Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
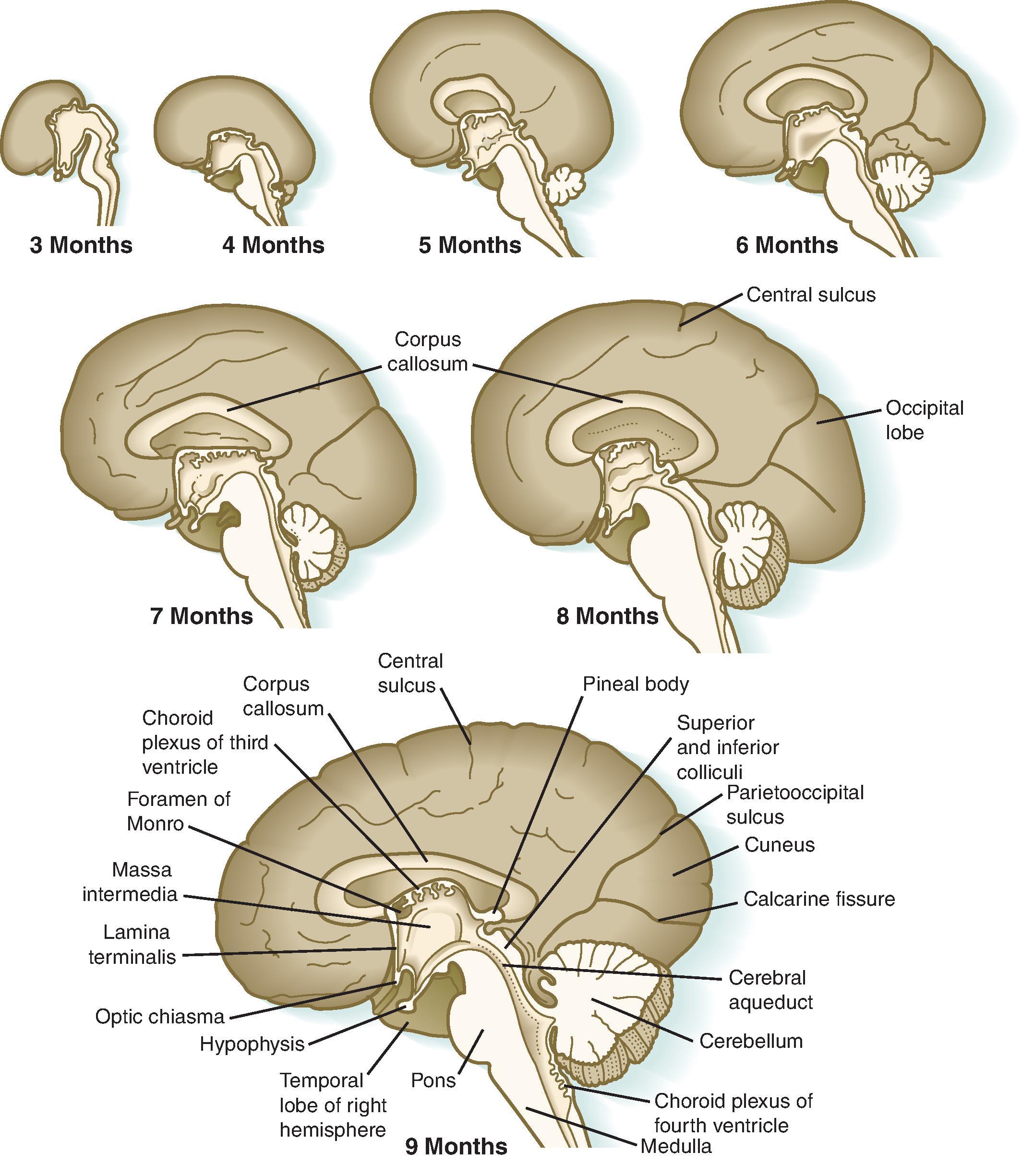
Brain malformations represent a disruption of normal development and can be caused by genetic, infectious, ischemic, and hemorrhagic factors. As seen in Table 2.1 , the time at which the insult occurs during development will determine the malformation. Because the majority of brain malformations occur early in development, the majority of malformations will be present when imaging is performed. Brain malformations are often first encountered on ultrasound and magnetic resonance imaging (MRI). In general, MRI provides the best assessment of brain malformation.
| Week | Major Developments | Appearance | Malformations |
|---|---|---|---|
| 3 | Neural groove and folds Three primary vesicles visible Cervical and cephalic flexures Motor neurons appear |
 |
Neural tube defects |
| 4 | Neural tube starts to close (day 22) Rostral end of neural tube closes (day 24) Caudal end of neural tube closes (day 26) Neural crest cells begin to migrate Secondary neurulation starts Motor nerves emerge |
 |
Neural tube defects, holoprosencephaly |
| 5 | Optic vesicle, pontine flexure Five secondary vesicles visible Sulcus limitans, sensory ganglia Sensory nerves grow into CNS Rhombic lips Basal nuclei begin Thalamus, hypothalamus begin Autonomic ganglia, lens, cochlea start |
 |
Holoprosencephaly, sacral cord abnormalities |
| 6–7 | Telencephalon enlarged Basal nuclei prominent Secondary neurulation complete Cerebellum and optic nerve begin Choroid plexus Insula |
 |
|
| 8–12 | Neuronal proliferation Cerebral and cerebellar cortex begin Anterior commissure, optic chiasm Internal capsule Reflexes appear |
 |
Migration/proliferation problems (e.g., abnormal cortex or gyri) |
| 12–16 | Neuronal proliferation and migration Glial differentiation Corpus callosum |
 |
Migration/proliferation problems (e.g., abnormal cortex or gyri) |
| 16–40 | Neuronal migration Cortical sulci Glial proliferation Some myelination (mostly postnatal) Synapse formation |
 |
Hemorrhage, other destructive events |
A pattern approach is necessary to detect malformations; more than one structure can be malformed. Major intracranial structures that should be evaluated include the following:
Size, sulcation, gyration, and myelination of the cerebral hemispheres
Size, formation, and proportions of the cerebellum, brainstem, and posterior fossa
Presence of the septum pellucidum and formation and size of the corpus callosum
Presence of the olfactory bulbs and sulci
Formation and size of the basal ganglia and thalami
Rotation of the hippocampi
Formation and size of the ventricles
Importantly, assessment of extracranial structures should include the eyes, ears, and facial bones as anomalies in these structures have associated brain anomalies. This chapter will focus on the imaging appearance of brain malformations and provide basic embryology underlying the malformation.
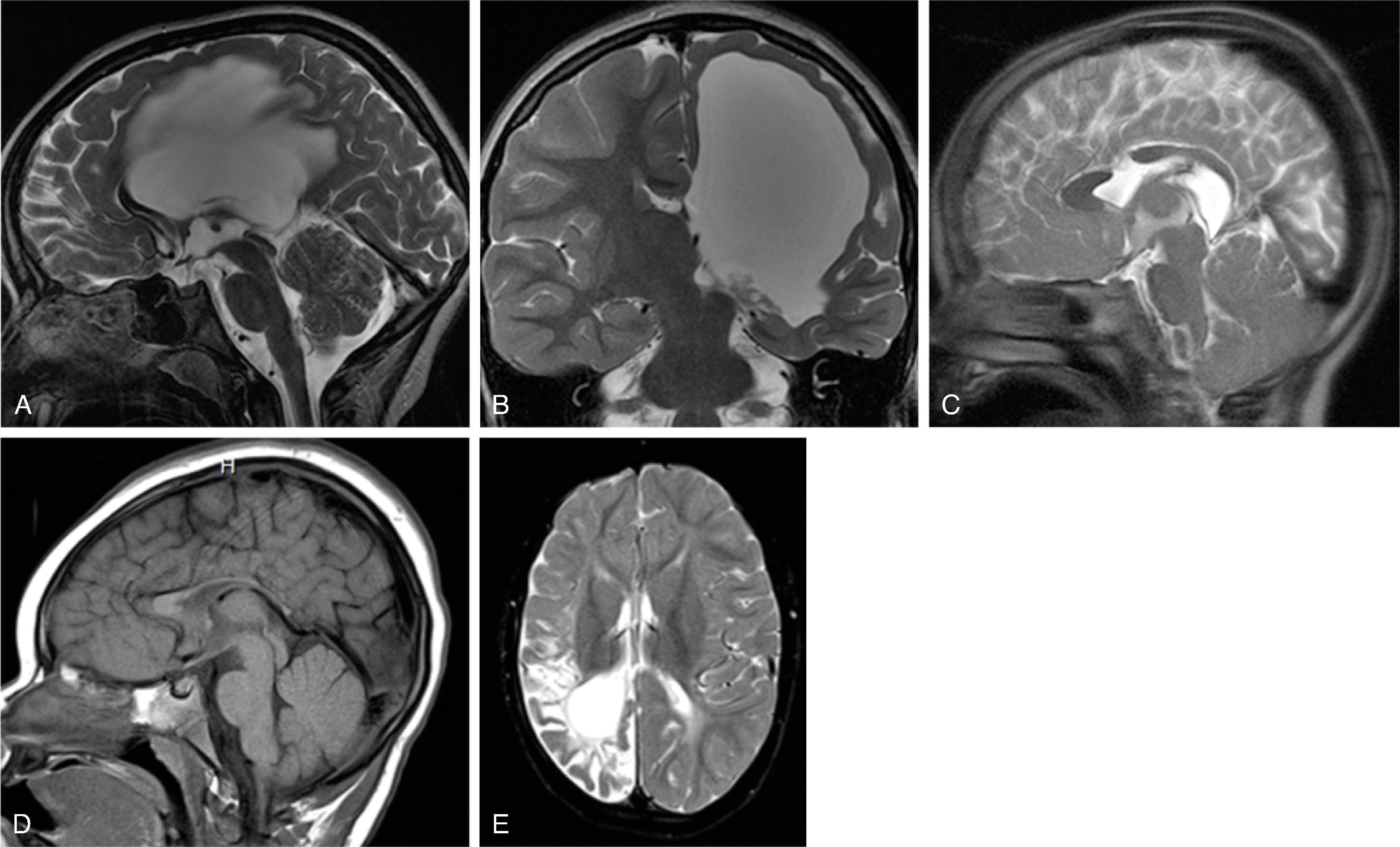
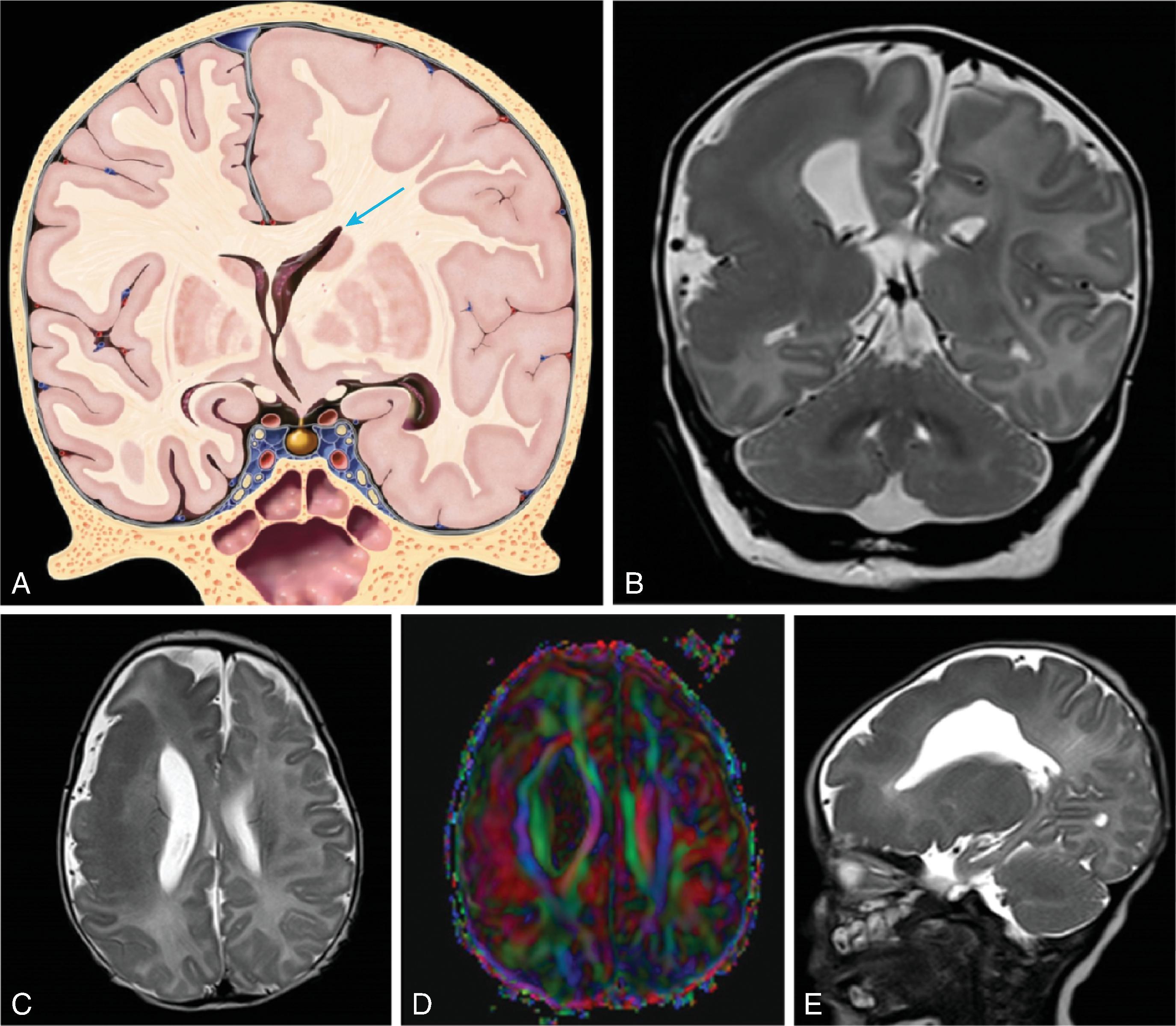
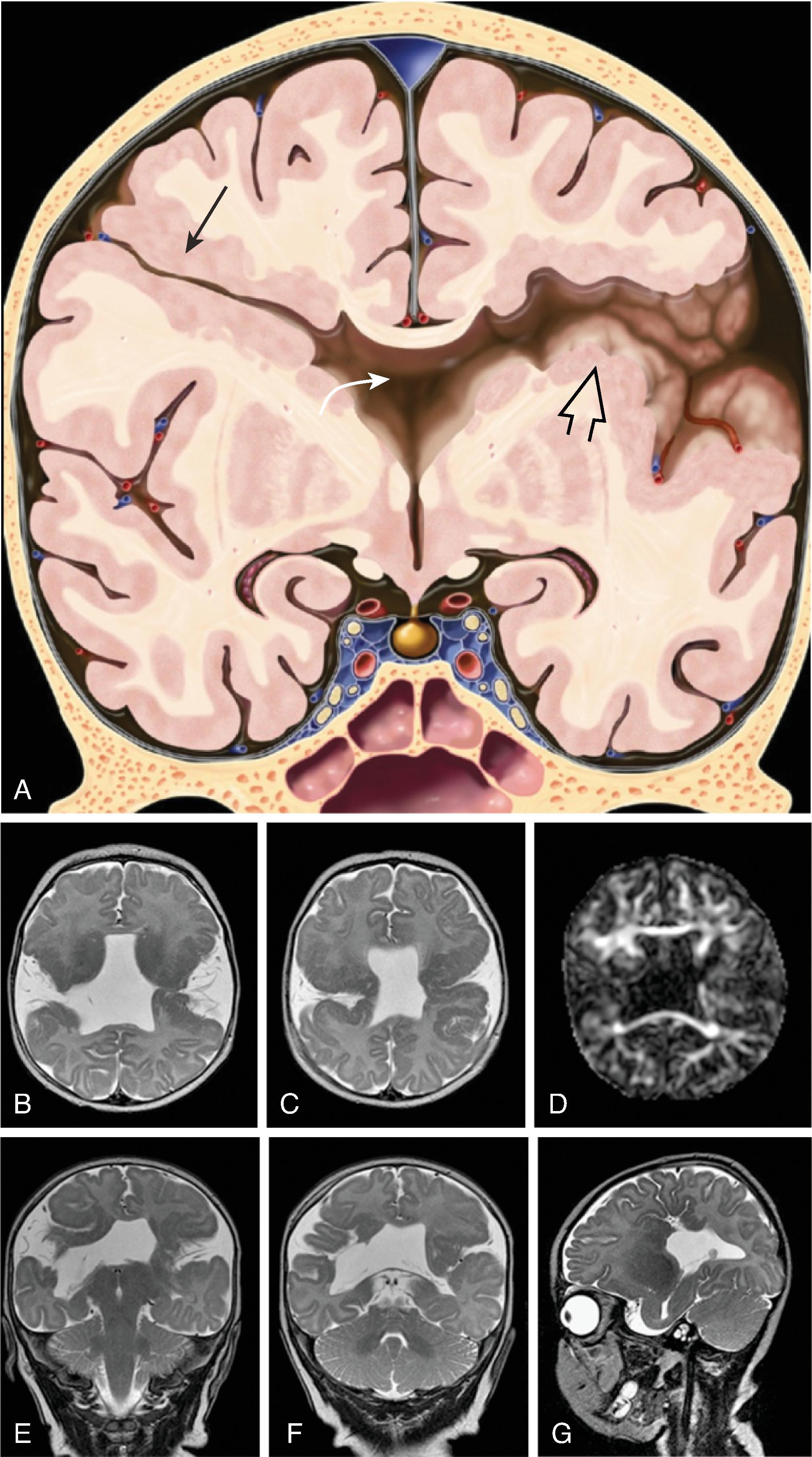
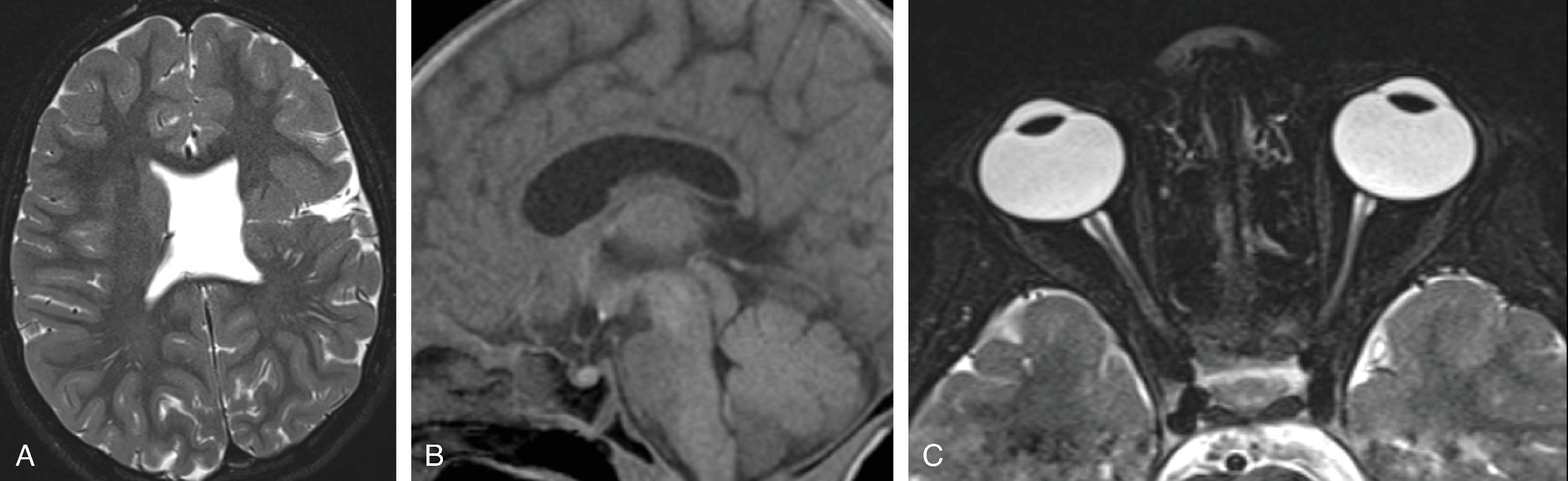
The corpus callosum is by far the largest commissure of the three telencephalic commissures and the principal anatomic and neurophysiologic connection pathway between the two hemispheres. The anterior commissure and hippocampal commissure represent the other two smaller telencephalic commissures and are closely related to the formation of the corpus callosum. The anterior commissure connects the olfactory cortex and crosses along the lamina terminalis. The hippocampal commissure is present but indistinguishable from the inferior margin of the posterior corpus callosum and connects the hippocampi. Formation of the corpus callosum is shown in Fig. 2.2 .
