Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Breast ultrasound can be used for both screening and diagnostic indications ( Table 5.1 ). Ultrasound evaluates breast tissue without the use of ionizing radiation or the injection of contrast material and is affordable, readily available, and well tolerated by patients. The following sections will review some common applications of breast ultrasound.
|
|
|
|
|
|
|
|
Even with the advancements in breast cancer screening, such as full-field digital mammography (FFDM) and digital breast tomosynthesis (DBT), breast ultrasound continues to be essential in breast cancer detection. Historically, the principal function of ultrasound was to discover if a mammographic mass was solid or cystic. Yet in recent years, the recognition of mammography’s diminished accuracy in women with dense breast tissue has changed the use of ultrasound from a fundamental diagnostic examination to a hybrid tool of screening and diagnosis.
Many single and multi-institutional screening studies, including the American College of Radiology Imaging Network (ACRIN) 6666 trial, confirm the utility of supplemental screening using ultrasound or magnetic resonance imaging (MRI) in women at elevated risk. The American College of Radiology (ACR) Appropriateness Criteria Practice Parameters and guidelines recommend bilateral whole breast ultrasound for women at elevated risk who qualify but are unable to undergo breast MRI or for women at increased risk solely due to breast density. (Breast MRI is discussed in Chapter 8: Breast MRI Indications, Interpretation, Interventions .)
Compared with screening mammography alone, supplemental screening breast ultrasound detects an additional three to four cancers per 1000 women screened ( Table 5.2 ). Most of these cancers are invasive and without evidence of lymph node involvement.
| Author (Year) | Center | Type | Examinations | Ultrasound-Only Cancers | Yield per 1000 |
|---|---|---|---|---|---|
| Lee et al. (2019) | Multi | HHUS | 6,081 | 33 | 5.4 |
| Ohuchi et al. (2016) | Multi | HHUS | 36,859 | 61 | 3.3 |
| Brem et al. (2014) | Multi | ABUS | 15,318 | 30 | 1.96 |
| Multi | HHUS | 4,814 | 32 | 4.28 | |
| Hooley et al. (2012) | Single | HHUS | 935 | 3 | 3.21 |
| Kelly et al. (2010) | Multi | AWBUS | 6,425 | 23 | 3.58 |
| Corsetti et al. (2008) | Multi | HHUS | 9,157 | 37 | 4.04 |
| Crystal et al. (2003) | Single | HHUS | 1,517 | 7 | 4.61 |
| Leconte et al. (2003) | Single | HHUS | 4,236 | 16 | 3.78 |
| Kolb et al. (2002) | Single | HHUS | 13,547 | 37 | 2.73 |
The added benefit of supplemental screening with breast ultrasound is balanced against an increase in false-positive findings relative to mammography. The ACRIN 6666 trial found an initial 8.1% false-positive rate for ultrasound compared with 4.4% for mammography. False positive biopsy rate for ultrasound screening decreased to ~5% on subsequent incidence screening rounds with only 7.4% of those biopsies positive for cancer. Many single and multicenter studies evaluating a whole breast ultrasound screening program in their practice show a decline of false-positive findings on subsequent screening rounds. The recommendation to perform an independent audit may change practice habits and improve outcomes.
Screening breast ultrasound may be performed manually by a technician or physician using a handheld device or by using an automated breast ultrasound system (ABUS). Each method has distinct advantages and disadvantages. The manual handheld technique relies on the individual’s experience acquiring the ultrasound images and directly corresponds to the study’s quality and diagnostic accuracy. Following the ACR practice parameter for performance of whole breast ultrasound, standard examination documentation for a negative examination consists of a single representative image of each quadrant plus an image of the retroareolar region. If an abnormality is missed by the technician or the physician during real-time scanning, this information is not available for interpretation or later review and could result in a false-negative examination.
