Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Bronchiolitis is a syndrome of inflammation and obstruction of the lower respiratory tract that usually is caused by a viral infection. A young child with bronchiolitis typically comes to medical attention during the winter months after 2–4 days of low-grade fever, nasal congestion, and rhinorrhea with symptoms of lower respiratory tract illness that may include cough, tachypnea, and increased respiratory effort as manifested by grunting, nasal flaring, and intercostal, subcostal, or supraclavicular retractions. Inspiratory crackles and expiratory wheezing may be heard on auscultation. Various definitions of bronchiolitis have been proposed, but the term generally is applied to a first episode of wheezing in infants <12 months of age. Apnea, especially in preterm infants in the first two months of life, may be an early manifestation of viral bronchiolitis. Reported rates of apnea among infants with bronchiolitis range 1%–24%, reflecting differences in the definitions of bronchiolitis and apnea in the presence of coexisting conditions.
Yearly hospital admissions attributable to bronchiolitis increased more than twofold 1980–1996, likely reflecting the increased use of childcare centers and changes in criteria for hospital admission for children with bronchiolitis. More recent reports indicate that bronchiolitis hospitalization rates have fallen by 20% to approximately 20–30 admissions per 1000 children <12 months of age. One explanation for lower rates of bronchiolitis hospitalization is the improved health of neonates at the time of discharge compared with several years earlier. This is attributed to several factors, including: the use of antenatal glucocorticoids, surfactant replacement, improvements in methods for ventilatory support, corrective cardiac surgery performed earlier in life, and a better understanding of neonatal nutrition. A variety of potential clinical markers have been proposed to identify infants who are at risk for severe disease, but current scoring systems do not predict severe complications.
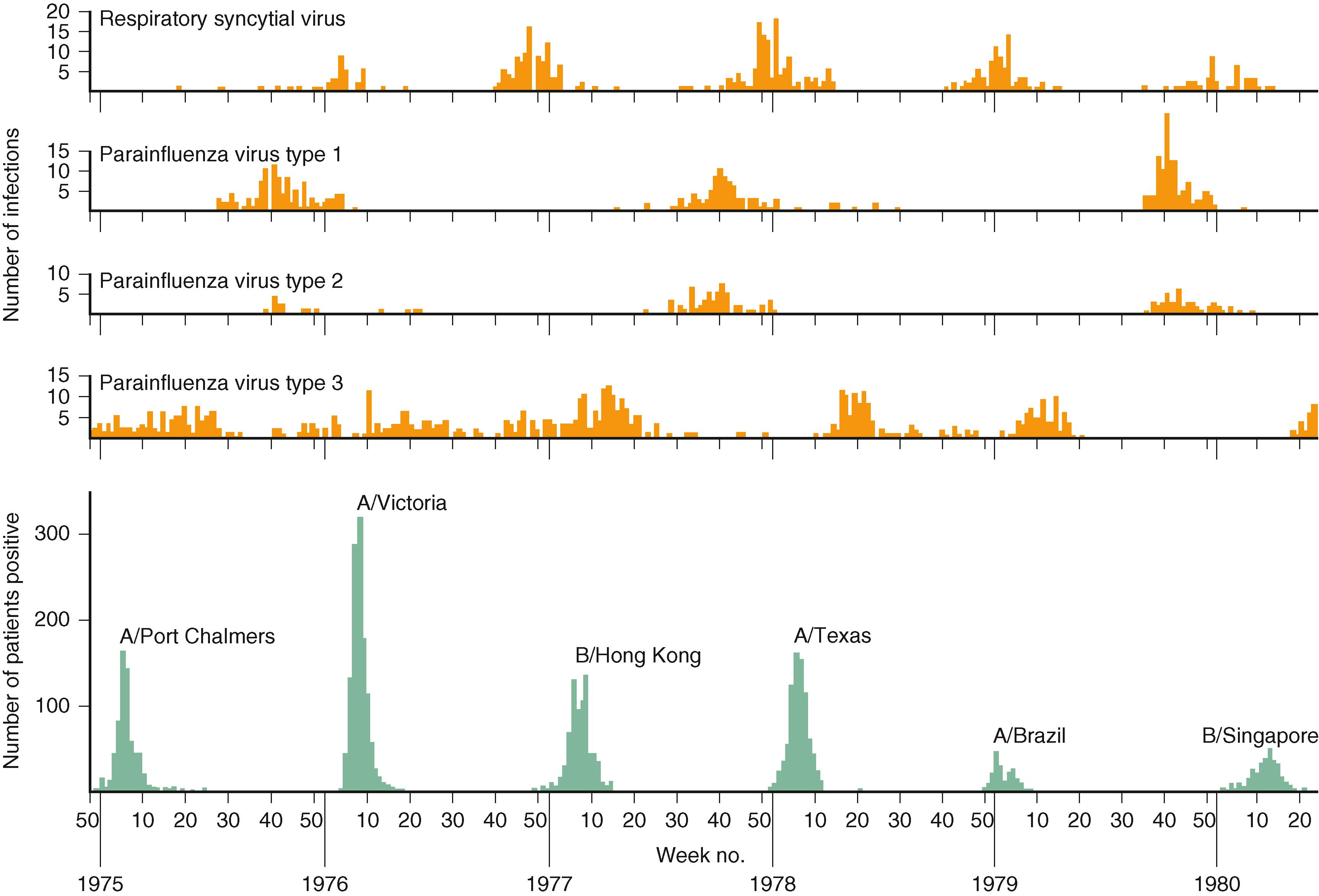