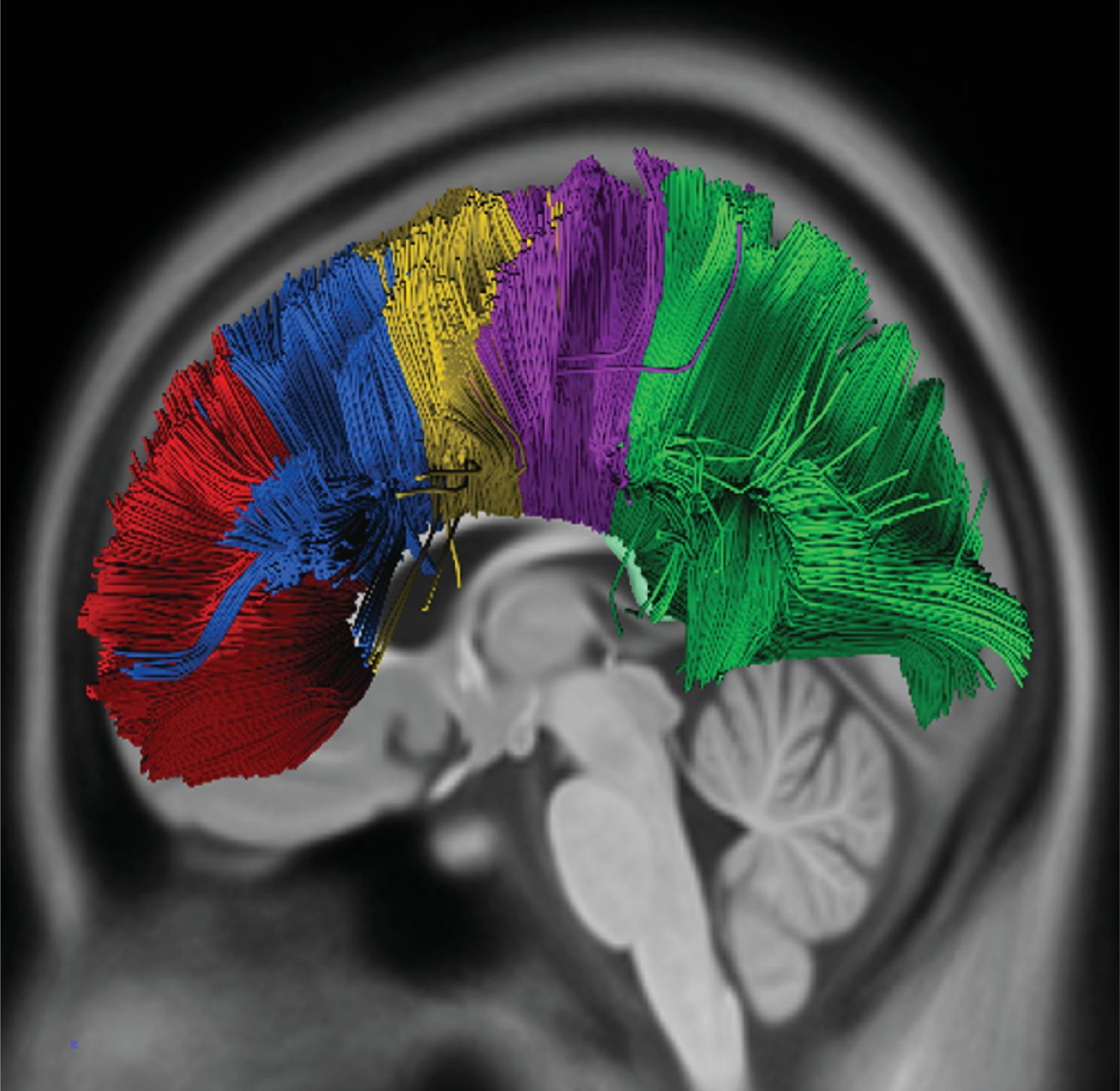
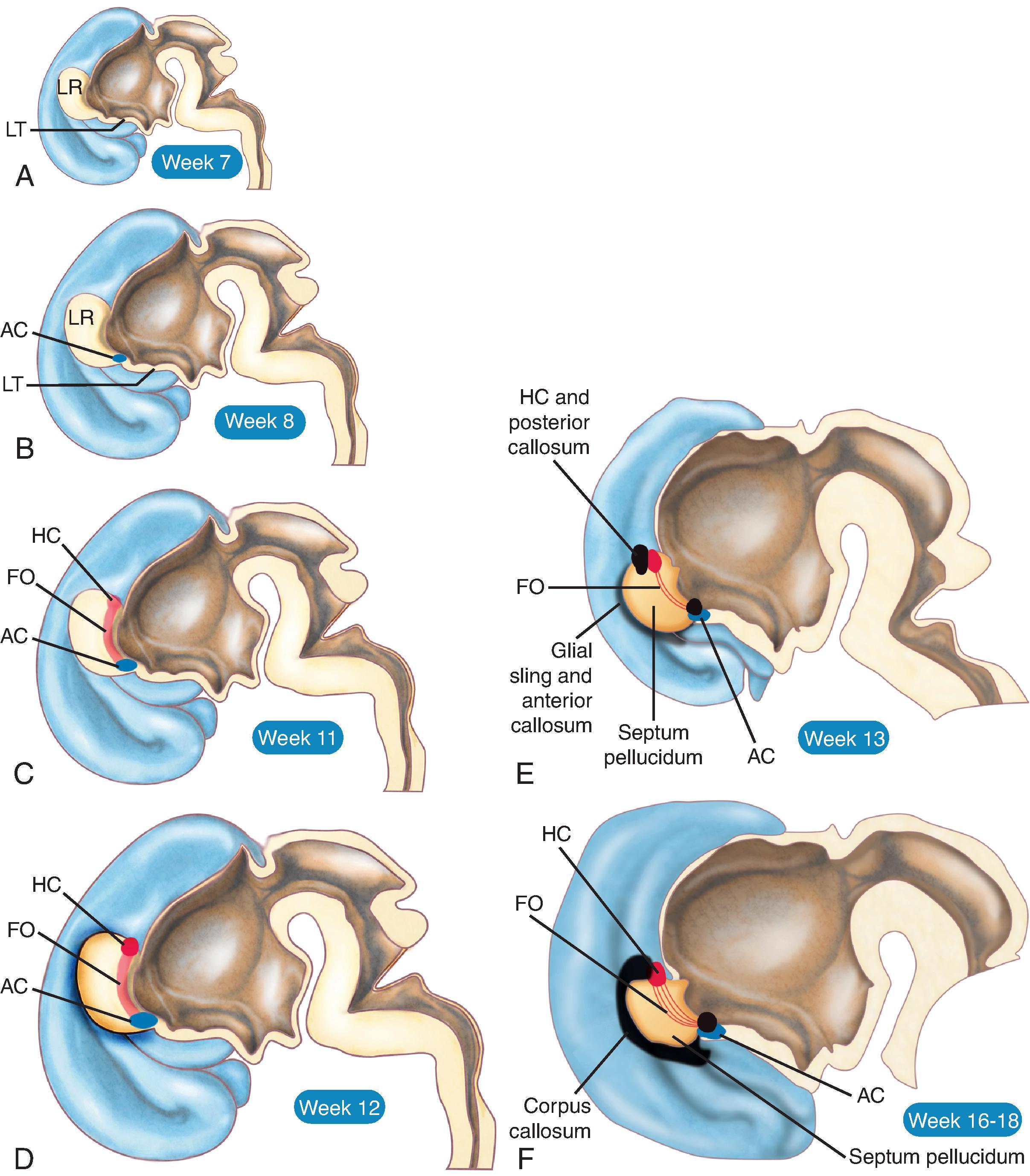
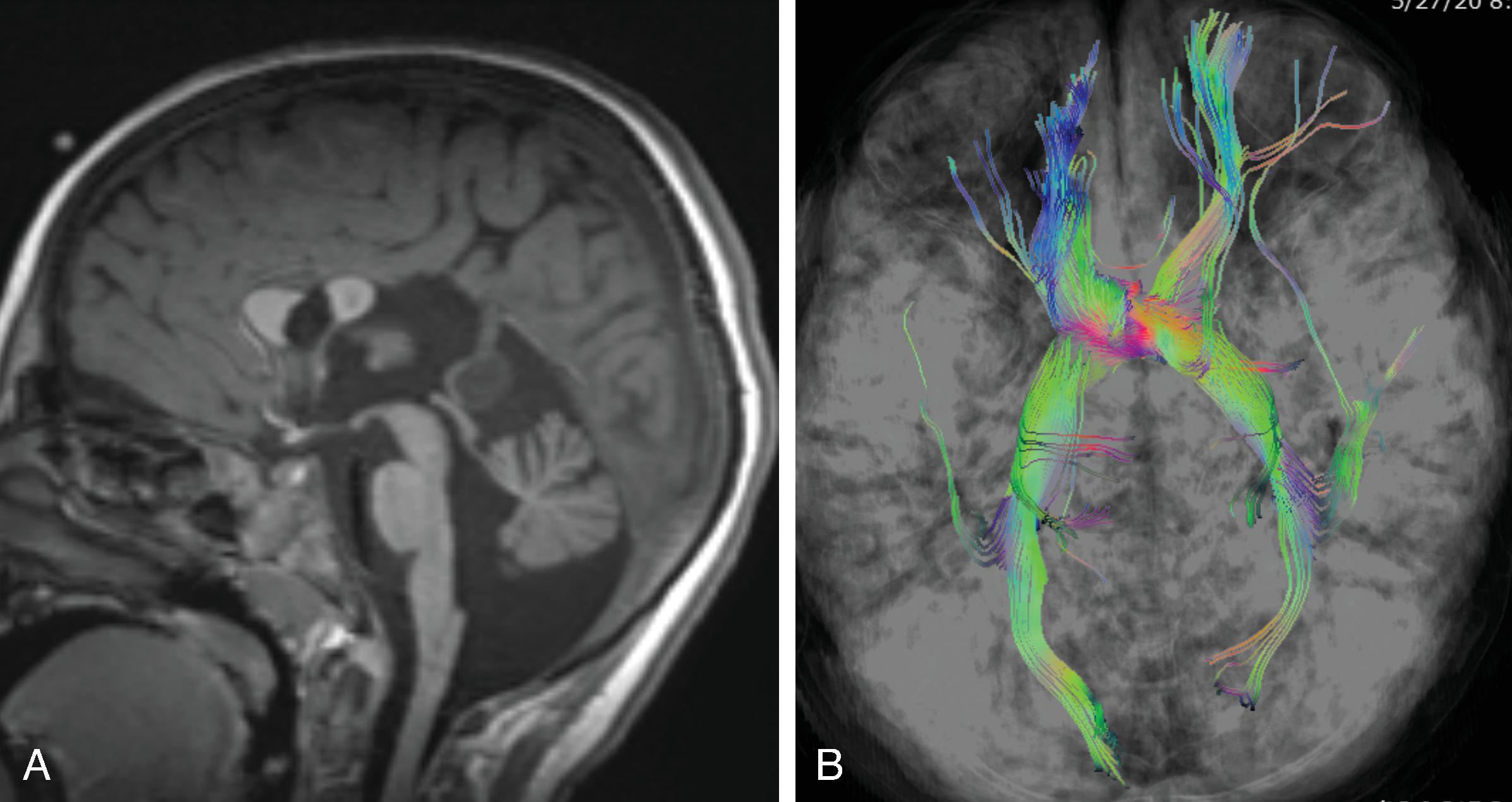

The anterior to posterior segmentation of corpus callosum includes the rostrum, genu, body, isthmus, and splenium.
The majority of callosal fibers join symmetric areas of the cerebral cortex.
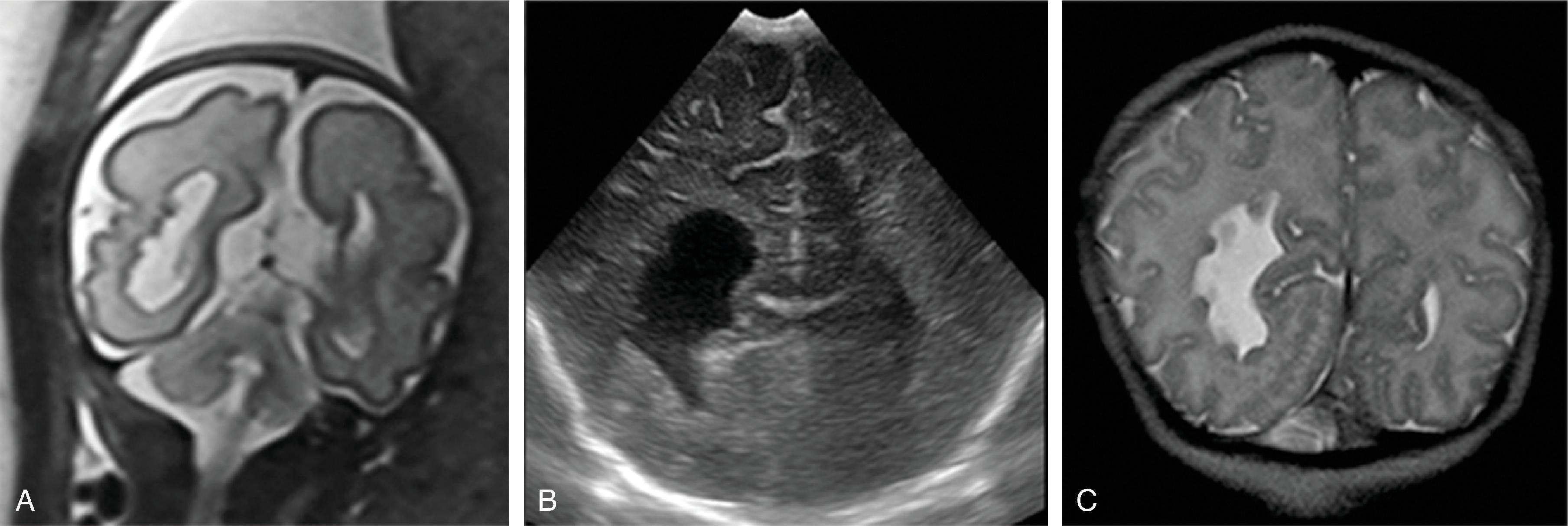
The corpus callosum should be present on imaging at 18 weeks’ gestation. The cavum septum pellucidum is closely associated with formation of the corpus callosum and should be visible by 18 weeks on ultrasound. Absence of the cavum septum pellucidum raises concern for a malformation of the corpus callosum. Sagittal T2W imaging is typically the best plane for visualizing the entire corpus callosum.
As the brain develops, the corpus callosum becomes longer and thicker. Biometry data of the corpus callosum indicate most of the postnatal enlargement of the corpus callosum occurs in the first 4 years of life, after which the size is essentially stable. While subjective assessment of callosal length and thickness is usually done in routine clinical care, biometry data can be useful for in some patients and are provided in Chapter 1 .
The following examples will demonstrate malformations of the corpus callosum on imaging.