Automated breast ultrasound systems resolve the operator dependence of the handheld technique and have the capacity to record the entire ultrasound examination using cine capability. The recording of the whole study decouples the experience and expertise of the individual acquiring the images from the physician’s diagnostic interpretation. Moreover, some automated systems use a large 15-cm transducer to obtain an extended field of view with axial/transverse images, which are immediately available for reconstruction into multiple planes (coronal and sagittal). The cross-sectional (three-dimensional) data facilitates understanding of the patient’s breast anatomy, including potential pathologic findings that may identify more extensive disease from adjacent normal tissue. The reconstructed coronal view is especially useful in detecting areas of architectural distortion that are not as conspicuous on the transverse (axial) images ( Fig. 5.1 ). Although the use of panoramic software found in a conventional ultrasound machine can achieve a similar transverse extended field of view image, multiplanar reconstruction is unattainable. The long transverse or panoramic view can improve visualization and distinguish abnormal findings from the surrounding tissue; however, this technique is conducted only on a focused portion of the breast that displays the abnormality ( Fig. 5.2 ).


Screening ultrasound, like screening mammography, can have a final Breast Imaging Reporting and Data System (BI-RADS) assessment code of 0. Because there are fixed images of the screening examination, any further evaluation would be consistent with a diagnostic study. An incomplete assessment code is not appropriate for a diagnostic ultrasound scan, since the goal of the study is to explain an abnormal finding, even if the second ultrasound examination takes place on the same day or later.
Besides screening in dense breast tissue, breast ultrasound is the initial examination performed in women younger than 30 years of age and pregnant or lactating women complaining of breast-related symptoms. According to ACR practice parameter guidelines for the performance of breast ultrasound examination, in women 40 years of age and older, mammography is recommended as the initial examination to evaluate a patient’s symptoms (see Chapter 14: The Symptomatic Breast ). Ultrasound provides a direct correlation between imaging and clinical findings. Its use gives a characterization of palpable lumps and breast-related symptoms such as focal noncyclical pain or nipple discharge ( Fig. 5.3 ). The complaint of nipple discharge is concerning when it occurs from one orifice of one breast. Bilateral nipple discharge is usually secondary to hormonal effects or fibrocystic changes. Evaluation with ultrasound with the guidance of color or power Doppler can be performed to search for an intraductal mass when nipple discharge is unilateral. Papillary lesions are common intraductal masses and can be single or multiple. A single intraductal papilloma within the milk duct can appear as an expansile hypoechoic mass or as a complex cystic and solid mass. Color Doppler imaging helps identify internal vascularity, which can differentiate a papillary lesion from an inflamed cyst. Papillary cystic neoplasms are more complex in appearance and may contain a mural nodule with or without a fibrovascular stalk and thick septations (see Fig. 5.3 ).

Diagnostic evaluation of palpable areas of concern is a common use of ultrasound. The two-finger technique, which surrounds the lump in question while using the ultrasound probe to scan in between will increase sensitivity. The sonographic appearance of the symptomatic area can differentiate between a dense island of tissue or an underlying abnormality and reassure the patient and clinician when there is a negative examination ( Fig. 5.4 ). Women who present for ultrasound examinations after a negative mammogram have an ultrasound finding that correlates 50% of the time, with most results categorized as benign, for example a cyst or ridge of dense glandular tissue. According to the ACR appropriateness criteria and best practices for palpable breast mass, it may be appropriate to perform an ultrasound examination as the initial test in symptomatic women with a recent mammogram in the previous 6 months. A repeat mammogram may be unnecessary, given that ultrasound is highly diagnostic in this setting.

Breast ultrasound is the most common adjunct tool used in the evaluation of a mammographic abnormality. When correlating between the two modalities, careful consideration is made to lesion location, lesion size, and lesion shape with a clear understanding of the surrounding tissue. Lesion location is the most important feature, since an upper outer ultrasound mass does not correlate with a lower inner mammographic mass, even if the masses appear similar in shape and size. Interrogation of the correct area requires understanding how positioning and acquisition of the mammographic examination can affect differing appearances on the two modalities.
