sides of the heart with Eisenmenger physiology. In the latter, the risk for SCD is a function of the severity of pulmonary vascular disease; also, pregnant patients with Eisenmenger syndrome have an extraordinarily high risk for maternal mortality during labor and delivery (see Chapter 92 ). Potentially lethal arrhythmias and SCD have been described as late complications after surgical repair of complex congenital lesions, particularly tetralogy of Fallot, transposition of the great arteries, and atrioventricular (AV) canal defects. These patients should be observed closely and treated aggressively when cardiac arrhythmias are identified, although the late risk for SCD may not be as high as previously thought.
Acquired disease of the AV node and His-Purkinje system and the presence of accessory AV pathways (see Chapter 68 ) may be associated with SCD. Clinical surveillance and follow-up studies have suggested that intraventricular conduction disturbances in coronary heart disease are one of the few factors that can increase the proportion of SCD in patients with coronary heart disease. Early studies demonstrated a very high risk for total mortality and SCD during the late in-hospital course and the first few months after hospital discharge in patients with anterior MIs and right bundle branch or bifascicular block. In a later study evaluating the impact of thrombolytic therapy versus the pre–thrombolytic era experience, the incidence of pure right bundle branch block was higher but that of bifascicular block was lower, as were late complications and mortality.
Primary fibrosis (Lenègre disease) or injury secondary to other disorders (Lev disease) of the His-Purkinje system is commonly associated with intraventricular conduction abnormalities and symptomatic AV block and less commonly with SCD. Identification of those at risk and the efficacy of pacemakers for prevention of SCD, rather than only amelioration of symptoms, have been subjects of debate. However, survival appears to depend more on the nature and extent of the underlying disease than on the conduction disturbance itself.
Patients with congenital AV block (see Chapter 68 ) or nonprogressive congenital intraventricular block, in the absence of structural cardiac abnormalities and with a stable heart rate and rhythm, have been characterized as being at low risk for SCD in the past. Later data have suggested that patients with the patterns of congenital AV block previously thought to be benign are at risk for dilated cardiomyopathy, and routine pacemaker implantation in patients older than 15 years, if not indicated sooner based on symptoms, has been suggested by at least one group. Confirmation from clinical trial data is not available. Hereditary forms of AV block have also been reported in association with a familial propensity to SCD. Sodium channel gene mutations have been associated with progressive conduction system disturbances and variants of Brugada syndrome (see Chapter 63 ). External ophthalmoplegia and retinal pigmentation with progressive conduction system disease (Kearns-Sayre syndrome), which is associated with mitochondrial DNA variants, may lead to high-grade heart block and pacemaker dependence.
The anomalous pathways of conduction in Wolff-Parkinson-White syndrome are commonly associated with nonlethal arrhythmias. However, when the anomalous pathways of conduction have short anterograde refractory periods, the occurrence of atrial fibrillation may allow the initiation of VF during very rapid conduction across the accessory pathway (see Chapter 65 ). Patients who have multiple pathways appear to be at higher risk for SCD, as do patients with a familial pattern of anomalous pathways and premature SCD.
Congenital long-QT syndrome is a functional abnormality usually caused by mutations affecting ion channel proteins and is associated with environmental or neurogenic triggers that can initiate symptomatic or lethal arrhythmias (see Chapter 63, Chapter 67 ). Such mutations may occur de novo or more commonly may be transmitted from an apparently normal parent. Syncope is the most common manifestation in symptomatic patients. SCD is less common, although data are limited by the absence of information on undiagnosed carriers in whom fatal cardiac arrest is the first clinical event. For example, the prevalence of long-QT variants in the population is generally cited to be in the range of 1/2000 to 1/2500, but a study from Japan reporting on routine ECG screening among first- and seventh-grade school children provides an estimated prevalence of 1/988, more than double the generally accepted figure from referral populations. Some patients have prolonged QT intervals throughout life without any manifest arrhythmias, whereas others are highly susceptible to symptomatic and potentially fatal ventricular arrhythmias. The concept of modifier genes interacting with the primary defect or physiologic contributors to expression is an area of active investigation.