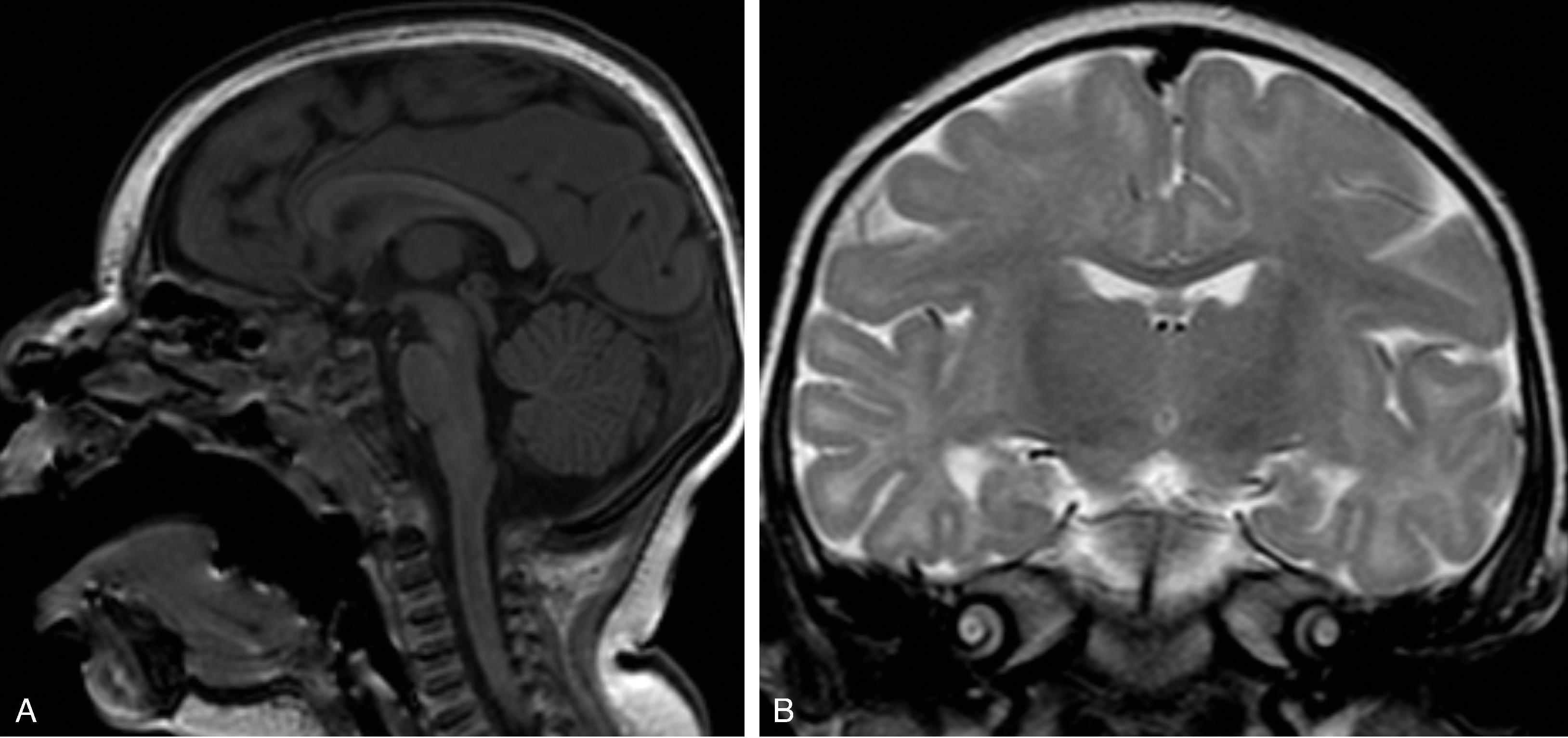
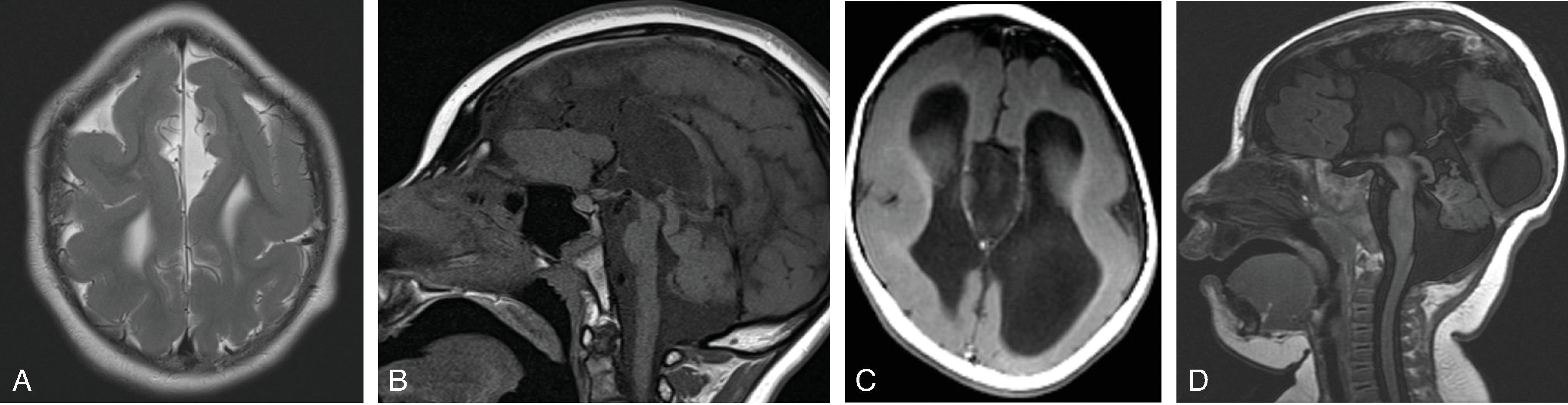
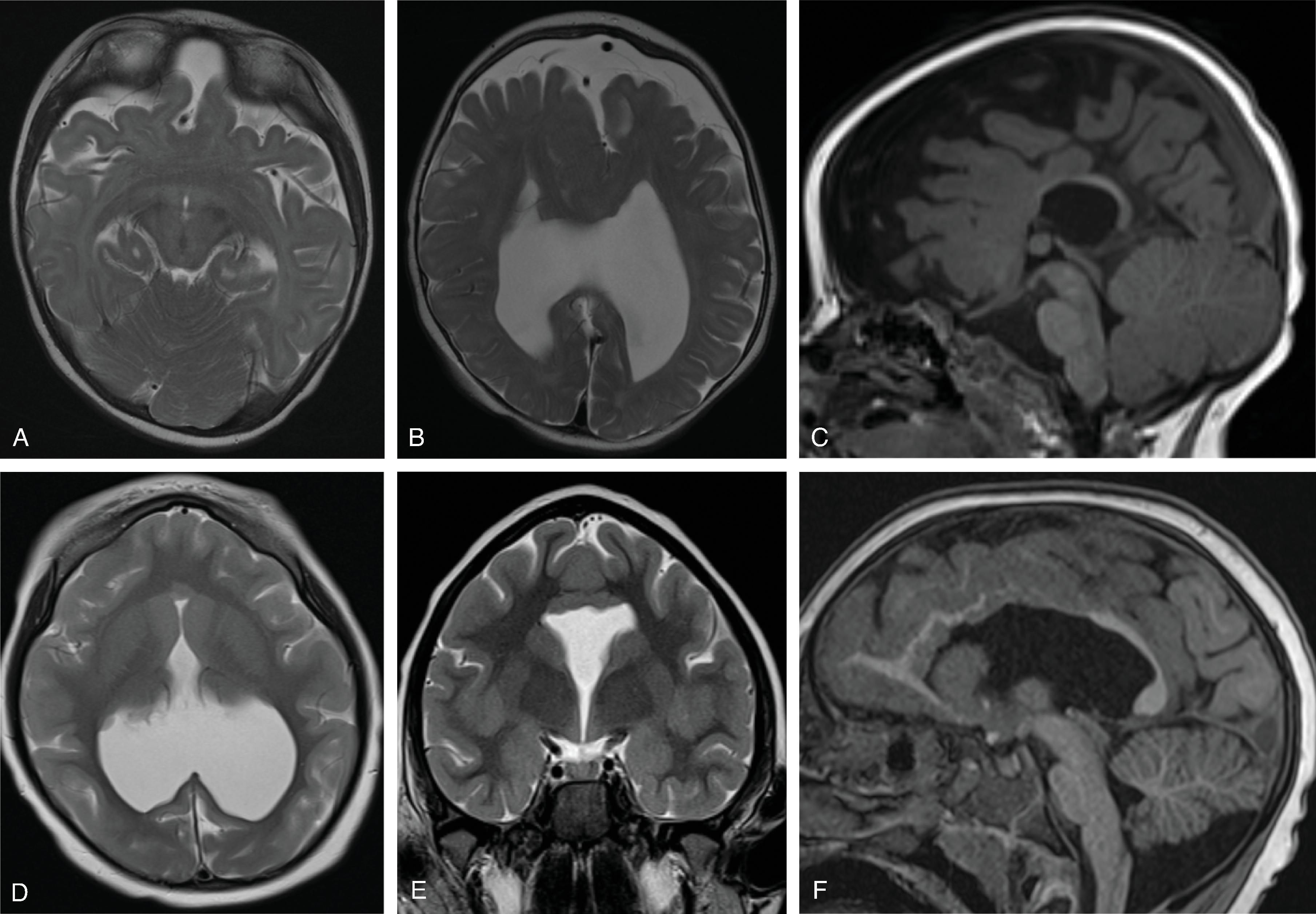
Agenesis, hypogenesis, and dysgenesis are malformations of the corpus callosum occurring from 8–20 weeks’ gestation. Agenesis refers to complete absence of the corpus callosum. Hypogenesis refers to partial formation of the corpus callosum. Dysgenesis refers to abnormal formation of the corpus callosum.
Prevalence is ∼1:10,000 live births.
Etiologies include genetic, infectious, and teratogenic causes. Corpus callosum malformations should be differentiated from destructive lesions (e.g., adjacent white matter ischemic lesion), which are discussed later in this chapter, or high-grade thinning (e.g., high-grade obstructive hydrocephalus).
Malformations of the corpus callosum can be isolated or associated with cerebral anomalies (∼50% of patients) and extracerebral anomalies (∼60% of patients).
Over 80 chromosomal, genetic, and sporadic syndromes are associated with malformation of the corpus callosum. Approximately 50% to 80% of patients have an associated syndrome and malformation, and chromosomal anomalies are found in 15% to 20% of patients.
Prognosis is variable for callosal malformations and ranges from normal to developmental delay, epilepsy, and behavioral disorders. Prognosis is influenced by the presence of additional anomalies. Outcomes in isolated agenesis of the corpus callosum are normal in ∼75%, borderline or moderate disability in 14%, and severe disability in 11% of patients. Outcomes in partial agenesis of the corpus callosum are normal in ∼66%, borderline or moderate disability in 7%, and severe disability in 27% of patients.