First, one must understand the varying degrees of obliquity and positioning when acquiring images for the two types of examinations. The interrogation of a mass in the radial (extending as rays from the nipple) and antiradial projections (perpendicular to the radial axis) on ultrasound will appear similar but not exact to the mediolateral oblique (MLO) and craniocaudal (CC) mammographic views. Second, a general awareness of lesion depth is useful, but it is critical to determine its related position to the mammary zone (anterior, middle, or posterior). A mammographic abnormality should resemble a similar positional depth on ultrasound with allowances for technique—mammographic compression pulls a lesion away from the chest wall, while the application of compression during the ultrasound examination is toward the chest wall. Compression and positional differences may give the false impression that a lesion is closer to the pectoralis muscle on the ultrasound examination compared with mammography. In this latter case, a detailed understanding of the adjacent tissue density is essential to correlate findings accurately. A mass that is nearly surrounded by fat density on mammography bears no resemblance to a finding surrounded by dense fibroglandular changes on ultrasound. The third discrepancy between the two modalities affects the orientation of the mass. Depending on the degree of compression applied during the ultrasound examination, an obliquely oriented mass can change to a horizontal position, which may not correlate to the mammographic finding.
Besides location, the size of the lesion on mammography must resemble the findings identified on ultrasound. In more difficult cases, such as asymmetry, it may be hard to correlate between the mammographic and sonographic findings. Asymmetry on mammography can represent a summation of smaller sonographic structures. One must consider the surrounding tissue to account for the asymmetry identified on mammography confidently. Ultrasound dimensions obtained should include the longest measurements in two views with calipers spanning the outside edges of the lesion. If you measure the inside of the lesion, the ultrasound lesion may not correlate and will always be smaller than the mammographic finding.
Lastly, the lesion shape on mammography must correlate with the shape of the lesion seen on ultrasound. An irregular mass on mammography is suspected to be irregular on ultrasound. Differences that cannot be accounted for by the adjacent surrounding tissue suggest there is no correlative finding. The ultrasound section of the ACR BI-RADS lexicon gives a guide to ultrasound interpretation and aids in determining the level of suspicion (see Ultrasound BI-RADS and Interpretation section in this chapter). Examples of mammography and ultrasound correlation are shown in Figs. 5.5 and 5.6 .


Targeted ultrasound has the potential to identify a suspicious lesion recognized on physiologically based imaging such as breast magnetic resonance imaging (MRI), breast-specific gamma imaging (BSGI), positron emission mammography (PEM), and contrast-enhanced spectral mammography (CESM) with the intent to biopsy. Ultrasound plays a significant role in characterizing suspicious findings identified on most, if not all, breast imaging modalities. Implementation of ultrasound in this way is sometimes referred to as a “second look” since ultrasound evaluates a lesion initially identified with a different modality, which may or may not be visible on the second review with sonographic interrogation. Figs. 5.7 and 5.8 show examples of targeted second-look ultrasound for BSGI and MRI findings.


Lesions detected with physiologic-based imaging may not be readily apparent on ultrasound imaging that relies on anatomic variations. The efficiency of second-look ultrasound is best investigated for MRI. Reported ultrasound detection rates for MRI findings vary between 22.6% and 82.1%. Ultrasound correlates are more likely to be found for mass lesions than nonmass lesions and for malignant masses versus benign. Technical differences, software advancements, and reader experience contribute to the range of published results. When performing the ultrasound examination, there must be consideration of multiple factors, such as surrounding landmarks, size, and shape of lesion and location in the breast. If there is no ultrasound correlate, then a biopsy should be performed using the modality that demonstrates the lesion best.
The postsurgical breast includes a spectrum of findings related to fat necrosis. Fat necrosis evolves and has a variable presentation on all modalities of breast imaging. Ultrasound characteristics consist of skin thickening and edema (hyperechoic appearance) of the tissue surrounding the surgical site. Additional findings include hematoma/seroma that develops in the lumpectomy bed and can be present for years. The seroma takes the shape of the surgical cavity and can have an anechoic ultrasound appearance or one that is more heterogeneous with low-level echoes (see Fig. 5.2 ). Both presentations are benign; however, a spontaneous increase in seroma size should raise suspicion. Any morphologic descriptor that describes the findings and is suspicious will also dictate an image-guided biopsy.
Over time, the fluid/debris within the surgical bed will get reabsorbed, and a fibrinous scar develops. Ultrasound characteristics of postoperative changes can mimic cancer and must be viewed in two orthogonal views to help differentiate disease from a scar. Scar tissue can usually be followed to the skin in an oblique line, whereas the orthogonal view may appear as a hypoechoic irregular mass with posterior acoustic shadowing ( Fig. 5.9 ). Worrisome ultrasound findings for disease recurrence include a new mass adjacent to the excisional site, increased volume of the hypoechoic scar (that persists in two projections), and eccentric thickening or nodularity of the seroma cavity. Doppler may help determine whether there is internal vascularity, which would be more indicative of recurrent disease.