Higher levels of risk are associated with female sex, greater degrees of QT prolongation or QT alternans, unexplained syncope, family history of premature SCD, and documented torsades de pointes or previous VF. Patients with the syndrome require avoidance of drugs that are associated with QT lengthening and careful medical management, which may include implantable defibrillators. Moreover, it is important to identify and to manage relatives medically who carry the mutation and may be at risk (see Chapter 63, Chapter 64, Chapter 67 ). While abnormalities in genes coding for ion channels are the most frequent causes of long-QT syndrome, there are now multiple LQT types coding for other proteins that are responsible for this syndrome (see Chapter 63 ).
Even in the absence of manifest long-QT syndrome, from an epidemiologic perspective, there is interest in whether QT interval abnormalities or the propensity thereto, interacting with acquired diseases, predisposes to SCD as a specific clinical expression. In many studies, QT prolongation has been associated with increased SCD, but it is interesting to note that individual components of the QT interval may bear more predictive utility. The hypothesis that common genetic variants may modulate QTc in unselected populations has stimulated interest in the relationship to selective risk for SCD in individuals with acquired diseases. However, a number of rare variants may be even more important.
The acquired form of prolonged–QT interval syndrome refers to excessive lengthening of the QT interval and the potential for the development of torsades de pointes (TdP) in response to environmental influences. As with congenital LQTS, it is more common in women. The syndrome may be caused by drug effects or an individual patient’s idiosyncrasies (particularly related to class IA or III antiarrhythmic drugs and psychotropic drugs; see Chapter 9, Chapter 99 ), electrolyte abnormalities, hypothermia, toxic substances, bradyarrhythmia-induced QT adjustments, and central nervous system injury (most commonly subarachnoid hemorrhage). It had also been reported in intensive weight reduction programs that involved the use of certain liquid protein diets and in patients with anorexia nervosa. Lithium carbonate can prolong the QT interval and unmask Brugada syndrome and has been reported to be associated with an increased incidence of SCD in cancer patients with preexisting heart disease. Drug interactions have been recognized as a mechanism of prolongation of the QT interval and TDP. Inherited polymorphisms or mutations with low penetrance involving the same gene loci associated with phenotypically expressed long-QT syndrome may underlie the acquired form, in many cases. In acquired prolonged-QT syndrome, as in the congenital form, torsades de pointes is commonly the specific arrhythmia that triggers or degenerates into VF.
A familial pattern of risk for SCD has been associated with abnormally short QT intervals, defined as a QTc shorter than 300 milliseconds (QT <280 milliseconds). Short-QT syndrome is much less common than long-QT syndrome, and there is little to guide risk profiling other than documented life-threatening arrhythmias and familial clustering of SCD. Several ion channel gene loci variants have been identified, but they account for a minority of cases (see Chapter 63 ).
This disorder, now considered part of the J wave syndromes, is characterized by an atypical right bundle branch block pattern and unusual forms of nonischemic ST-T wave elevations in the anterior precordial leads ( Fig. 70.9 ). It is a familial disorder associated with risk for SCD and occurs most commonly in young and middle-aged men (see Chapter 63, Chapter 67 ). Mutations involving the cardiac Na + channel gene ( SCN5A ) are the most commonly observed variants but are identified in only a minority of cases, and a number of other ion channel defects have been associated with the syndrome. A variant in SCN10A has been observed in more than 16% of affected individuals. The right bundle branch block and ST-T wave changes may be intermittent and evoked or exaggerated by Na + channel blockers (e.g., ajmaline, flecainide, procainamide). Individual risk for SCD is difficult to predict. Persistent type I electrocardiographic patterns, syncope, sex, and life-threatening arrhythmias, in various combinations, are thought to be the best predictors. Though the role of programmed ventricular stimulation to identify patients at high risk for SCD is debated, a pooled analysis of eight studies incorporating 1312 patients found a hazard ratio of 2.66 (95% confidence interval [CI], 1.44 to 4.92) for inducible sustained or hemodynamically significant polymorphic VT or fibrillation.