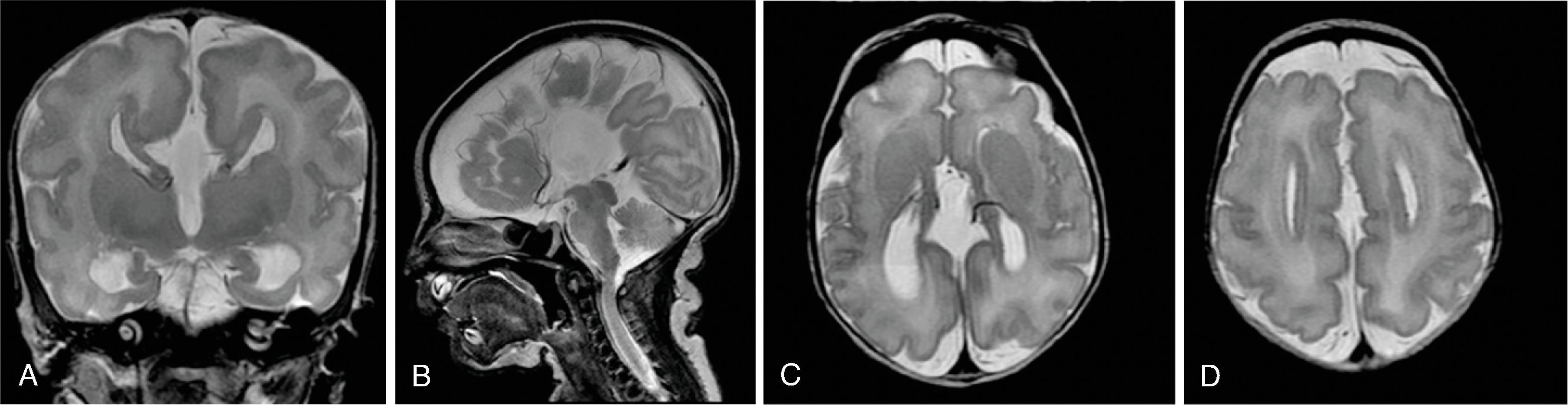
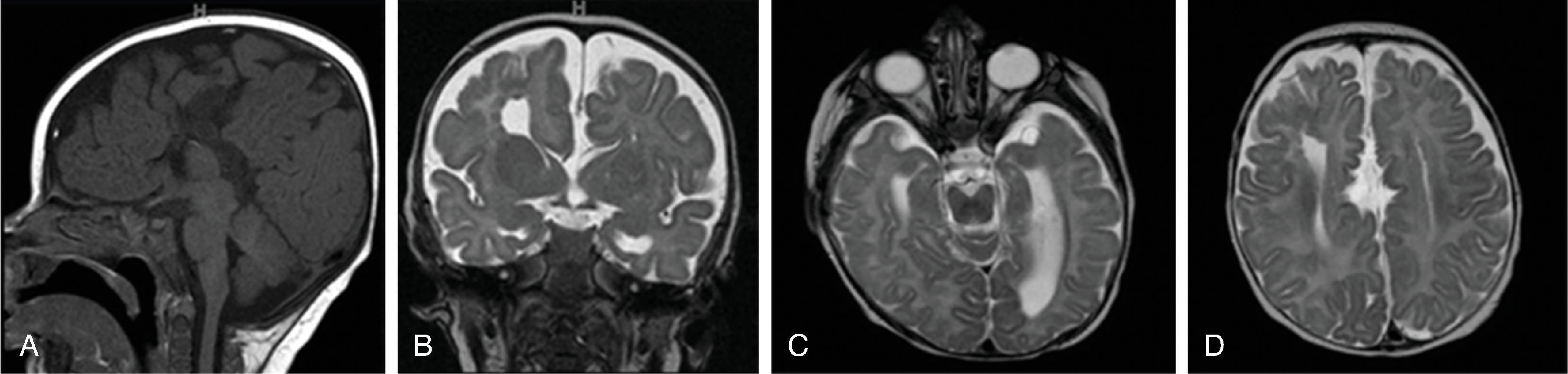
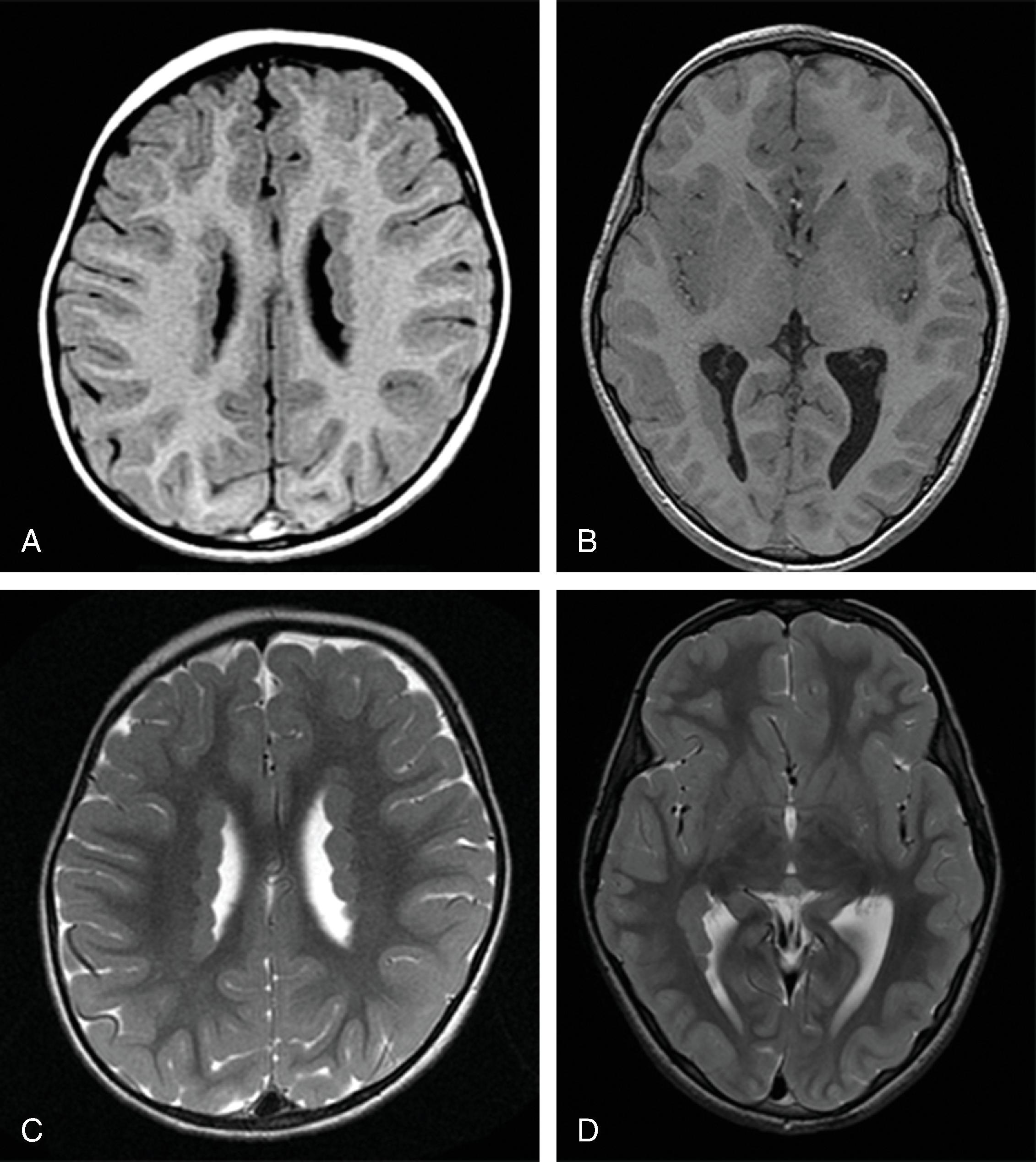
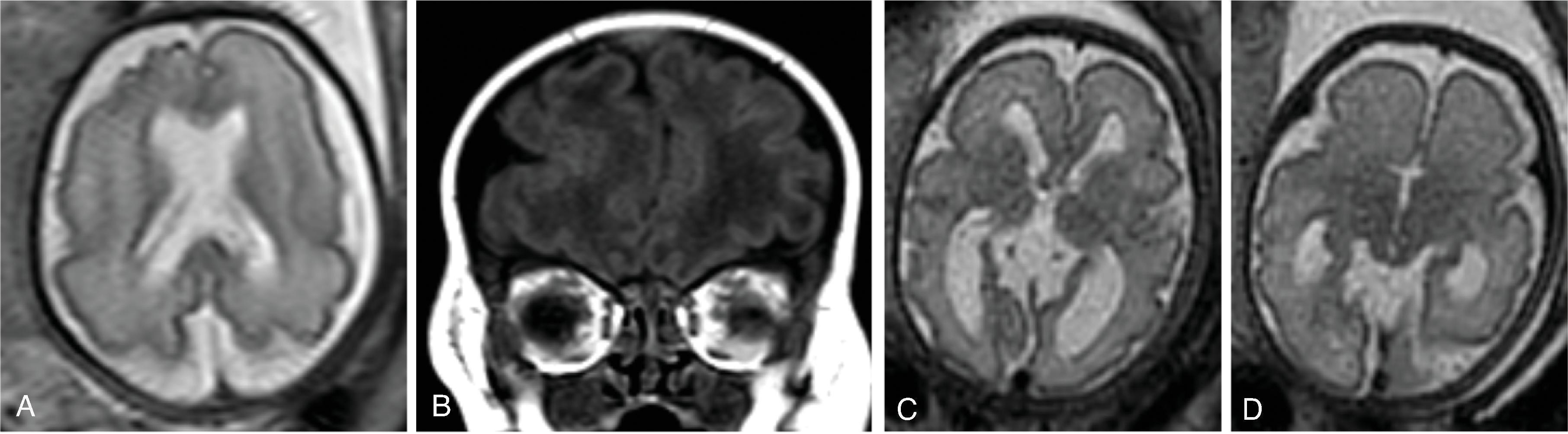
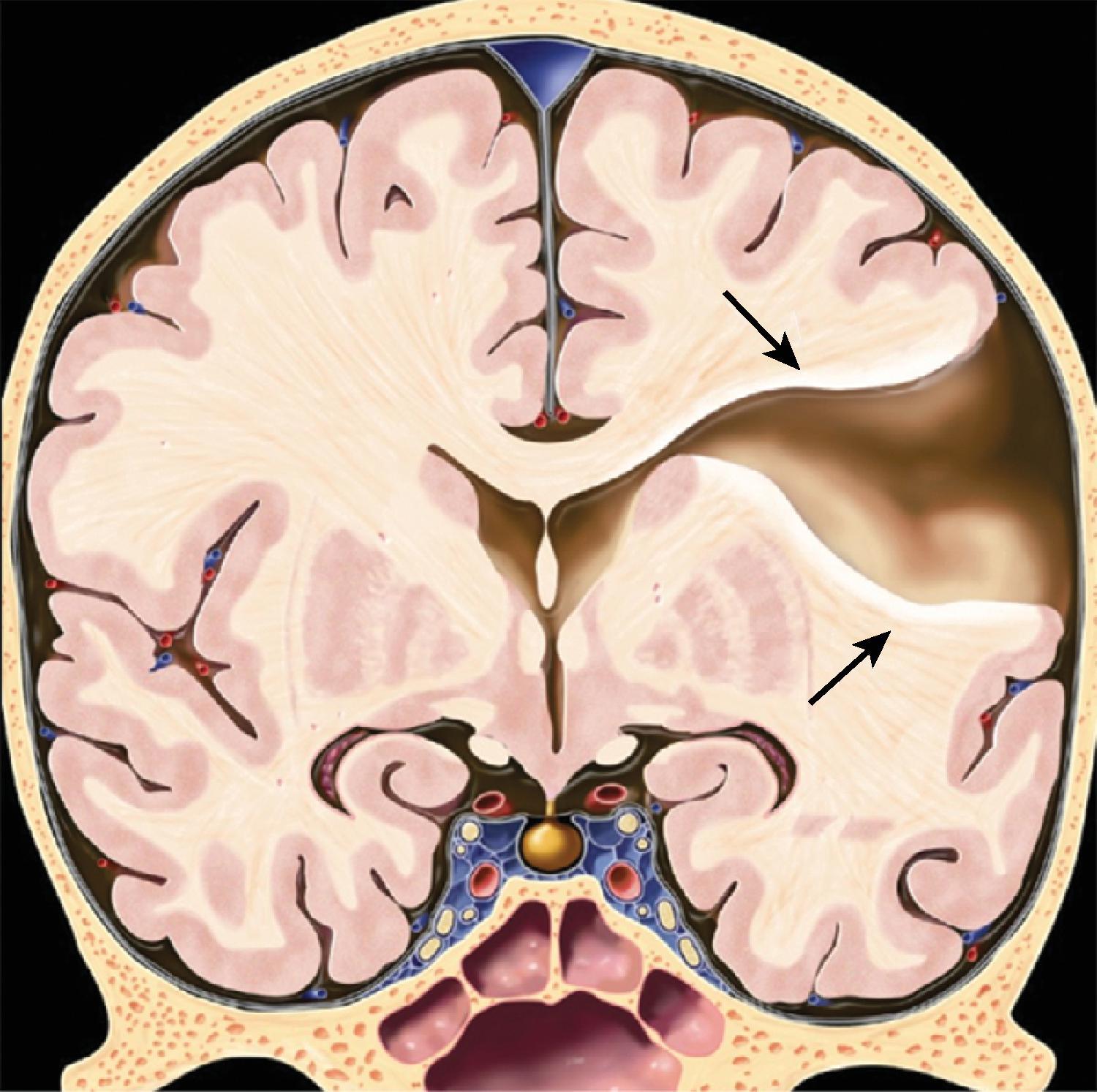
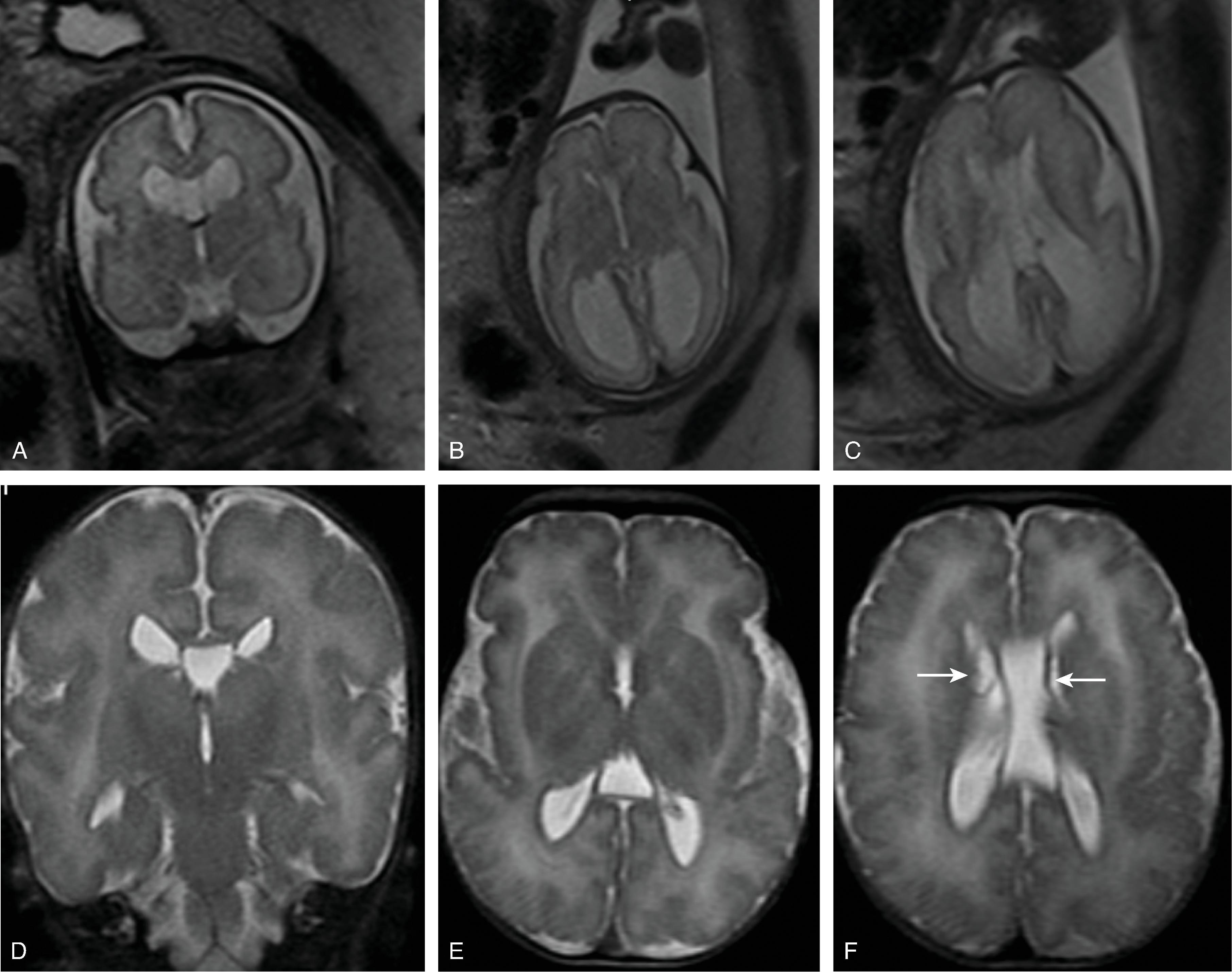
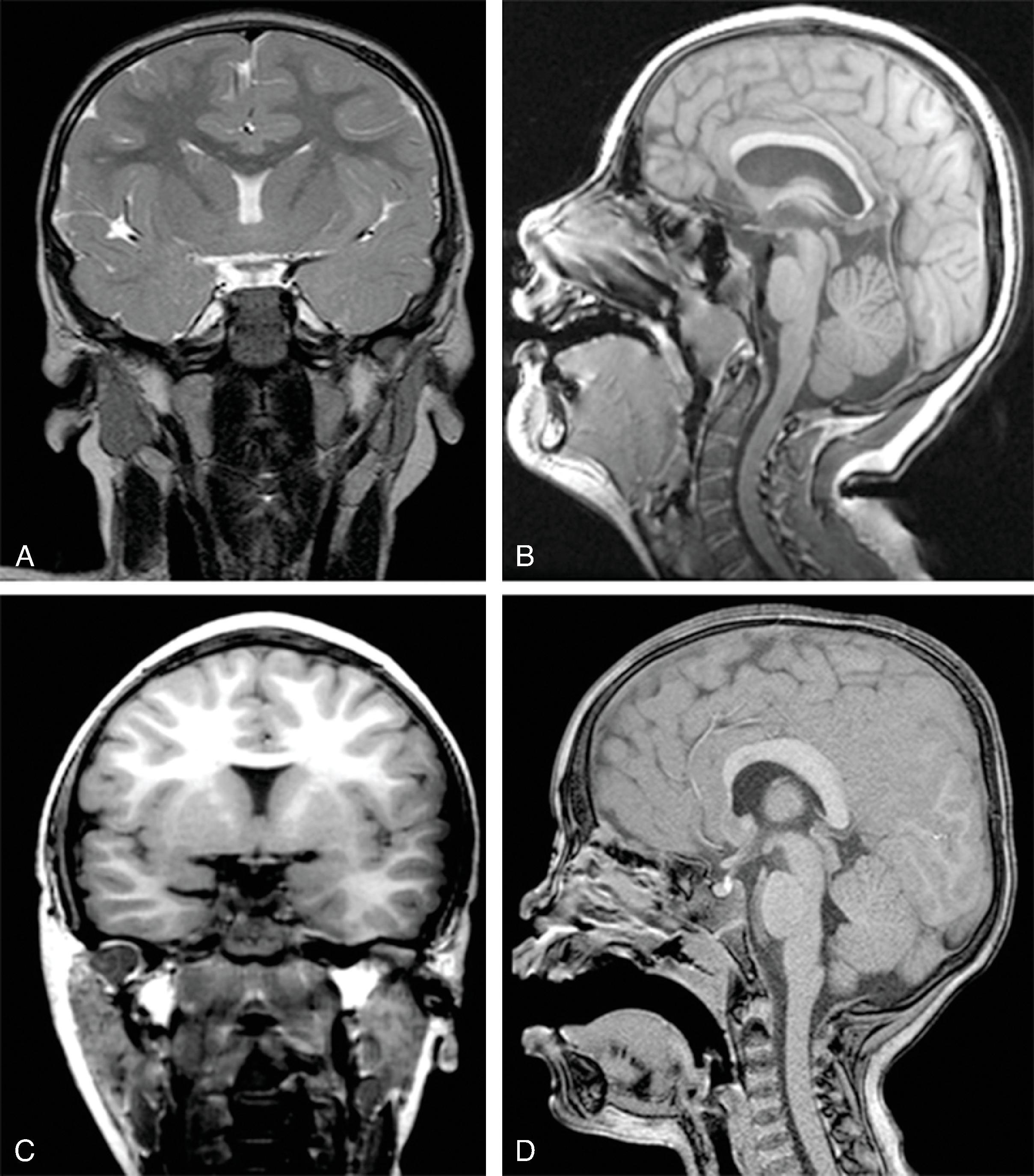
In agenesis of the corpus callosum, the corpus callosum and hippocampal commissure are absent, and the anterior commissure is absent in about half of patients.
In hypogenesis of the corpus callosum, a variable amount of corpus callosum is present from anterior to posterior, the hippocampal commissure may be absent depending on the degree of dysgenesis, but the anterior commissure is always present.
Imaging findings include the following:
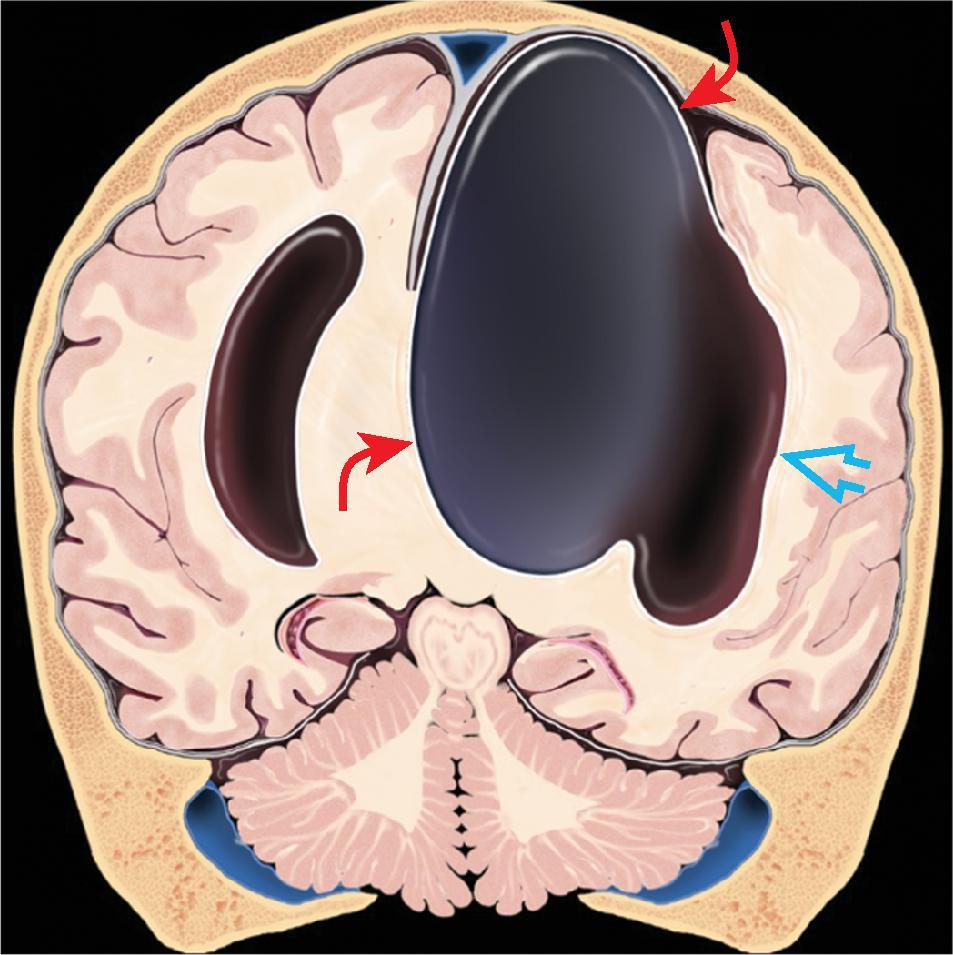
Partial or complete absence of the corpus callosum
Absent cingulate gyri and sulci
Perpendicular radiation of midline gyri from the third ventricle to the periphery
Widened interhemispheric fissure
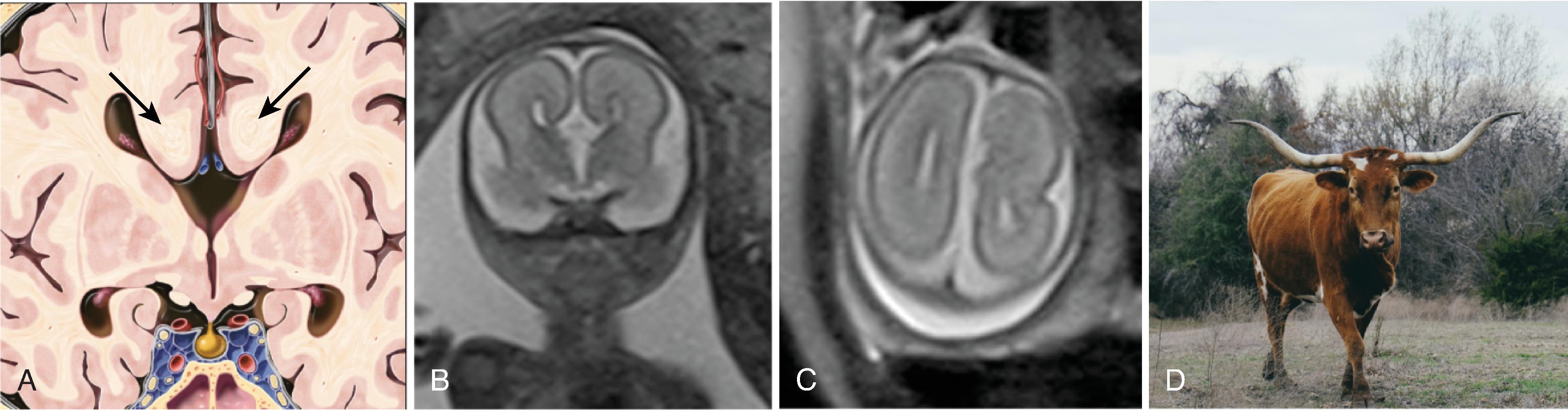
Parallel orientation of the lateral ventricles and widened atria of the lateral ventricles (colpocephaly)
White matter bundles known as the Probst bundles course along the superomedial border of the lateral ventricles
Elevated third ventricle and lateral ventricles mimic a Texas longhorn configuration on coronal imaging
Absent septum pellucidum
Absent or hypoplastic anterior and hippocampal commissures
Malrotated, vertically oriented ventral hippocampi
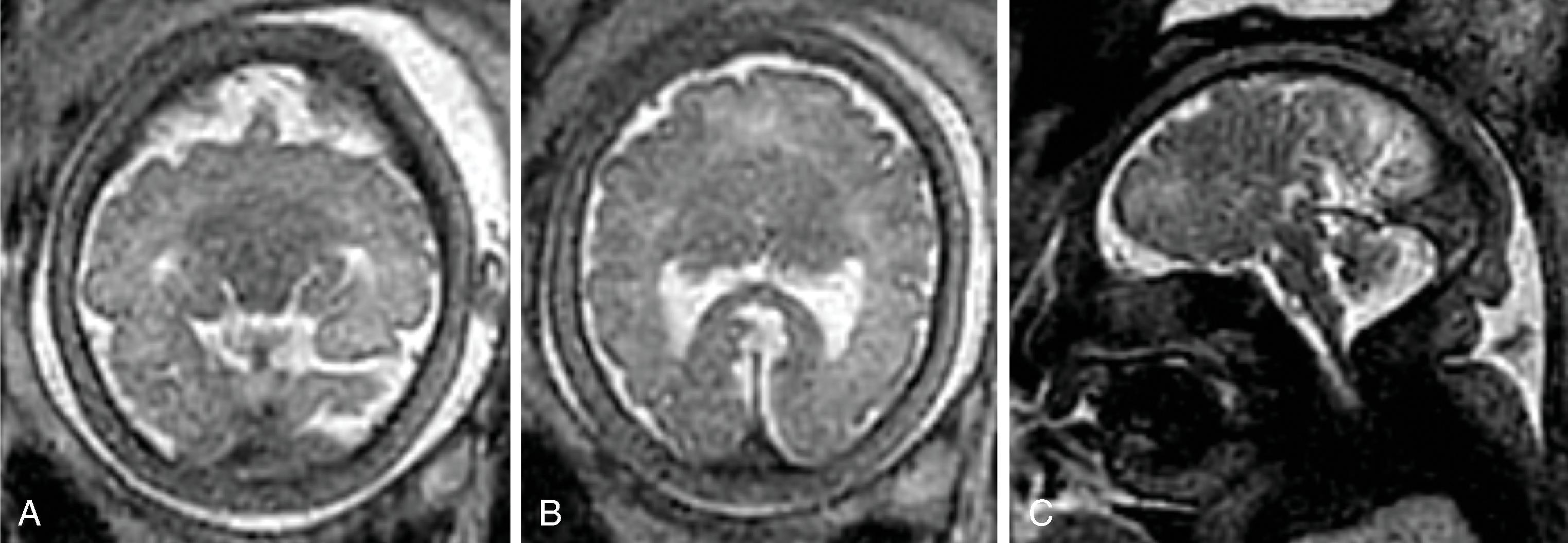
Diffusion tensor imaging show a lack of fibers crossing the midline matching the area of the missing corpus callosum.