During seasonal outbreaks of bronchiolitis from November through March in North America, respiratory syncytial virus (RSV) is identified as the etiologic agent in up to 80% of hospitalized children ( Table 33.1 ). Other respiratory viruses that cause a largely indistinguishable syndrome include human rhinoviruses, parainfluenza viruses, human metapneumoviruses, coronaviruses, adenoviruses, influenza viruses, enteroviruses, and human bocaviruses. Some differences in the severity of disease have been reported due to different viruses. For example, it has been observed that rhinovirus associated bronchiolitis may result in a shorter length of hospitalization than bronchiolitis attributable to RSV. The yearly cycles of respiratory virus circulation are depicted in Fig. 33.1 . Rates of viral coinfection in hospitalized children with bronchiolitis vary widely among studies, ranging from 10% to more than 30%. Studies that have used nucleic acid amplification assays suggest that one or more viral respiratory pathogens can be isolated from the upper respiratory tract of as many as 30% of asymptomatic young children. It is not fully understood whether the detection of a viral genome in asymptomatic children represents prolong shedding after an infection has resolved, a persistent low-grade infection producing small amounts of virus or infection by a serotype with limited ability to cause disease.
| Infectious Agent | Occurrence a |
|---|---|
| Respiratory syncytial virus | ++++ |
| Rhinovirus | ++ |
| Human metapneumovirus | ++ |
| Parainfluenza virus 3 | ++ |
| Bocavirus | + |
| Parainfluenza virus 1 | + |
| Parainfluenza virus 2 | + |
| Coronaviruses | + |
| Adenovirus | + |
| Influenza virus (A or B) | + |
| Mycoplasma pneumoniae | + |
| Enterovirus | + |
a Relative importance varies with season and epidemic disease.
No evidence has been found for a primary role for bacteria as a cause of bronchiolitis. However, Chlamydia trachomatis and Mycoplasma pneumoniae are considered in the differential diagnosis of a young child with lower respiratory tract infection.
The peak incidence of severe disease occurs before 6 months of age, and the highest rates of hospitalization occur among infants 60–90 days of age. Most infants hospitalized for bronchiolitis are term infants with no known risk factors. Chronologic age is the single most important determinant for severe bronchiolitis; about two-thirds of hospitalizations occur before 6 months of age. , Several reasons may account for this age distribution. Birth shortly before or soon after the onset of the RSV season results in a longer period of exposure to RSV earlier in life. Maternal antibody concentrations to RSV also show seasonal variation, and infants born early in the RSV season are more likely to be born to mothers with low neutralizing antibody concentrations.