With the increasing use of neoadjuvant chemotherapy and localized brachytherapy, ultrasound has played a pivotal role in management and pretreatment decisions. Ultrasound evaluation can explore the potential for multifocal/multicentric disease, cancer involvement of the skin, and axillary lymph nodes, which are all-important prognostic indicators. Chest wall involvement is best identified with breast MRI, which has the advantage of discriminating the prepectoral fascial plane, chest wall musculature, and tumor margin. There are competing issues that may limit ultrasound visibility, such as shadowing from the mass and decreased penetration of the beam in deeper tissue that will make it less favorable in this evaluation.
The normal appearance of a lymph node, whether in the axilla or the breast, is a circumscribed hypoechoic oval (mostly reniform) mass containing an echogenic fatty hilum. Size is usually not taken into consideration, except for a new or enlarging lymph node. Benign reactive lymph nodes have morphologic characteristics that overlap with more suspicious features such as enlargement or cortical thickening, albeit these characteristics are more uniform in appearance ( Fig. 5.10 ). When such questionable overlapping features are present, evaluation of the contralateral axilla can be helpful. The discovery of bilaterally enlarged lymph nodes would suggest an underlying systemic process such as lymphoma, human immunodeficiency virus (HIV), sarcoid, or rheumatoid arthritis. Please also refer to Chapter 17: Lymph Node Evaluation in Breast Imaging .

Signs suspicious for metastatic involvement of a lymph node include eccentric cortical thickening >3 mm and hilar effacement or replacement. The underlying pathology coincides with imaging whereby metastatic cells are carried to the lymph node through the capsule and lodge in the cortical and subcapsular sinuses. This creates an eccentric thickening of the lymph node ( Fig. 5.11 ). Perinodal spread of tumor beyond the capsule accounts for the loss of the outer capsule, presenting as cortical irregularity and a finding that warrants a biopsy to confirm a diagnosis.

According to the recent guidelines from the National Comprehensive Cancer Network, systemic treatment in the adjuvant or neoadjuvant setting is recommended for locally advanced cancer that measures more than 5 cm (stage IIB and III) with axillary lymph nodes demonstrating significant tumor burden, or cancer with direct extension and involvement of the chest musculature and skin (NCCN guidelines). The administration of preoperative (neoadjuvant) systemic treatment can improve the odds of breast-conserving surgery and reduce disease recurrence rates. Compared with physical examination or mammography alone, breast ultrasound is superior at assessing the extent of disease. Its use during the neoadjuvant setting can help tailor treatment based on tumor response. Favorable signs, such as decreased size and reduced vascularity of cancer while undergoing treatment, translates to improved outcomes. Breast MRI, which includes morphologic and physiologic information, is extremely sensitive in assessing tumor response in this setting but is more expensive ( Fig. 5.12 ). After neoadjuvant treatment, the residual tumor surrounding the biopsy clip is removed through image-guided localization. If there is no longer a visible tumor on imaging, then the biopsy marker is removed along with the surrounding tissue. A complete pathologic response is an excellent prognostic indicator.