Postnatal MRI is recommended in patients as ∼15% of prenatally diagnosed isolated agenesis of the corpus callosum are found to have additional anomalies on postnatal imaging.
Additional malformations, such as pituitary gland anomalies, migrational abnormalities (most commonly gray matter heterotopia), or bilateral schizencephaly, should be excluded.
Corpus callosum malformations may also be part of complex brain malformations, including Aicardi syndrome, Chiari II malformation, or Dandy-Walker syndrome.
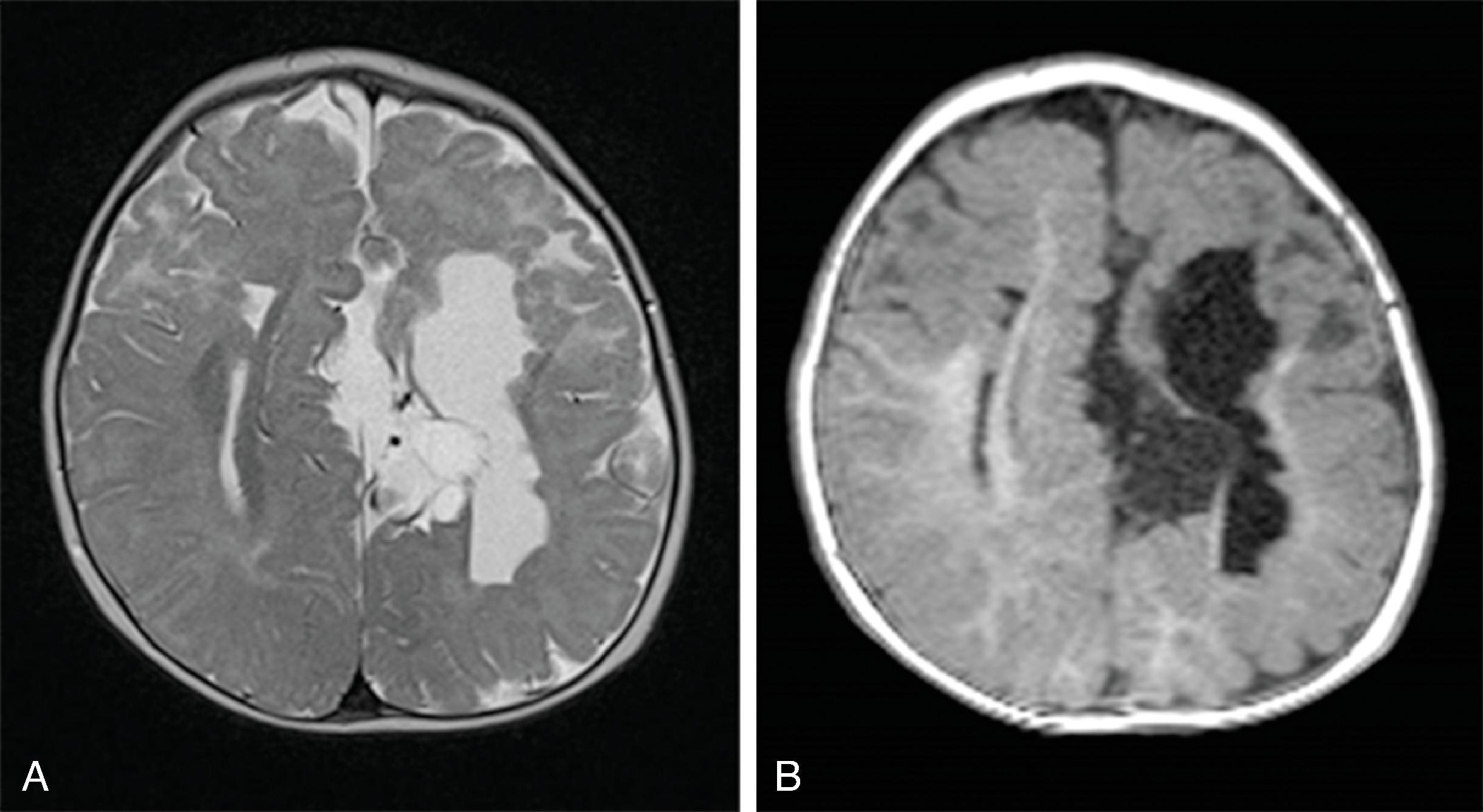
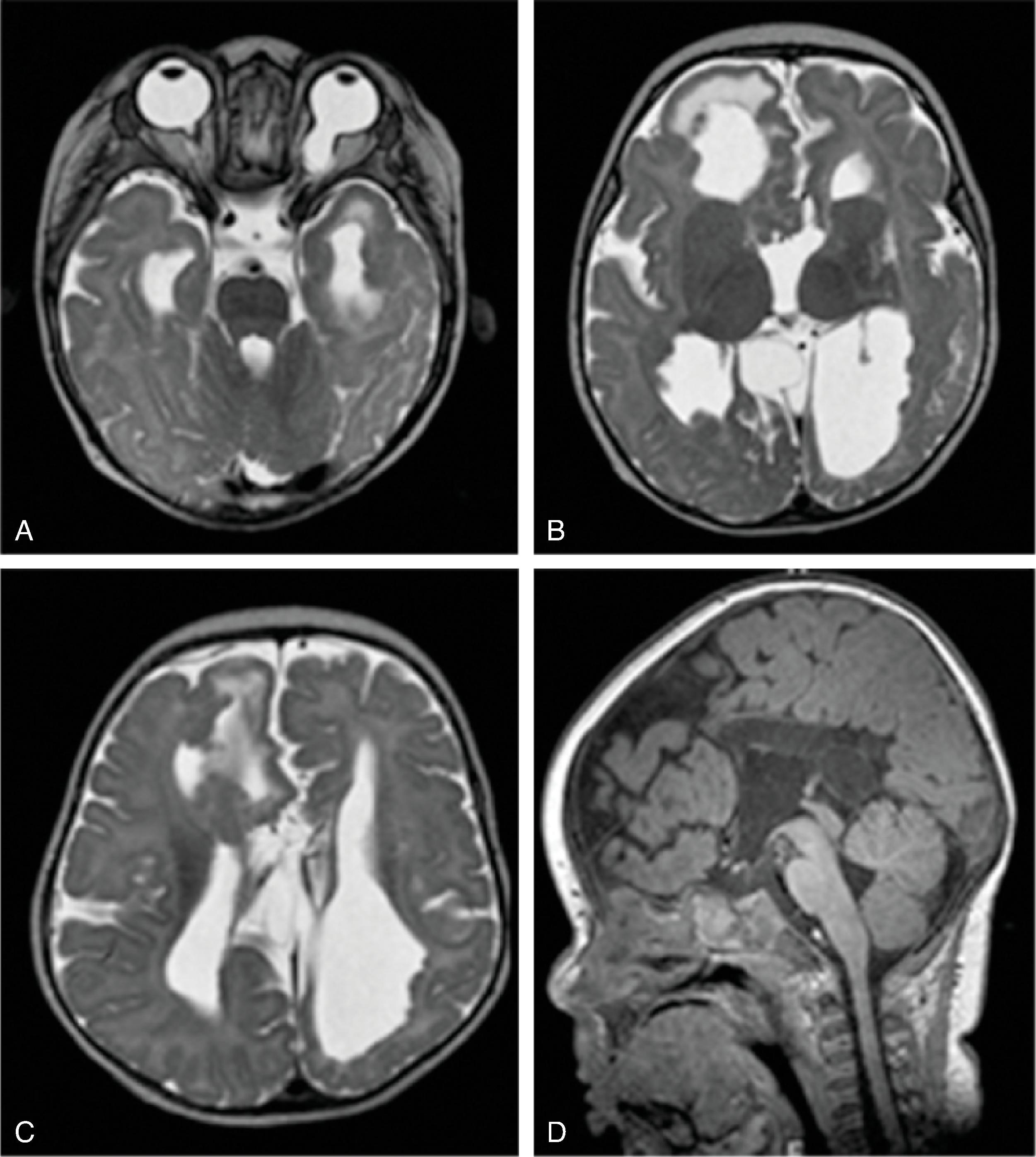
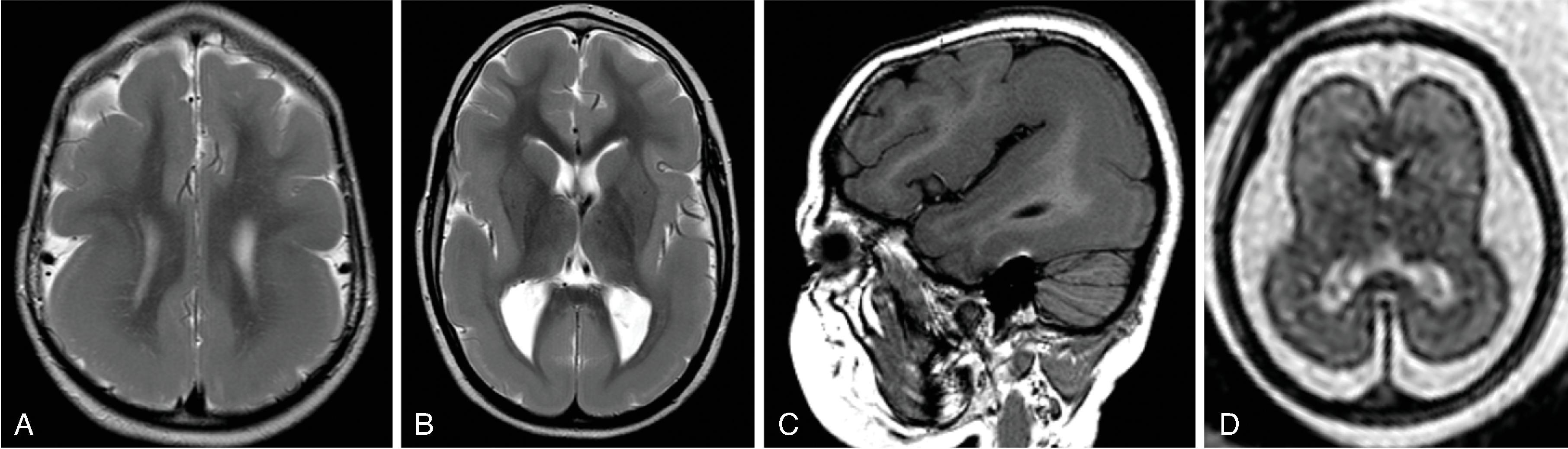
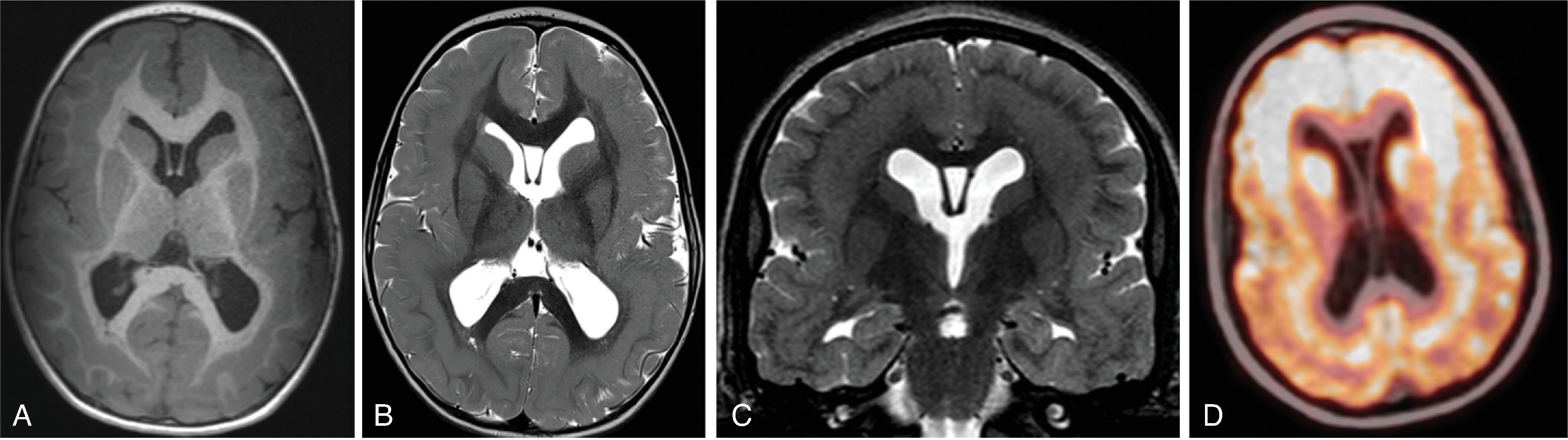
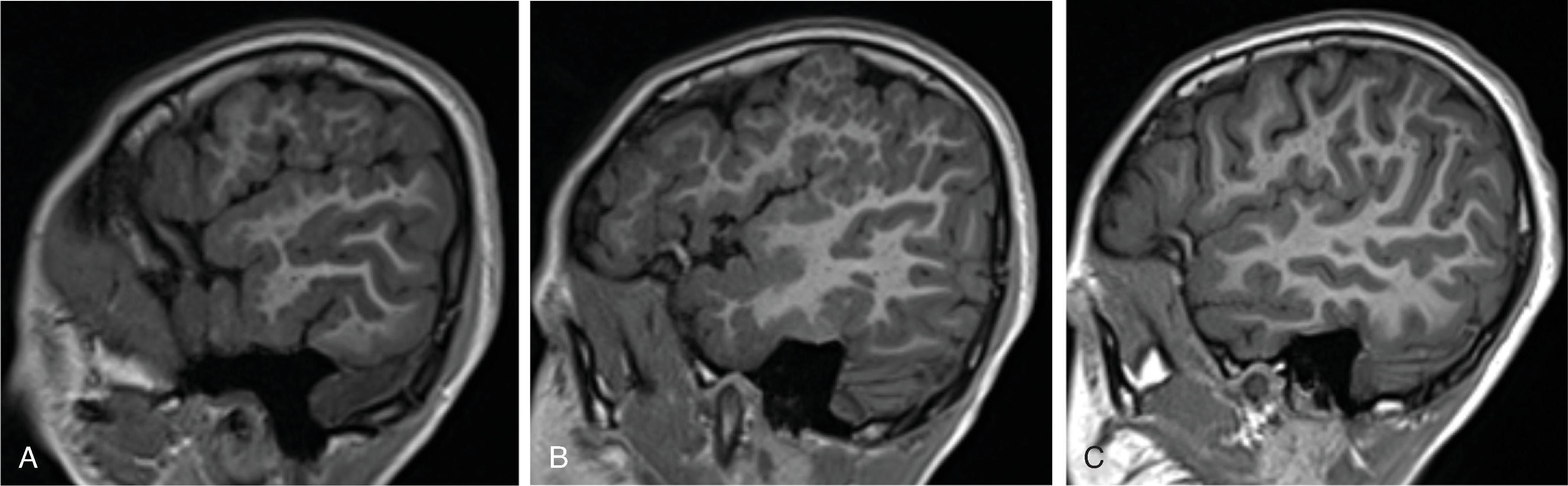
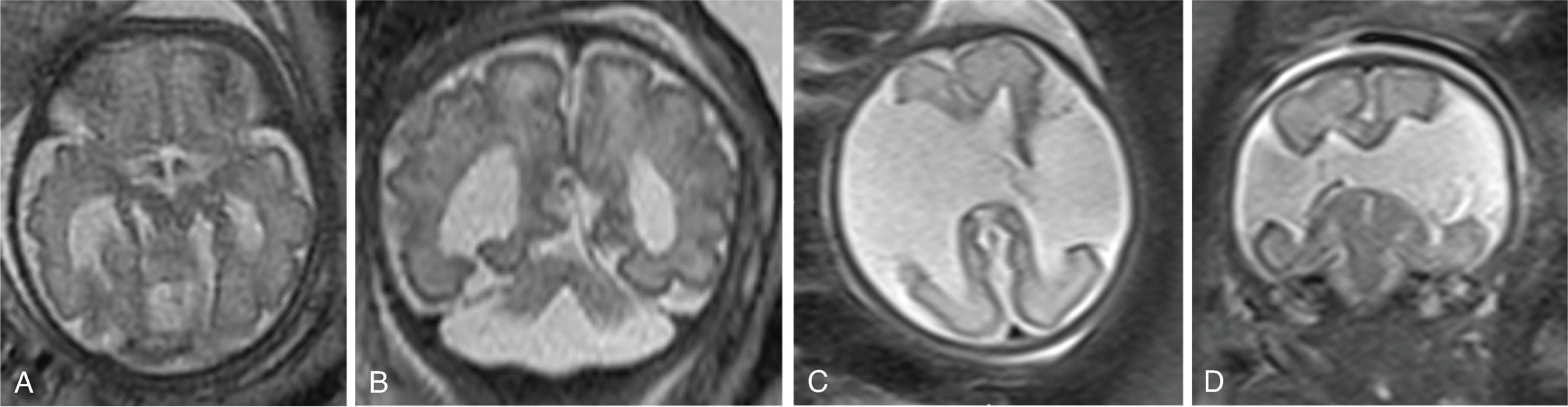
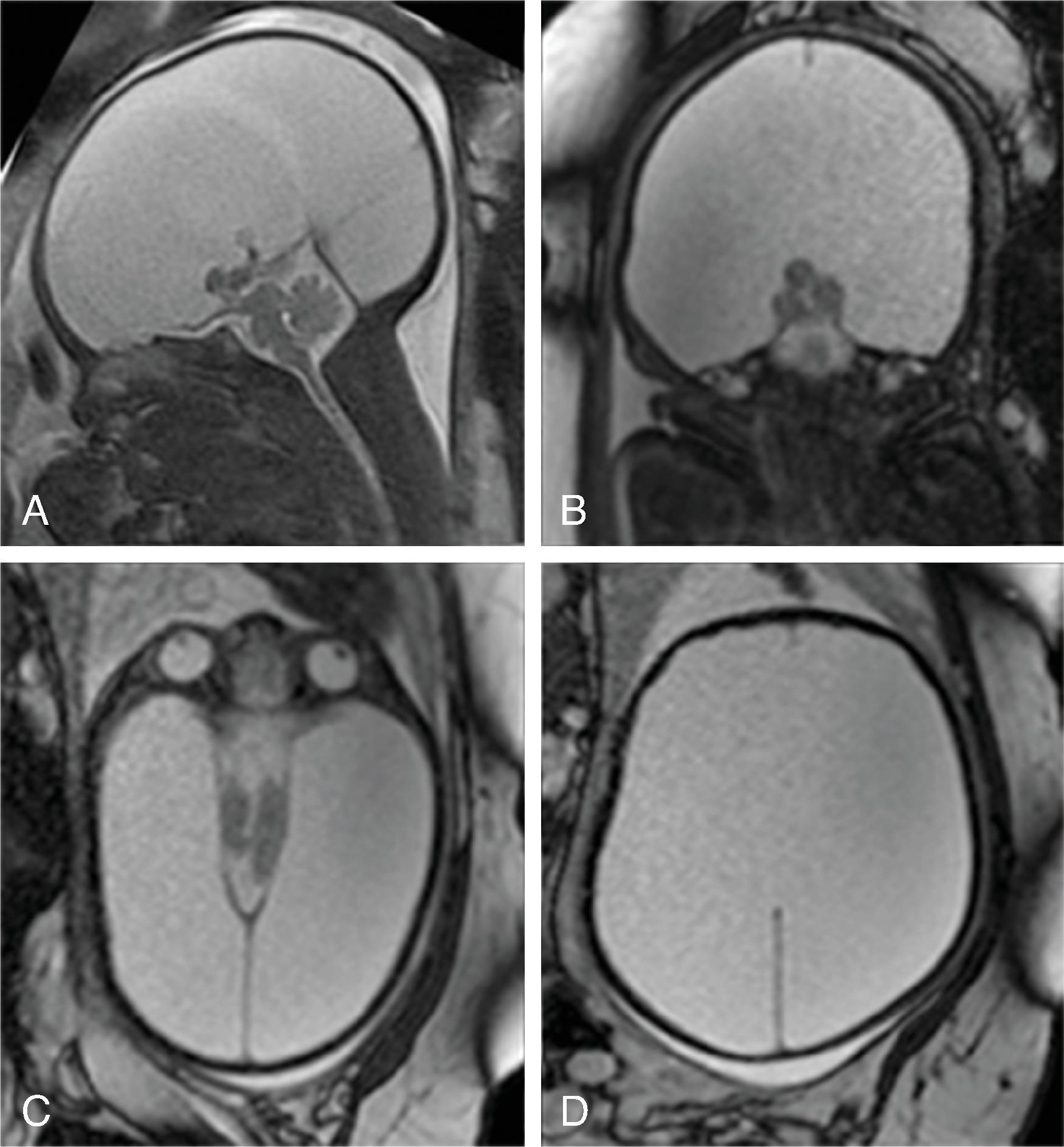
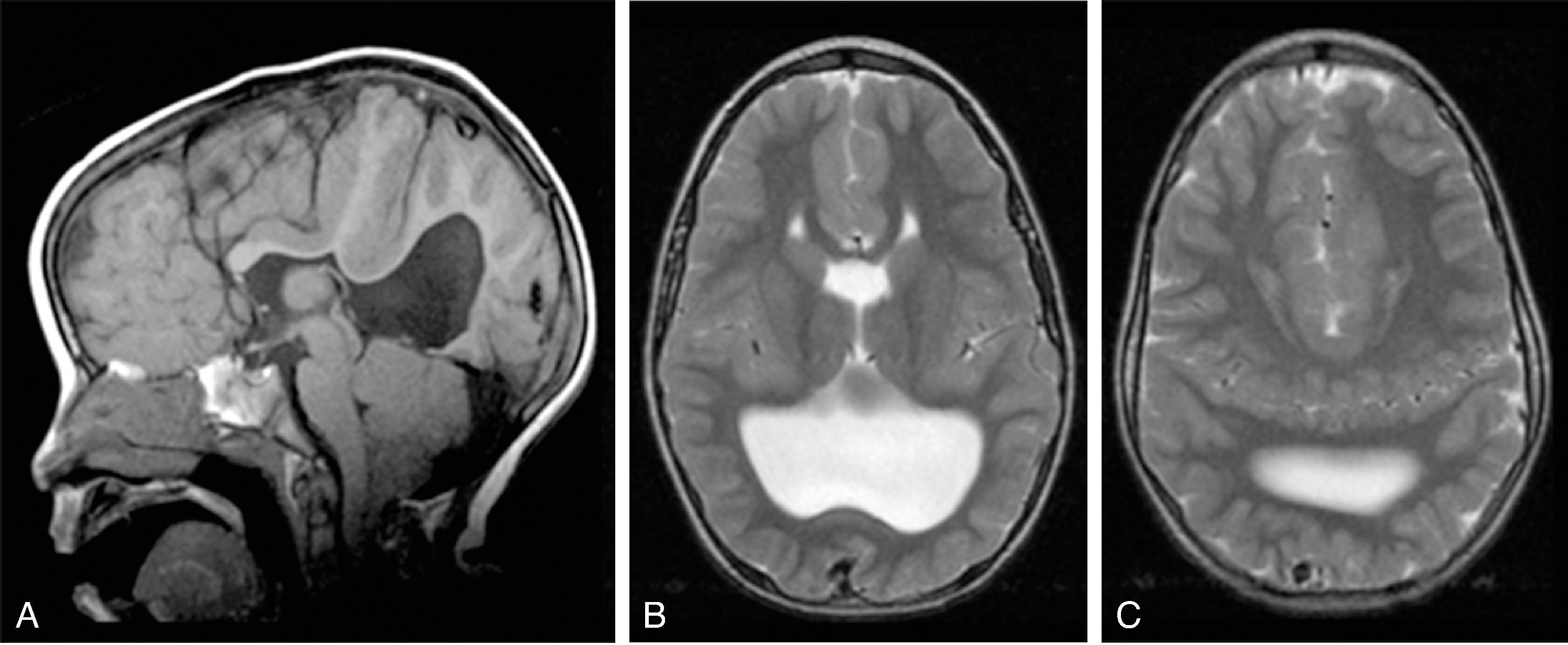
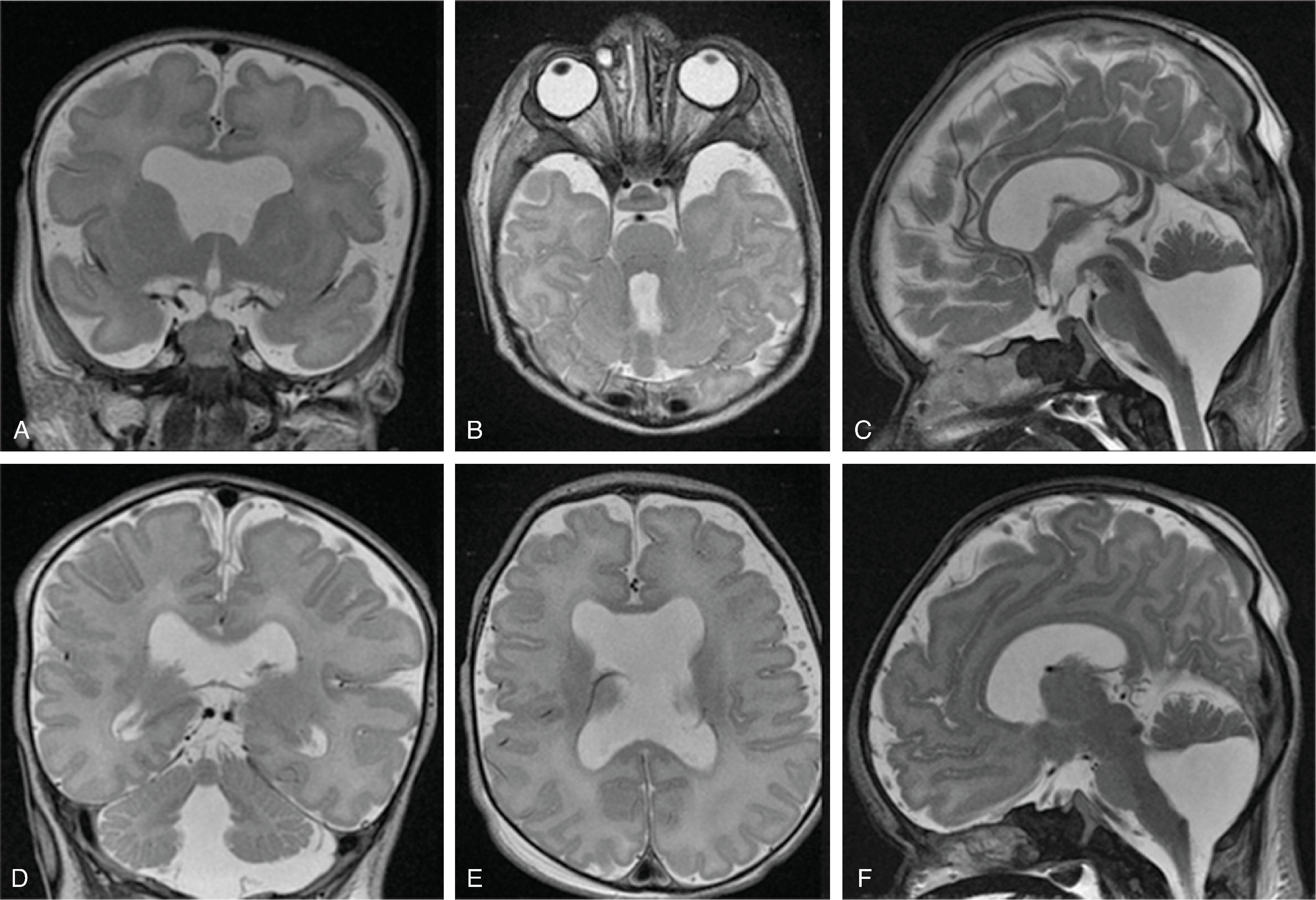
Corpus callosum malformations may be associated with interhemispheric cysts, also known as cystic meningeal dysplasia.
This malformation is believed to be secondary to a disruption of the normal development of the commissural plate and adjacent cortex. The cysts can occur from expansion of the roof of the third ventricle due to lack of an overlying corpus callosum or multiple cysts due to abnormal differentiation of the primitive meninges.
Often presents early with macrocephaly.
Prognosis is variable, ranging from normal development to epilepsy, developmental delay, and neurologic deficits.
In a series of 40 patients with asymmetric ventriculomegaly, interhemispheric cyst and agenesis or dysgenesis of the corpus callosum (known as AVID), 26 (65%) survived more than 2 years of age and of these 26, 12 (46%) had epilepsy, 14 (54%) could sit independently, 4 (16%) were in mainstream schools, 16 (62%) had expressive language problems, and 7 (28%) had near normal development without seizures.