Specific comorbidities, including prematurity (<29 weeks’ gestation), chronic lung disease of prematurity and hemodynamically significant congenital heart disease, may result in more severe disease compared with children without these comorbities. , Worldwide, 65,000–200,000 deaths due to bronchiolitis were estimated to occur in 2005 among children <5 years of age. , The US Centers for Disease Control and Prevention (CDC) estimates <500 pediatric deaths occur annually in the US.
Occurrence of the respiratory virus season is predictable even though the severity of the season, the date of onset, the peak of activity, and the end of the season cannot be predicted with precision. There can be variation in timing of community outbreaks of disease due to RSV from year to year in the same community and among neighboring communities, even in the same season. Southern US communities tend to experience the earliest onset of RSV activity, and those in the Midwest tend to experience the latest onset. The duration of the season in western and northeastern areas is typically between that in the South and the Midwest. Nevertheless, these variations occur within the overall pattern of RSV outbreaks, usually beginning in November or December, peaking in January or February, and ending by the end of March or April.
Limited numbers of cases of bronchiolitis may occur during late spring, summer, and early fall, and they often are caused by viruses other than RSV, such as rhinoviruses or parainfluenza viruses. These cases often are milder than RSV-related cases. In tropical countries, the annual epidemic of RSV coincides with the rainy season, although cases can occur throughout the year.
Household crowding is a risk factor for severe viral lower respiratory tract illness due to RSV and other respiratory viruses. As the number of household members increases, the likelihood of close exposure to infectious respiratory secretions also increases. Childcare attendance has been correlated with an increased risk of bronchiolitis in some studies.
Unlike other respiratory viral infections, exposure to passive household tobacco smoke has not been associated with an increased risk of RSV hospitalization on a consistent basis. In contrast to the well-documented benefit of breastfeeding against some viral illnesses, existing data are conflicting regarding the specific protective effect of breastfeeding against RSV infection.
Other reported risk factors for bronchiolitis include poverty, malnutrition, maternal smoking during pregnancy, congenital malformation of the airways, neuromuscular impairment, Down syndrome, male sex, meconium aspiration, absence or short duration of breastfeeding, household crowding, childcare attendance and number of children in the childcare facility, lower level of maternal education, and living at an increased altitude. However, many of these risk factors are inconsistent from study to study, and in most instances, the impact on increased hospitalization is small.
The immune response to RSV infection is complex. Pulmonary infection initiates a host inflammatory response that recruits immune cells required for viral clearance but also contributes to the severity of disease. The interaction with viral proteins initiates both a protective and a pathogenic response. RSV infection of the epithelial cells of the human respiratory tract mucosa results in a lymphocytic infiltration of the bronchiolar walls and edema of the surrounding tissue. Disease progression is associated with proliferation and necrosis of the bronchiolar epithelium. The sloughed necrotic epithelium and the increased mucus production lead to obstruction of the lumen of the infant’s small airways. Compared with inspiration, air movement is more restricted during expiration, when the airway lumen is compromised by positive expiratory pressure. This results in expiratory wheezing. Obstruction results in air trapping and the characteristic appearance of hyperinflation on chest radiographs. As this air is absorbed, the radiographic pattern evolves to show atelectasis. , Chapter 225 discusses the pathophysiology of RSV bronchiolitis in greater detail.
Upper respiratory tract symptoms consisting of nasal congestion and discharge and a low-grade fever begin about 3–5 days after the onset of infection. Bronchiolitis represents the later stage of a respiratory viral infection that develops after 2–4 days of illness. Approximately 30%–40% of RSV-infected infants experience progression of disease to the lower respiratory tract. Spread to the lower airway occurs by aspiration of sloughed RSV-infected epithelial cells from the upper airway or by cell-to-cell spread of the virus.