![Fig. 5.12, Indications: neoadjuvant setting. A 32-year-old woman with grade 3 invasive ductal carcinoma (IDC), Estrogen receptor/progesterone receptor (ER/PR), and human epidermal growth factor receptor (HER2) negative. (A) Ultrasound and mammogram demonstrate a microlobulated antiparallel oriented mass ( arrows ) biopsy proven to represent IDC. (B) Postneoadjuvant images (ultrasound, axial T1 postcontrast magnetic resonance imaging [MRI], and postbiopsy mammogram) demonstrates presence of the clip and surrounding soft tissue. There is no abnormal enhancement suggesting fibrosis and not residual disease. Both ultrasound and mammography examinations are good at predicting the presence of disease; however, MRI is superior in determining pathologic complete response. Fig. 5.12, Indications: neoadjuvant setting. A 32-year-old woman with grade 3 invasive ductal carcinoma (IDC), Estrogen receptor/progesterone receptor (ER/PR), and human epidermal growth factor receptor (HER2) negative. (A) Ultrasound and mammogram demonstrate a microlobulated antiparallel oriented mass ( arrows ) biopsy proven to represent IDC. (B) Postneoadjuvant images (ultrasound, axial T1 postcontrast magnetic resonance imaging [MRI], and postbiopsy mammogram) demonstrates presence of the clip and surrounding soft tissue. There is no abnormal enhancement suggesting fibrosis and not residual disease. Both ultrasound and mammography examinations are good at predicting the presence of disease; however, MRI is superior in determining pathologic complete response.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/BreastUltrasoundIndicationsandInterpretation/11_3s20B9780323758499000059.jpg)
Although women with implants have similar breast complaints as women who do not, the use of ultrasound can improve visualization of the overlying breast tissue, axilla, and the anterior edge of the prosthesis. Both silicone and saline implants appear anechoic on ultrasound. The outer shell of the implant can have a variable appearance depending on the type of implant (double or single lumen), and shell surface (textured or smooth). The shell of a single lumen implant appears as two parallel hyperechoic lines separated by an anechoic intermediary layer. A third outer hyperechoic layer represents the fibrous capsule and can appear as one with the implant’s outer shell ( Fig. 5.13 ). In the subcapsular region, surrounding the implant is a small but variable amount of normal anechoic fluid. The disruption of this appearance and relationship of these layers can signify pathology and possible implant rupture.

Ultrasound is sensitive in evaluating implant integrity; however, limitations in beam focusing with increasing depth of the tissue can reduce sensitivity when evaluating intracapsular rupture. The rupture of saline implants is a clinical diagnosis without any need for imaging, yet the majority of silicone implant ruptures are clinically silent. In symptomatic patients, the investigation of implant integrity is performed with an ultrasound or MRI. MRI is more sensitive than ultrasound in this evaluation. The classic sonographic appearance of intracapsular rupture resembles a stepladder, with alternating hyperechoic and anechoic parallel lines. This finding corresponds to the implant shell floating in the anechoic viscose fluid. Extracapsular silicone implant rupture refers to free silicone