Often requires ventricular shunting.
Callosal agenesis or dysgenesis, absent septum pellucidum, and an interhemispheric cyst are the hallmark imaging findings. Often seen as asymmetric ventriculomegaly as the cyst communicates with one ventricle. Diencephalic-mesencephalic junction dysplasia and aqueductal stenosis can be associated with agenesis of the corpus callosum with interhemispheric cyst.
Based upon their morphology they can be classified in two types:
Type 1: The cyst is communicating with the ventricle , likely represents a diverticulum of the ventricles, and is T1- and T2-isointense to CSF.
Type 2: The cyst is not communicating with the ventricle . Typically multiple cysts are present and the cysts are T1-hyperintense relative to CSF. Type 2 cysts are often associated with migrational abnormalities, including heterotopias and cortical malformations like polymicrogyria, or may be part of syndromes. Type 2 cysts often require ventricular shunting. Aicardi syndrome can have this appearance.
Differential diagnosis:
Porencephaly: Should have normal head size, associated gliosis or hemorrhage, and no mass effect
Schizencephaly: Gray matter lined cleft without mass effect
Arachnoid cyst: Located over the convexity rather than midline
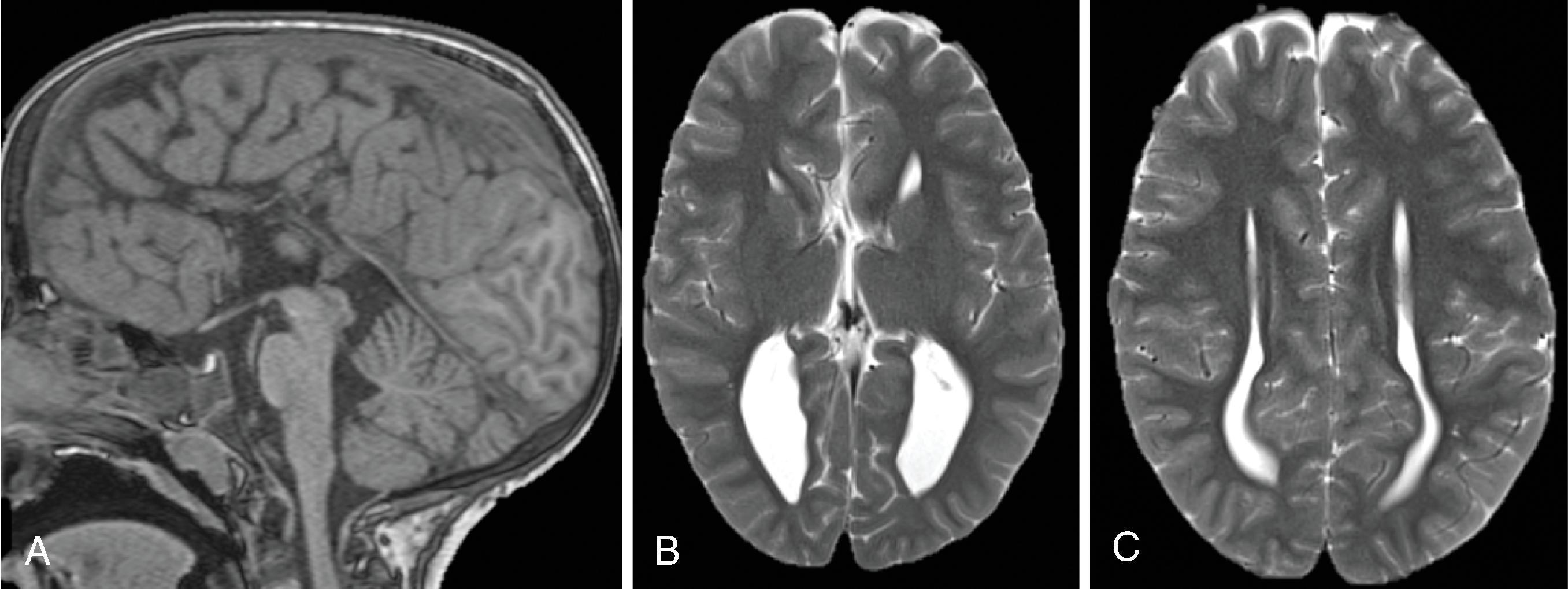
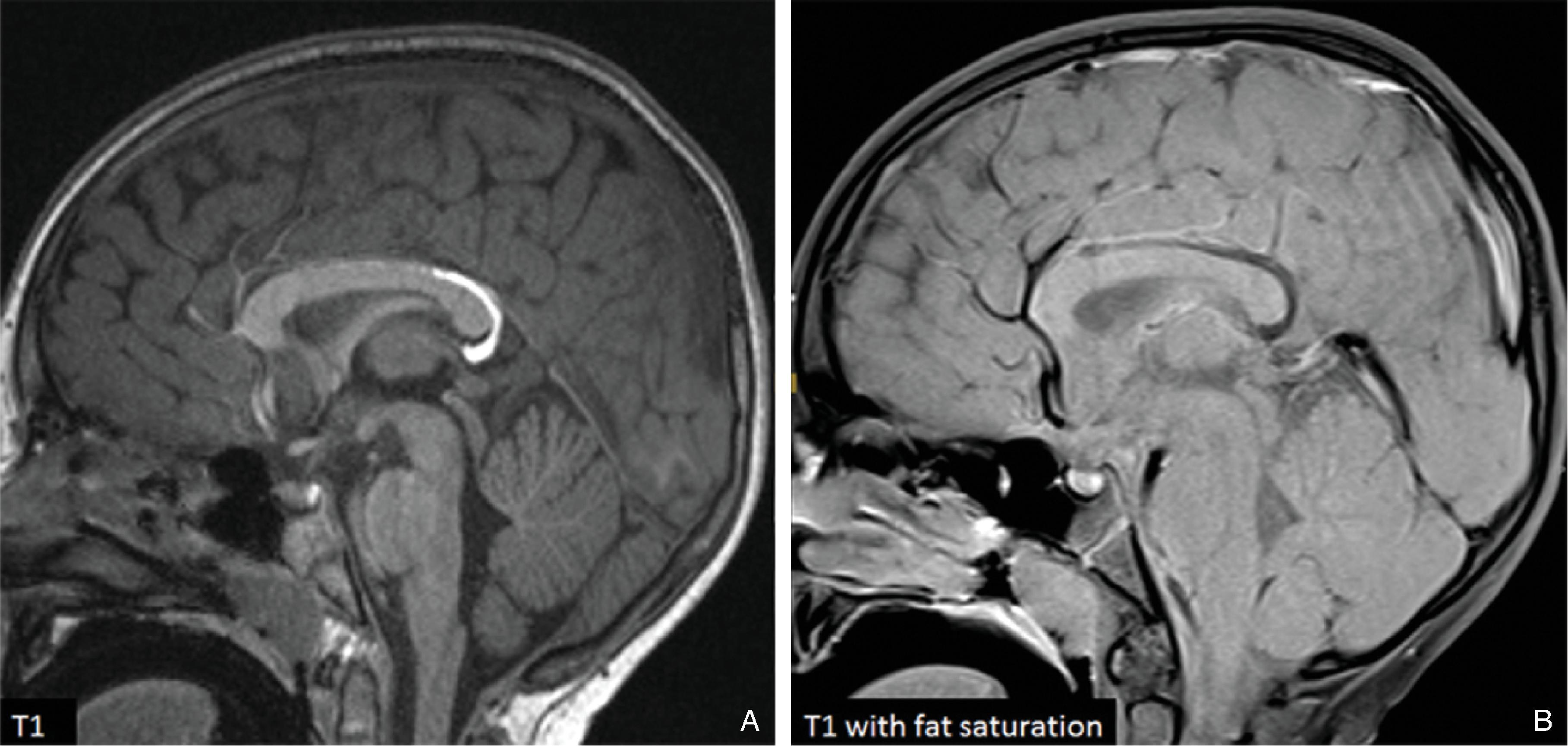
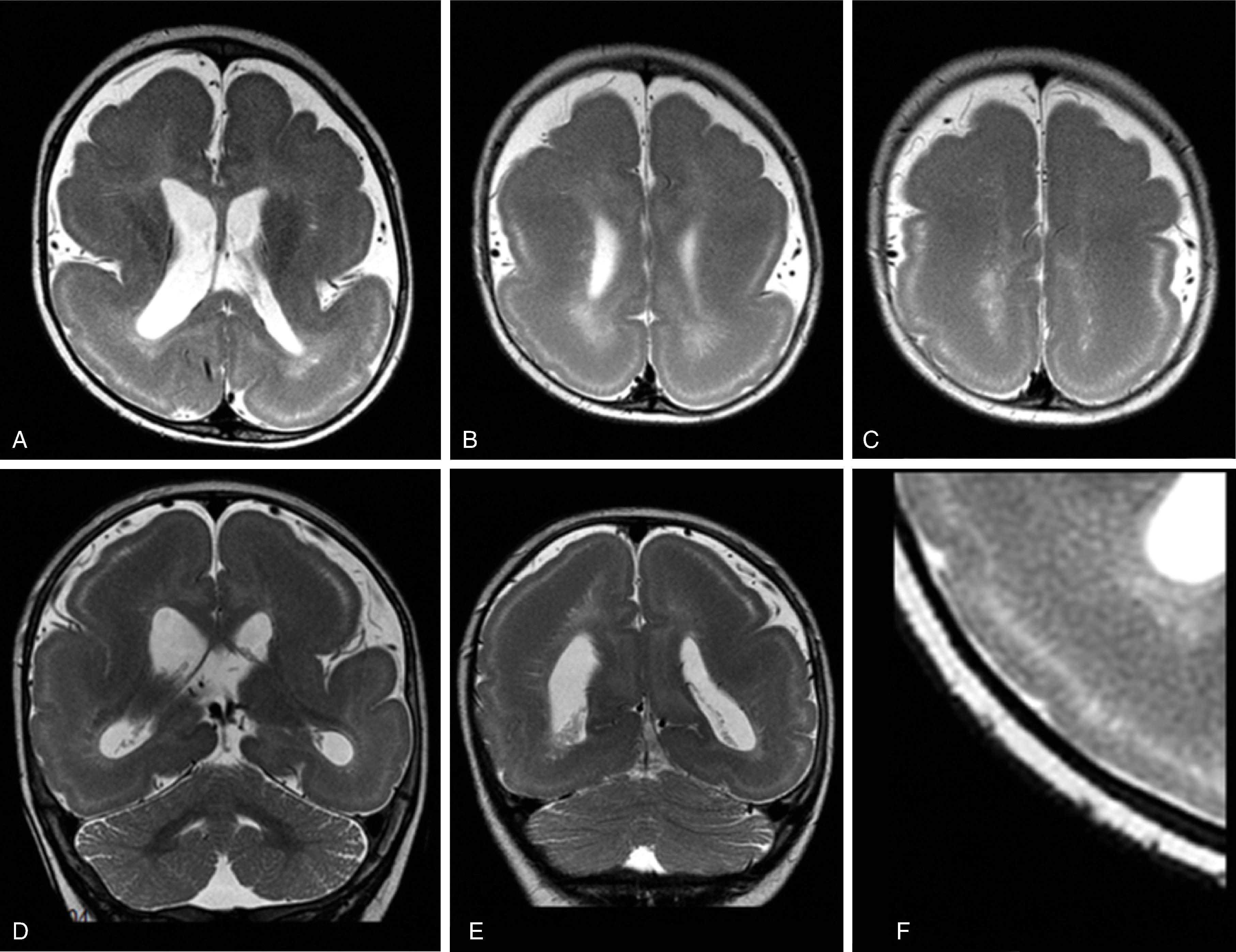
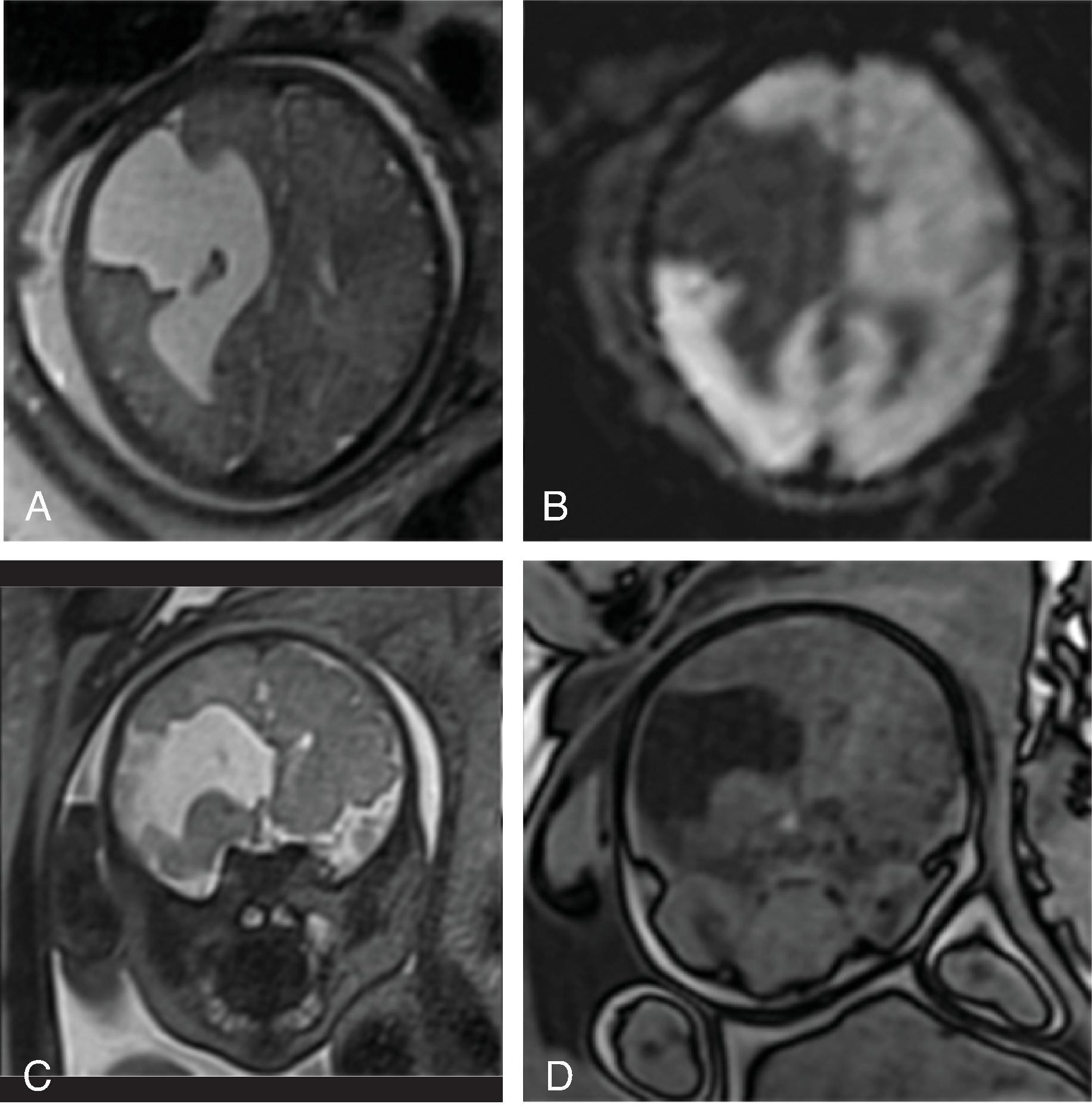
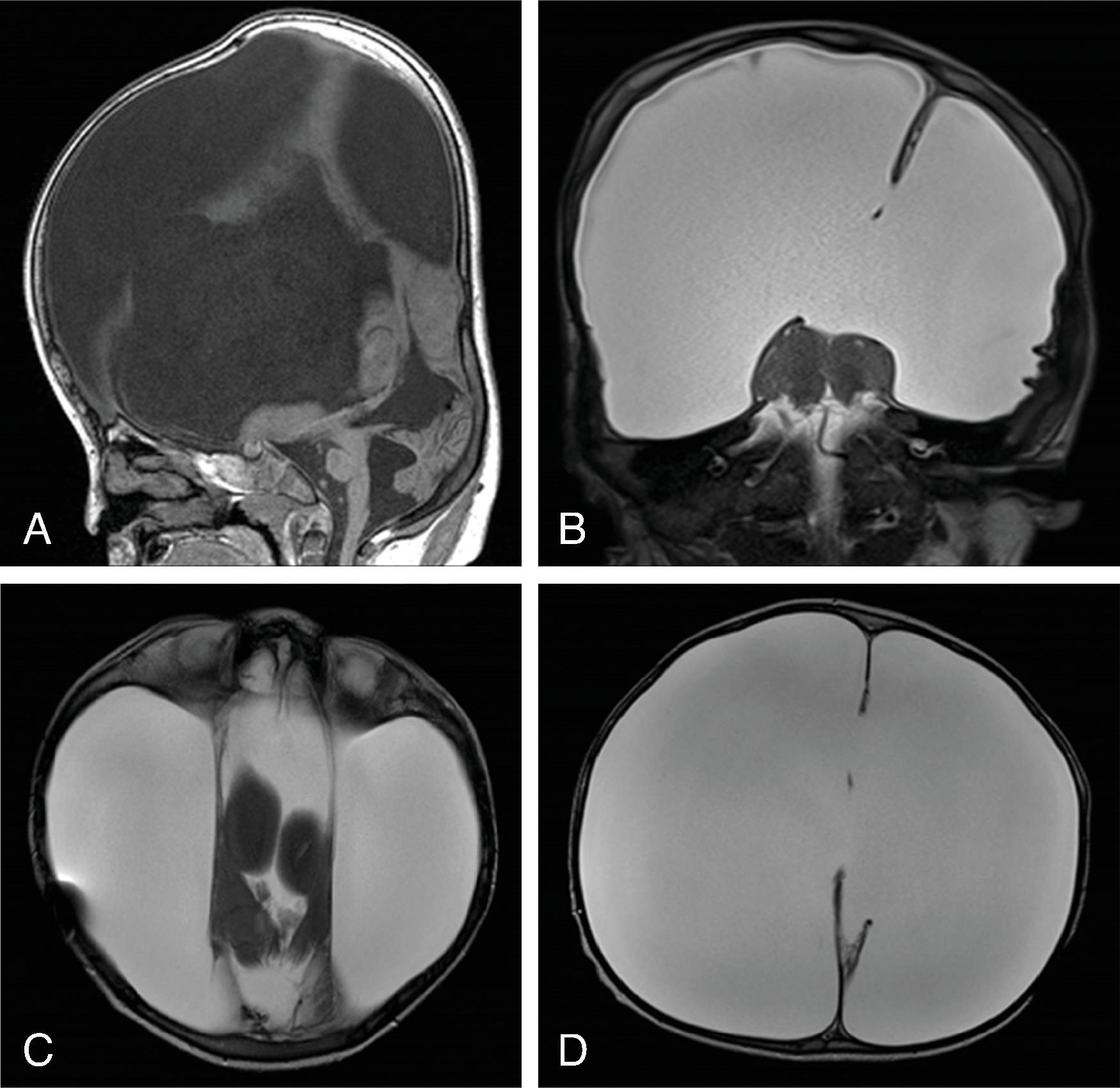