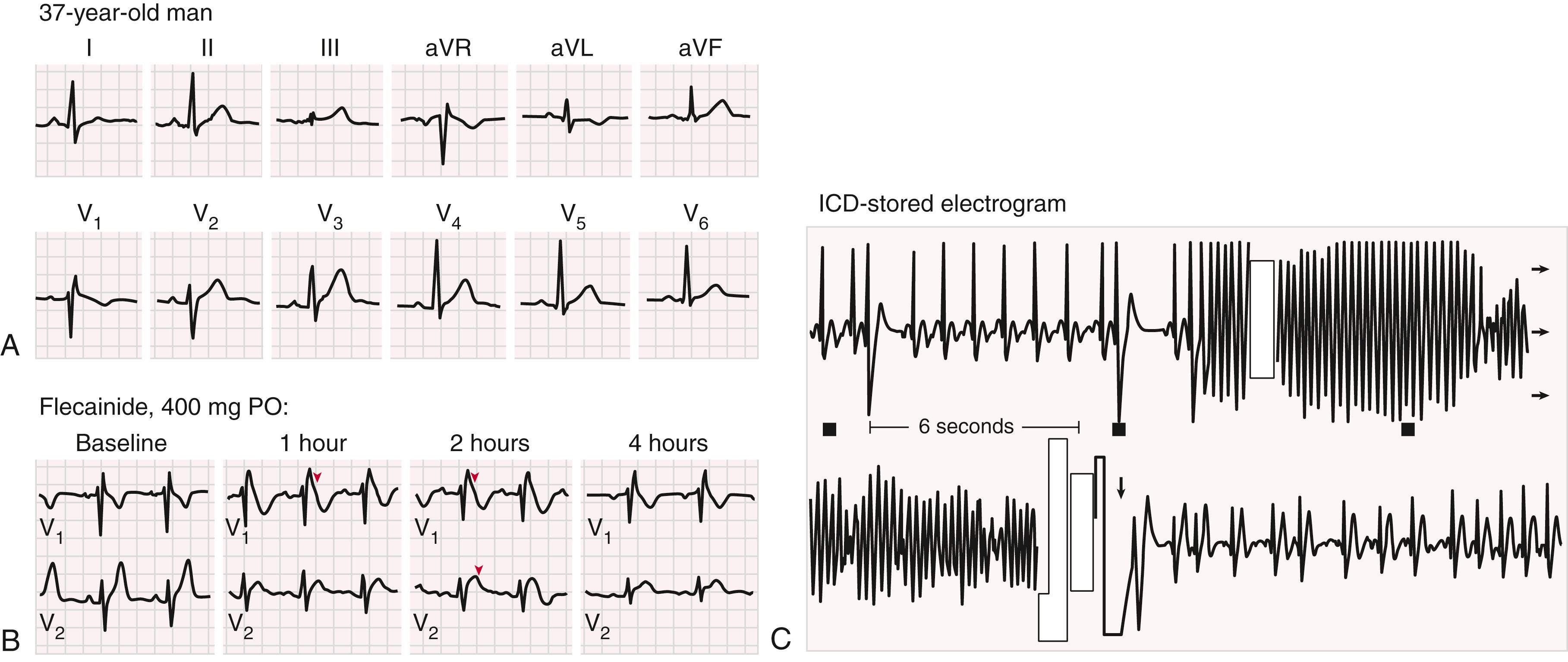
An association between the electrocardiographic pattern of early repolarization (ER) and risk for idiopathic VF has been described (J wave syndrome; see Chapter 67 ). ER was limited to the inferior and lateral leads, in contrast to the anterior leads, which associated with the conventional definition of benign ER. The magnitude of J point elevation was significantly greater in cardiac arrest survivors than in controls with ER. It has been estimated that ER accounts for an increase of 139.6 cardiac arrests per 100,000 subjects per year.
The observation that excess risk is expressed later in life suggests a possible interaction between the physiology of ER and structural heart disease, such as coronary heart disease. An association between ER and a higher mortality during acute MI has been reported.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here