Lower airway involvement is marked by an increase in the work of breathing, cough, tachypnea, wheezing, crackles, use of accessory muscles, and nasal flaring. The respiratory rate often exceeds 60–70 breaths/min in young infants. Intercostal, supracostal, and subcostal retractions are evident. Initially, wheezing occurs during the expiratory phase only and is audible only through a stethoscope. As wheezing progresses, it can be heard without a stethoscope. The chest becomes hyperexpanded and hyperresonant, respirations become more labored, and retractions become more severe. Mild hypoxemia occurs in most infants with bronchiolitis. Respiratory failure can occur due to progressive hypercapnia and respiratory muscle fatigue.
Disease severity may be recognized by the absence of audible air exchange on auscultation; flaring of the alae nasi; expiratory grunting; severe subcostal, supraclavicular, and intercostal retractions; and hypoxemia. A child with these findings may require intubation and ventilatory support. Apnea can be an early manifestation of RSV infection, sometimes resulting in respiratory failure. Because the severity of bronchiolitis often waxes and wanes before consistent improvement, serial assessment of respiratory status should be performed. The ability of a young infant to breastfeed or bottle-feed without distress over time often provides a practical guide to disease severity and management. An infant who has substantial difficulty feeding as a result of respiratory distress has moderate or severe illness and usually requires hospitalization.
Otherwise healthy infants younger than 2 months of age, infants born prematurely (<29 weeks′ gestation), and infants with chronic lung disease of prematurity are most likely to experience severe RSV disease. Infants born with hemodynamically significant congenital heart disease also are at increased risk of more severe bronchiolitis. , , , Infants with hemodynamically insignificant heart disease, including secundum atrial septal defect, small ventricular septal defect, pulmonic stenosis, uncomplicated aortic stenosis, mild coarctation of the aorta, and patent ductus arteriosus, and infants with lesions adequately corrected by surgery (unless they continue to require medication for management of congestive heart failure) are not considered to be at increased risk for hospitalization. Severe respiratory distress with bronchiolitis can be the presenting manifestation of previously unrecognized congenital heart disease.
The variable course of bronchiolitis and the inability to predict whether supportive care will be needed often results in hospital admission for infants even when symptoms are not severe. Among otherwise healthy infants, intensive care unit admission because of respiratory deterioration is less common than among infants with pre-existing conditions. The decision to admit to the intensive care unit is based on the possible need for intubation because of progressive hypercapnia, increasing hypoxemia despite supplemental oxygen, or episodes of apnea.
The typical course for a previously healthy infant older than 6 months of age is one of improvement over 2–3 days, as evidenced by a lower respiratory rate and fewer retractions. Pulmonary function abnormalities and evidence of mild desaturation may persist for several weeks. The differential diagnosis of bronchiolitis includes airway hypersensitivity to environmental irritants, anatomic abnormality of the airway, cardiac disease with pulmonary edema, cystic fibrosis, foreign-body aspiration, and gastroesophageal reflux.
A diagnosis of bronchiolitis should be based on the history and physical examination. Serial examinations may reveal fluctuations in disease acuity over a short period, reflecting rapid changes in blockage of the lumens of the small airways. Specific signs and symptoms at the time of presentation have a limited ability to predict disease severity. Assessment of the likelihood of progressive disease should consider the risk factors: age <12 weeks, a history of prematurity, underlying cardiopulmonary disease, or immunodeficiency.
Routine radiographic studies are not recommended. Infants with bronchiolitis may have abnormalities (e.g., atelectasis) on chest radiography, but the changes have little correlation with disease severity. , Radiography should be reserved for infants who require intensive care management and for those who do not improve as expected.
Routine virologic testing is not recommended because the result is unlikely to influence management. Respiratory isolation recommendations are similar for most viral infections and are based largely on the symptoms, not the specific cause. In the last decade, nucleic acid amplification test polymerase chain reaction (PCR) tests for the common viruses have been developed and are available for point of care testing. Routine testing has been proposed as a means to reduce antimicrobial use (differentiate between bacterial and viral disease) and to limit radiography. However, evidence that PCR testing reduces use of radiography on increases antibiotic stewardship has not been documented. The absence of demonstrable benefit may reflect difficulty in interpretation of a positive PCR test result in terms of indicating causation, asymptomatic infection, or prolonged postinfection viral shedding.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here