Midline lipomas are believed to be secondary to an abnormal differentiation of the meninx primitive. The meninx primitive can disrupt the development of the corpus callosum and adjacent cortex.
Larger lipomas associated with callosal malformations are more likely to be associated with neurologic disability compared to thin, curvilinear lipomas.
Lipomas may grow as the child grows.
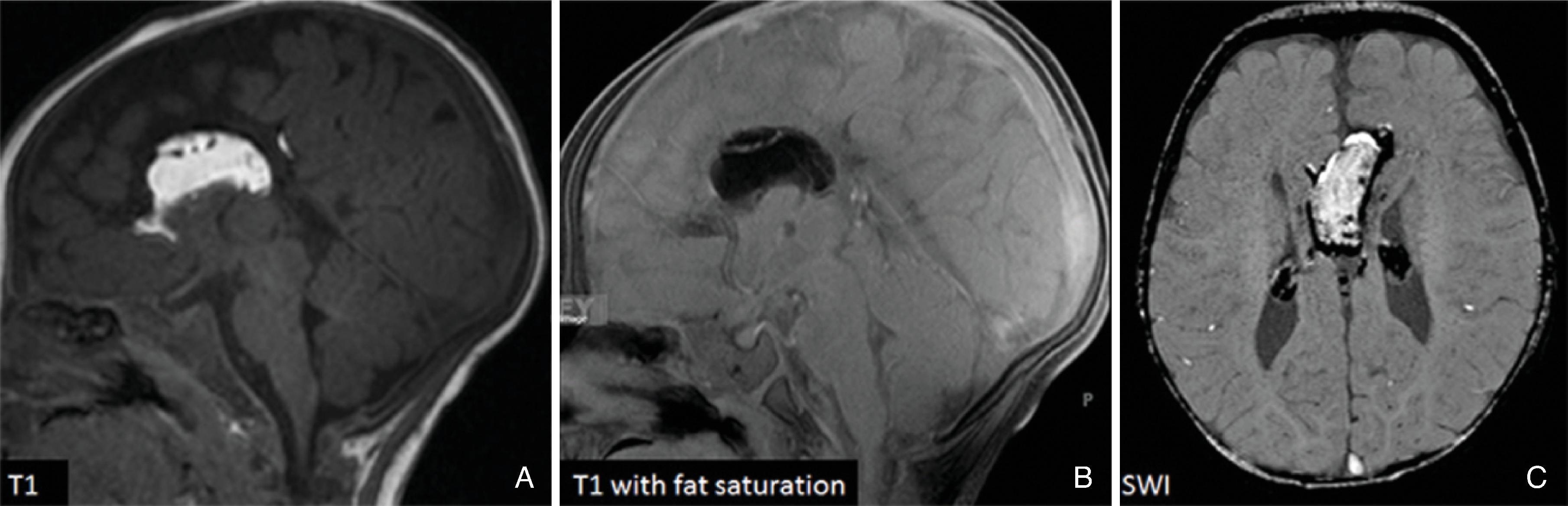
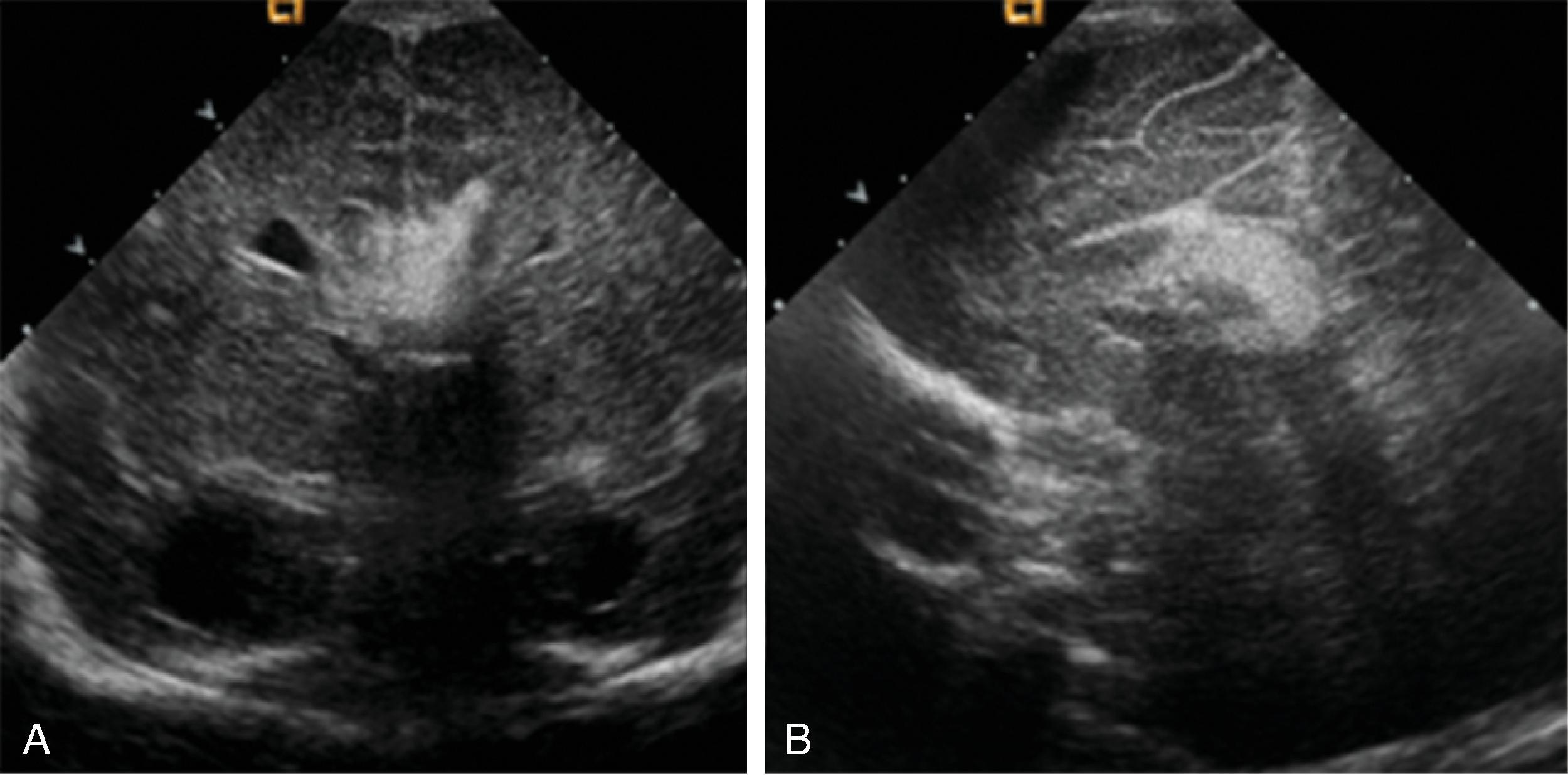
A pericallosal or interhemispheric lipoma appears hyperechogenic on ultrasound and hyperintense on T1-weighted MRI; the MRI signal is suppressed on fat-saturated MR sequences. Uncommonly, on fetal MRI the lipoma can sometimes lack the T1W hyperintensity due to immature fat cells.
On gradient echo MR imaging, marked fat-related chemical shift signal loss may be seen along the periphery of the lesion along the direction of the frequency encoding gradient.
The lipomas may be of various sizes and typically follow the course of the corpus callosum.
Lobular , mass-like focal lipomas are usually associated with callosal malformations.
Thin, curvilinear lipomas are less likely associated with callosal malformations.
Corpus callosum may appear “dysplastic” on a midsagittal image secondary to a remote injury. Examples include the following:
Chronic porencephalic cyst after neonatal periventricular hemorrhagic infarction
Neurosurgical interhemispheric transcallosal approach to the third ventricle
Periventricular white matter injury due to a neonatal hypoxic ischemic event
Differentiation from a true malformation is essential for prognosis.
Depending on the timing of injury (prenatal versus postnatal), differentiation must be made between malformation, disruption, and destruction.