outside of the implant with deposition in the breast tissue. Extracapsular rupture appears as an indistinct hyperechoic mass with variable posterior acoustic shadowing, typical “snowstorm” appearance ( Fig. 5.14 ). Recently an increasing number of breast implant–associated lymphocytic lymphoma cases has prompted evaluation and tissue sampling with ultrasound. Sonographic findings such as heterogeneity, asymmetry, or an enlarging peri-implant effusion are suspicious (see Chapter 18: The Augmented and Reconstructed Breast ).
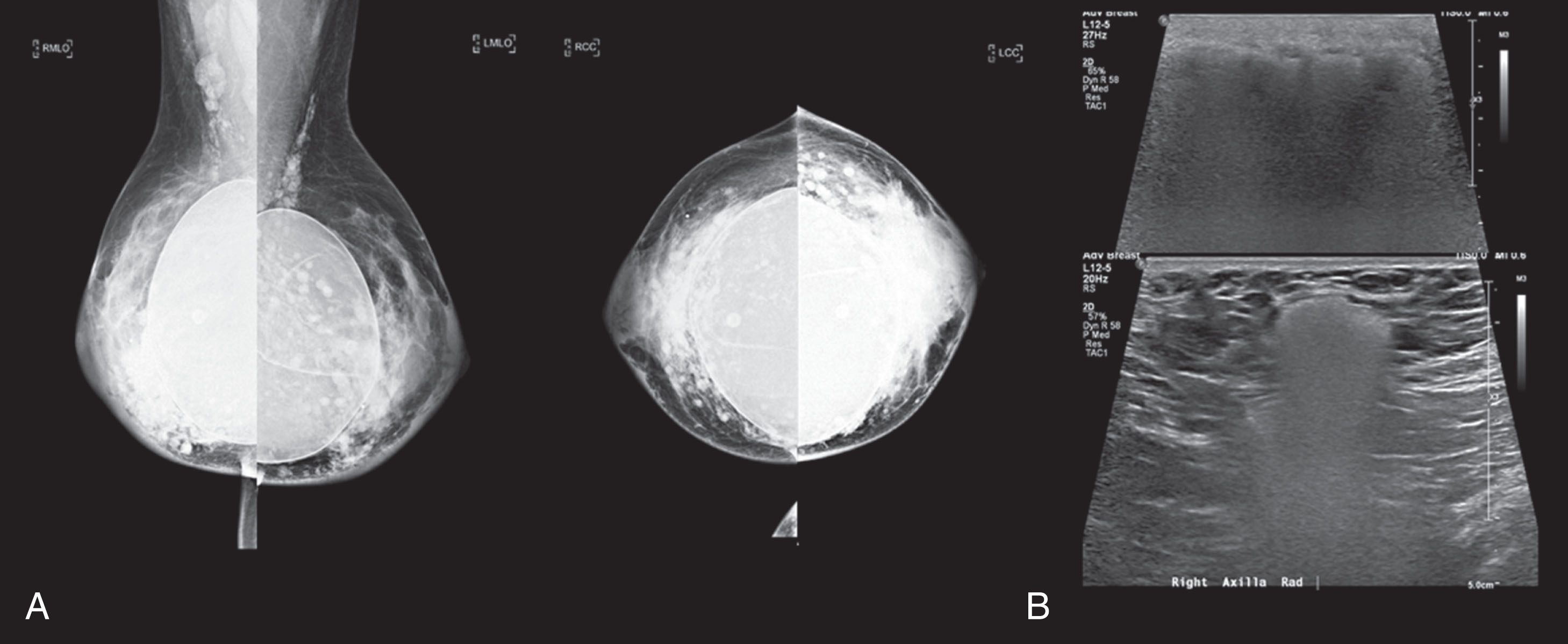
There are a number of ultrasound-guided percutaneous procedures, including fine-needle aspiration, core needle biopsy, and presurgical localization of nonpalpable masses. Using ultrasound assistance is preferred, since these procedures are less invasive, less expensive, and comparable in accuracy to surgical excision. The advantage of improved patient comfort with no contrast injection and no additional radiation makes it widely employed. The benefit of performing ultrasound procedures in real time makes it safer, since there is full control of the needle position. During the process, the intermittent use of color Doppler can identify and avoid complications and reduce bleeding risk. Optimal use of ultrasound-guided procedures improves access to lesions in difficult locations such as the axilla, lesions near the chest wall, or breast implants.
Procedural complications include hematomas, arteriovenous malformations, pseudoaneurysm, and abscess formation. The use of the ultrasound probe to apply compression and visualize improvement is one distinct advantage. Ultrasound is more than 95% accurate in diagnosing pseudoaneurysm ( Fig. 5.15 ). For further information on image-guided procedures, see Chapter 7: Mammographic and Ultrasound-Guided Breast Biopsy Procedures .

Proper technique and optimal position are needed to maximize the quality of the ultrasound image. B mode ultrasound, or brightness mode, is used to construct the conventional two-dimensional (2D) grayscale image. The formation of an ultrasound image occurs after a spectrum of frequencies is emitted, and the resulting echoes received to the transducer. The brightness of the gray value of the ultrasound image corresponds to the energy of the returning echoes.
During the ultrasound examination, the patient is positioned supine and oblique on a wedge-shaped cushion with the arm raised above the head. This position allows for the reduction in breast thickness and helps maximize the ultrasound beam’s penetration. Using this technique, the maximum penetration of the ultrasound beam is approximately 5 cm deep through a highly attenuating structure such as the breast. Gel or lotion is used as a medium to allow sound waves to propagate from the transducer into the tissue. Gentle transducer pressure is applied while scanning the patient to reduce artifactual shadowing from typical normal structures. Real-time ultrasound scanning allows the operator to change the transducer’s angle to improve the overall quality of the examination and, thus, interpretation. Per the ACR appropriateness guidelines and practice parameters, a recorded ultrasound image should have a permanent identification that labels the facility name, patient name and ID (or date of birth), examination date, laterality of the breast, and sonographer’s initials.
Image quality relies on three fundamental principles, transducer frequency, resolution, and contrast. The ACR practice parameter for the performance of breast ultrasound examination recommends a transducer with a broad bandwidth operating at a center frequency of at least 12 MHz and preferably higher. A high-frequency ultrasound transducer provides superior contrast and spatial resolution. The penetration of breast tissue is inversely proportional to transducer frequency. Ultrasound beams are composed of a range of frequencies determined by the number of sound waves in a unit time. The width of the spectrum of frequencies transmitted defines the bandwidth. The more sound waves to interrogate a mass, such as in broad bandwidth, the better. The transducer with a higher frequency improves resolution but has less penetration, whereas lower frequency provides greater penetration and less resolution ( Table 5.3 ). Thus, when evaluating deep tissue in patients or large breasts, it may be helpful to select lower frequency settings for the beam to penetrate deeper tissue and give better characterization.
| Action | Result | ||
|---|---|---|---|
| Transducer frequency | ↑ Frequency | ↑ Attenuation | Improves near field |
| ↓ Frequency | ↓ Attenuation | Improved depth penetration | |
| Compound imaging | ↑ Mass margin interrogation | ||
| Harmonics |
|
||
| Spatial resolution | ↑ Transducer frequency | Axial resolution improves | |
| ↑ Number of focal zones and position of ultrasound beam | Lateral resolution improves | ||
| Use standoff gel/pad |
|
The field of view must include visualization of the skin to the pectoralis muscle and is optimized to visualize an abnormality ( Fig. 5.16 ). Image labeling requires annotation describing the laterality (right or left breast), location of the lesion (clockface and distance from the nipple), and transducer orientation in relation to the breast. This information may also be designated using a breast pictogram. Documentation of the lesion’s largest size in two orthogonal planes (radial/antiradial or transverse/longitudinal) can record its maximum dimensions. Ultrasound features are essential and give an accurate assessment of a breast mass, so images with and without caliper measurements are recommended. An additional set of images with and without color/power Doppler will help assess the presence or absence of vascularity.

Artifacts are routinely encountered in breast ultrasound imaging ( Table 5.4 ). Speckle (noise) artifact refers to the diffuse granular appearance of breast tissue on the ultrasound image. This common artifact occurs from the interference of acoustic soundwaves with scattered low-amplitude echoes originating from the many microstructural surfaces of the breast. This artifact reduces image contrast and lesion characterization. Similarly, clutter is also considered a noise artifact that can degrade contrast resolution. Clutter is evident in anechoic or hypoechoic structures such as cysts presenting as artifactual echoes or “filling” in of cysts ( Fig. 5.17 ). Harmonic imaging can help reduce this artifact. Refraction artifacts occur from a change in the direction of the ultrasound beam when it encounters a curved interface. The two different sound speeds lead to edge shadowing in circumscribed structures such as cysts or breast implants. Refraction can also occur at the interface of fat lobules and Cooper ligaments. In the latter, changing the transducer’s angle or increasing transducer pressure can sometimes mitigate this artifact. Reverberation artifact is identified in anechoic structures and results from the ultrasound beam bouncing between two highly reflective parallel interfaces. It appears as multiple parallel hyperechoic (bright) lines in a cystic structure ( Fig. 5.18 ).
| Artifact Type | Description | Examples | Resolution | |
|---|---|---|---|---|
| Reverberation | Occurs due to multiple reflections | Anechoic cyst | Add THI | |
| Speckle artifact | Decreases image contrast and lesion characterization | ↑ Noise of image | Add THI | |
| Clutter | Decreases contrast resolution | ↑ Noise of image | Add THI | |
| Acoustic shadowing | Occurs behind a highly attenuating object creating a “shadow” | Solid mass | ||
| Acoustic enhancement | Occurs behind a minimally attenuating object causing increased echo intensity | Cyst | ||


While artifacts can degrade an image, common attenuation artifacts seen in breast ultrasound, such as acoustic shadowing and enhanced through transmission, help further characterize findings. Sound attenuation as it passes through a solid structure determines a solid mass from a cystic lesion. Shadowing occurs when there is a highly attenuating structure relative to the surrounding tissue. The low amplitude of the returning ultrasound beam distal to the highly attenuating structure causes a hypoechoic or dark band. In breast ultrasound, shadowing is indicative of a solid mass. However, it is essential to note that some masses can have enhanced through transmission. In this case further characterization with color Doppler imaging can aid in differentiating a solid lesion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here