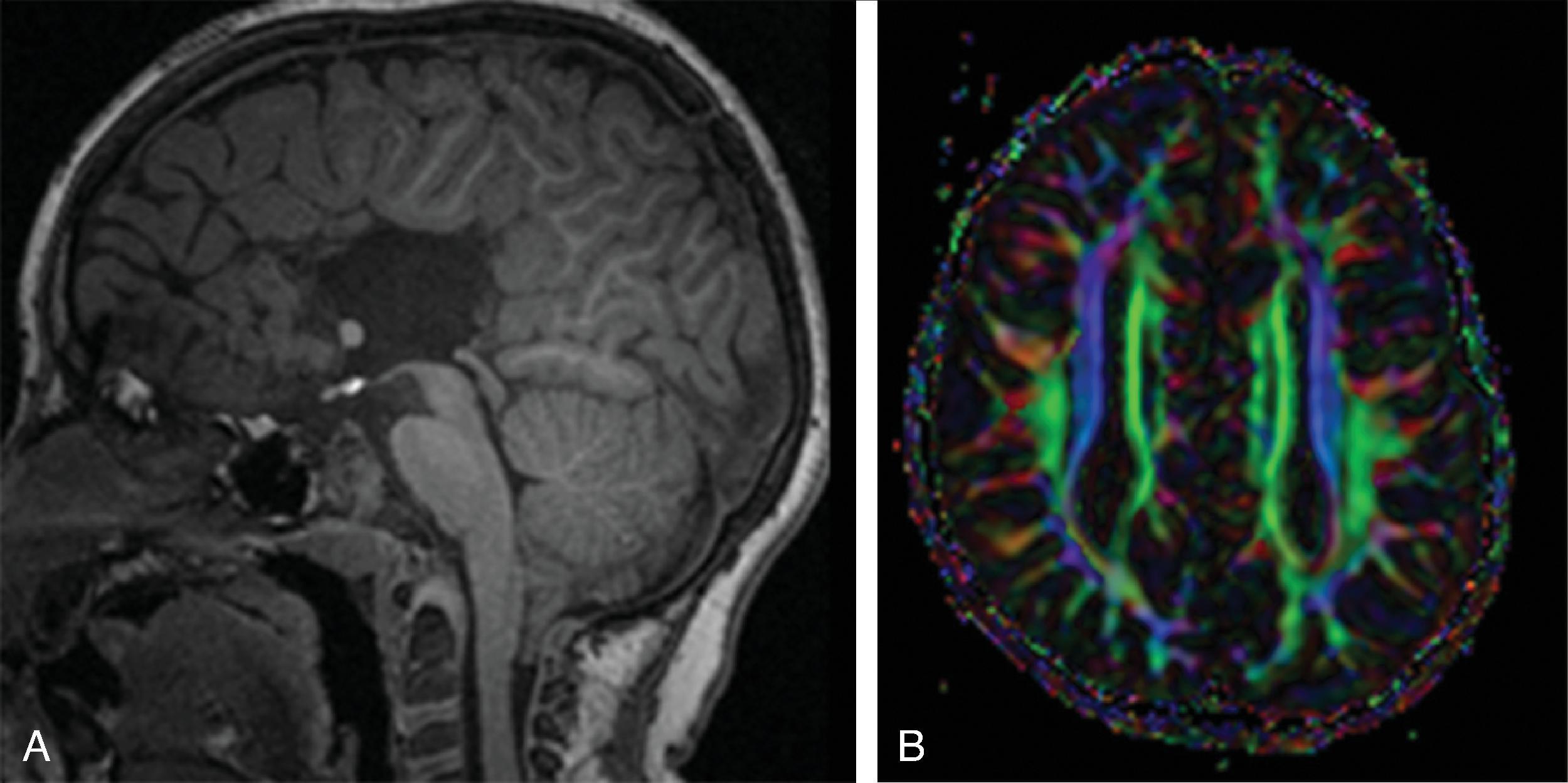
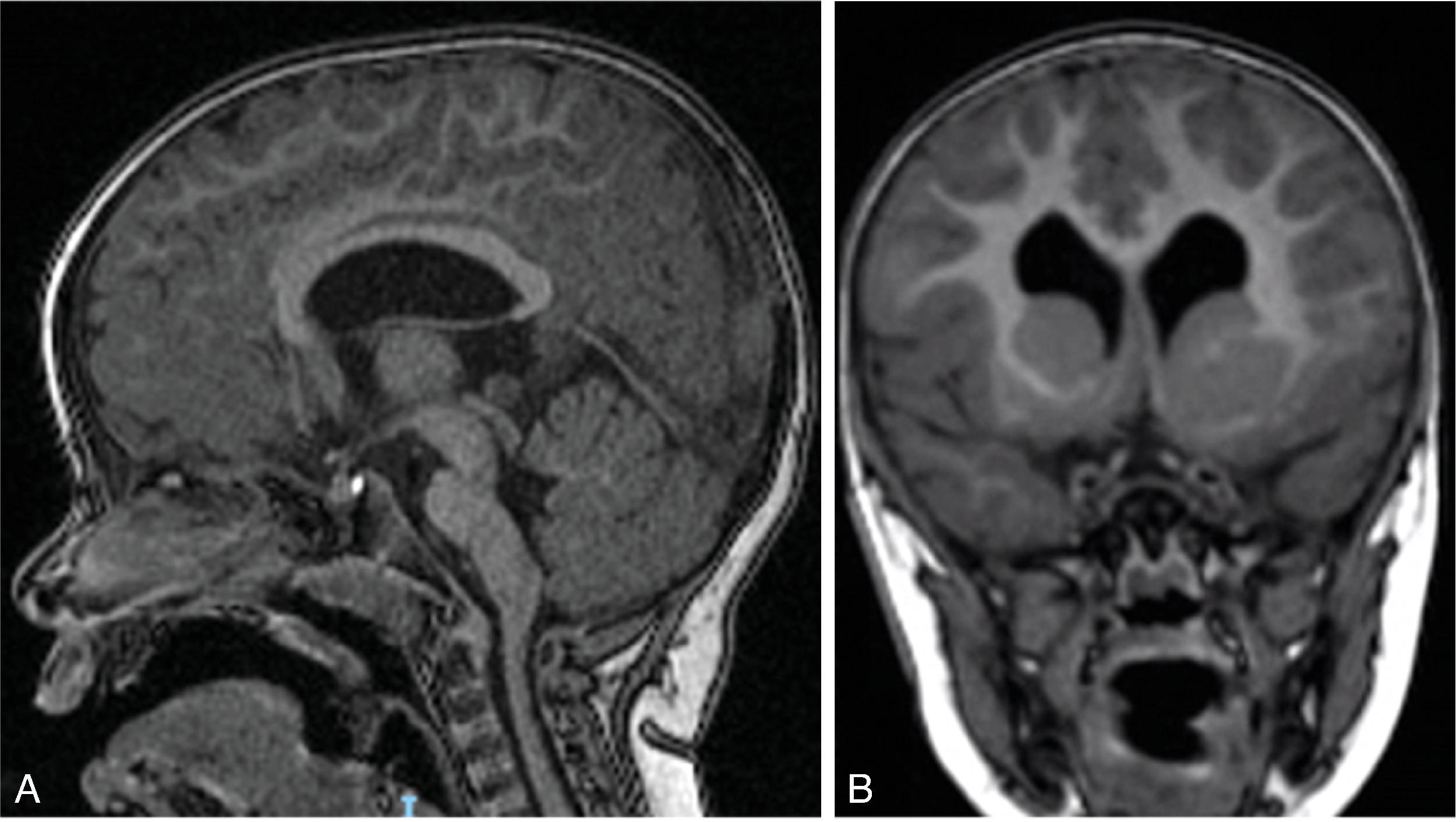
Defined by Dr. Jean Aicardi as an association of infantile spasms, agenesis of the corpus callosum, and chorioretinal lacunae in infant girls.
Aicardi syndrome is an X-linked dominant disorder and lethal in 46 XY males . Consequently, Aicardi syndrome is seen in females. The gene causing Aicardi syndrome, however, is unknown.
Poor prognosis with severe neurologic and cognitive impairments, epilepsy, and reduced life span, on average ranging from 8 to 18 years.
Infants with Aicardi usually have infantile spasms, which are characterized as single jerks of the body that occur usually while awake. Children typically grow out of infantile spasms and have generalized tonic-clonic seizures or other types of seizures.
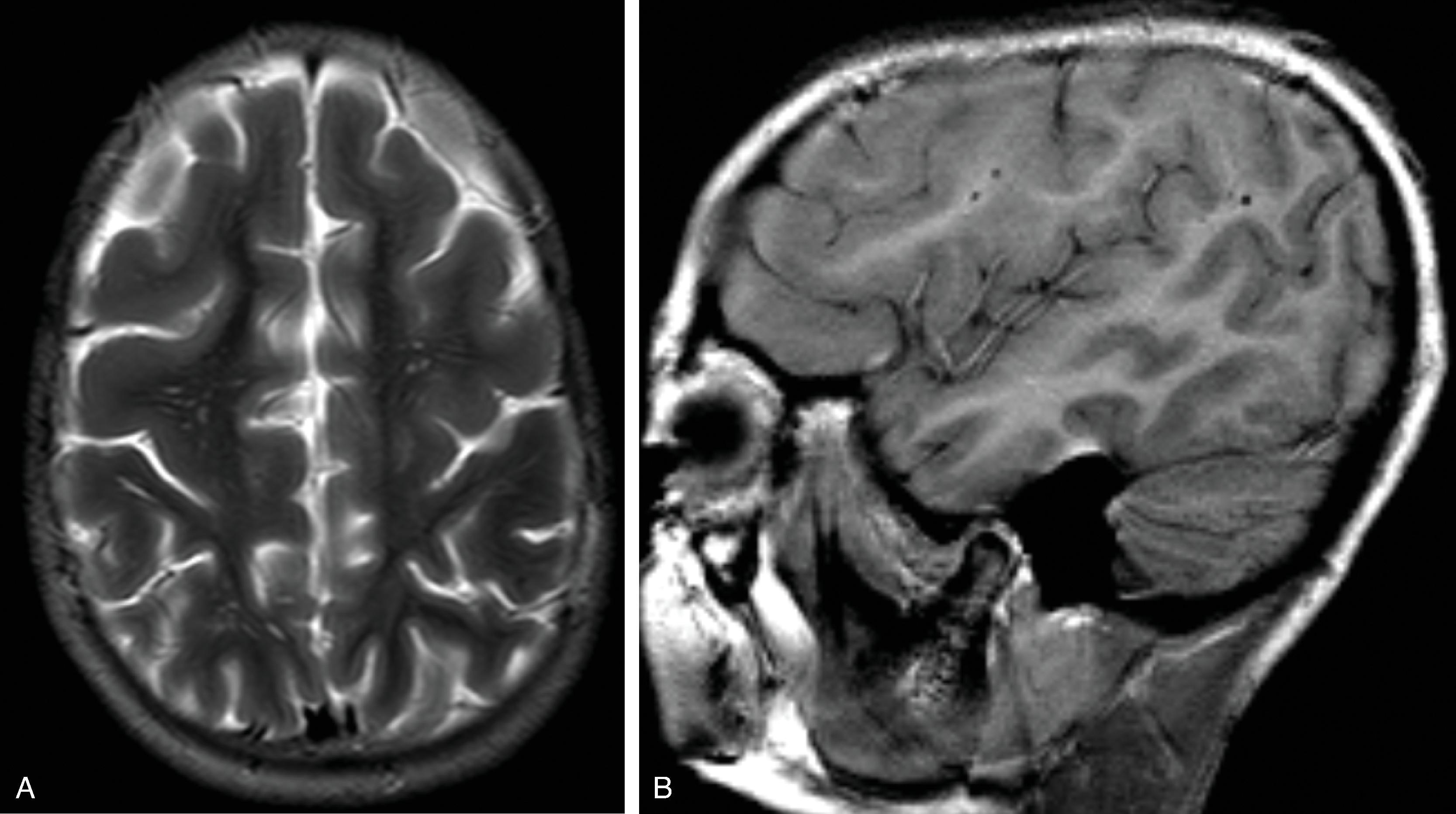
Findings include agenesis of the corpus callosum , migrational abnormalities , including gray matter heterotopia, polymicrogyria or pachygyria, choroid plexus cysts or papillomas, and optic nerve colobomas or microphthalmia .
White matter dysmyelination may also be seen.
Vertebral (block or hemivertebrae) and rib abnormalities may coexist.
Interhemispheric cyst when present is usually multilocular.
With exception of the corpus callosum agenesis, imaging findings may be subtle on early fetal MR imaging and become more obvious on follow-up MR imaging.
Understanding cortical malformations requires an understanding of basic cortical development.
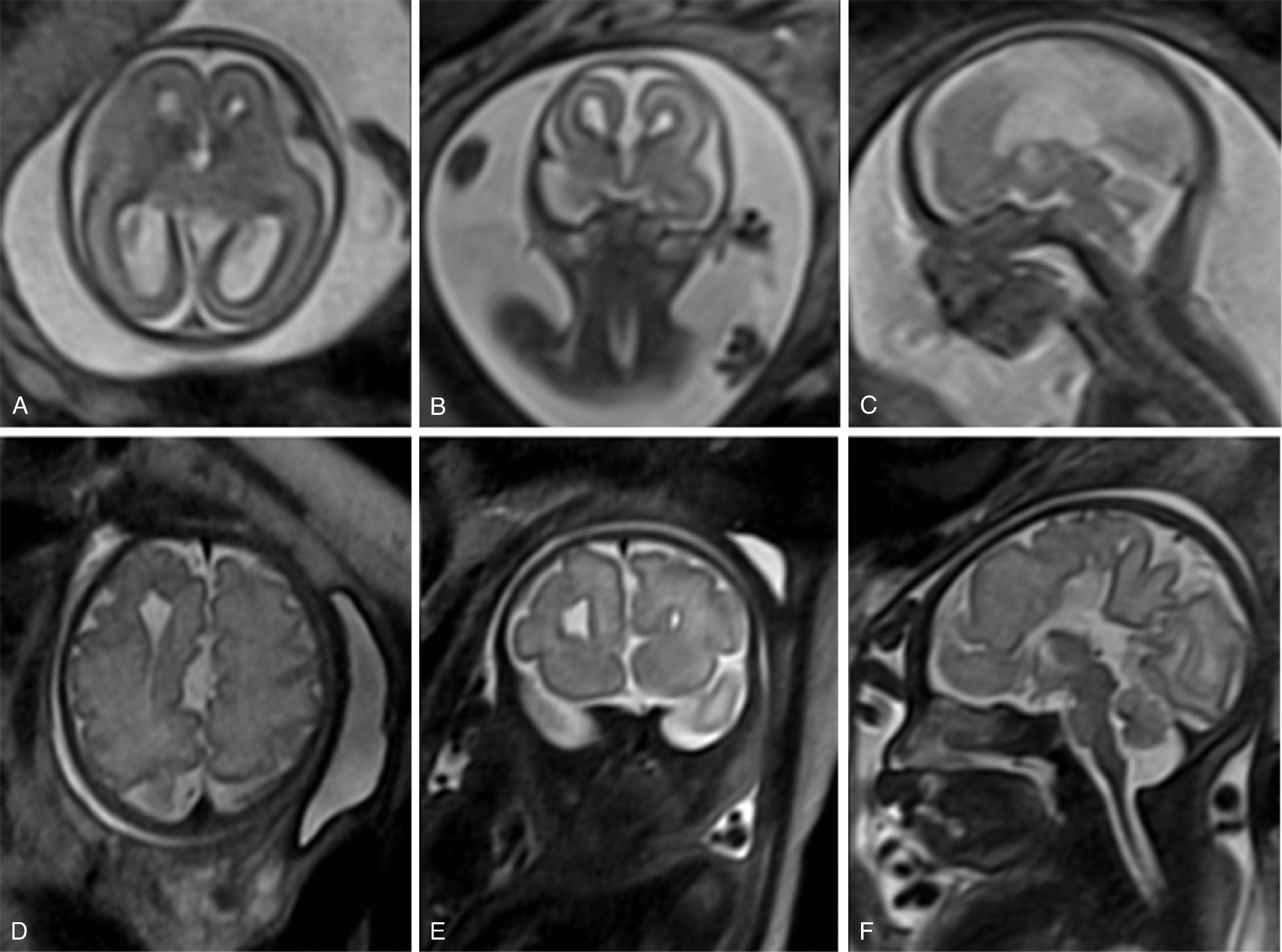
The germinal matrix develops in the walls of the telencephalon. Areas known as the ganglionic eminences develop as larger regions of the germinal matrix. As the neurons from the germinal matrix proliferate and migrate to the outer cortex, they form permanent and transient axonal connections, and the zones in the telencephalon are visible during this stage of development. These zones change in appearance during development and peak between 15 and 24 weeks of gestation ( Fig. 2.21 ).
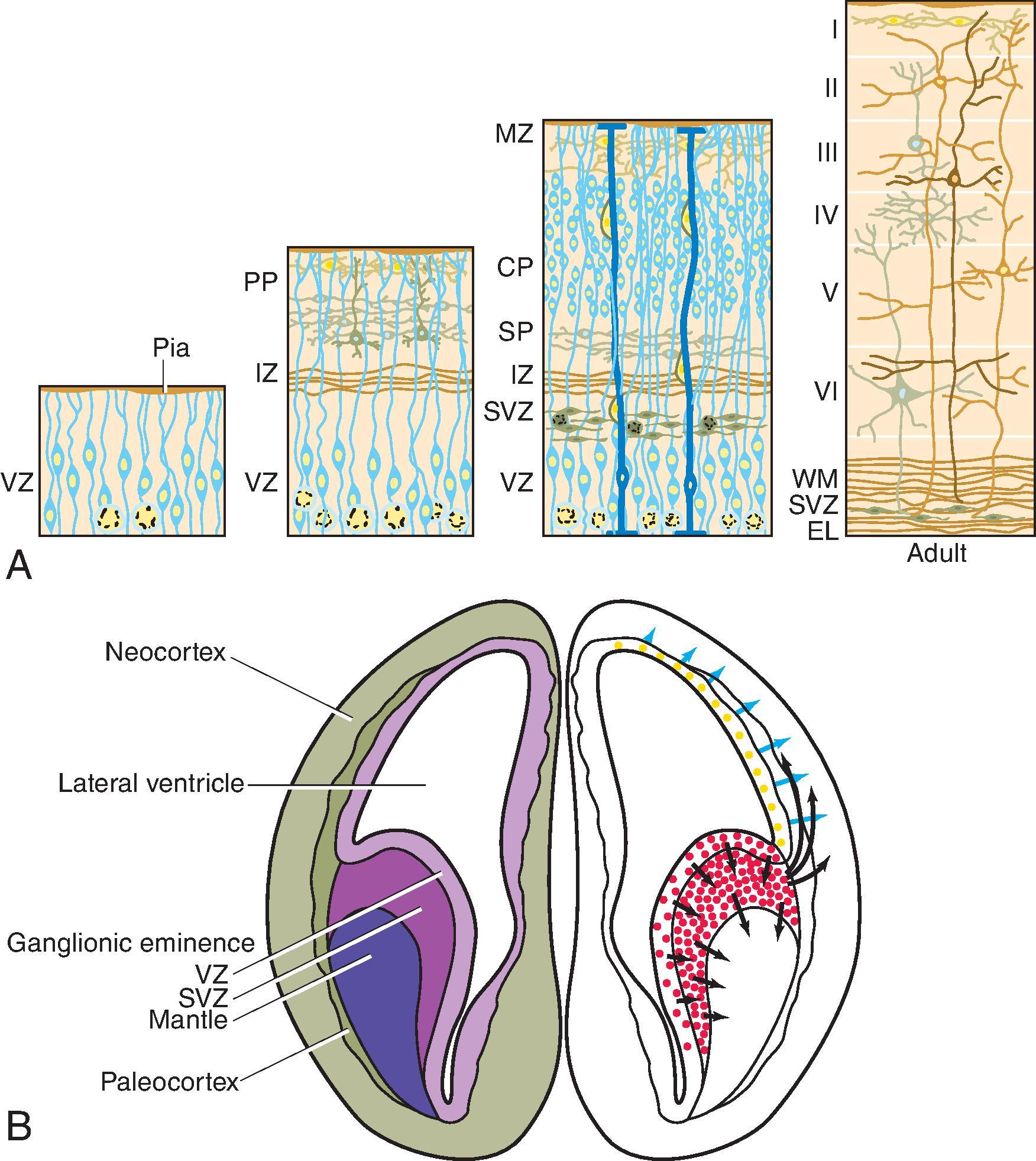


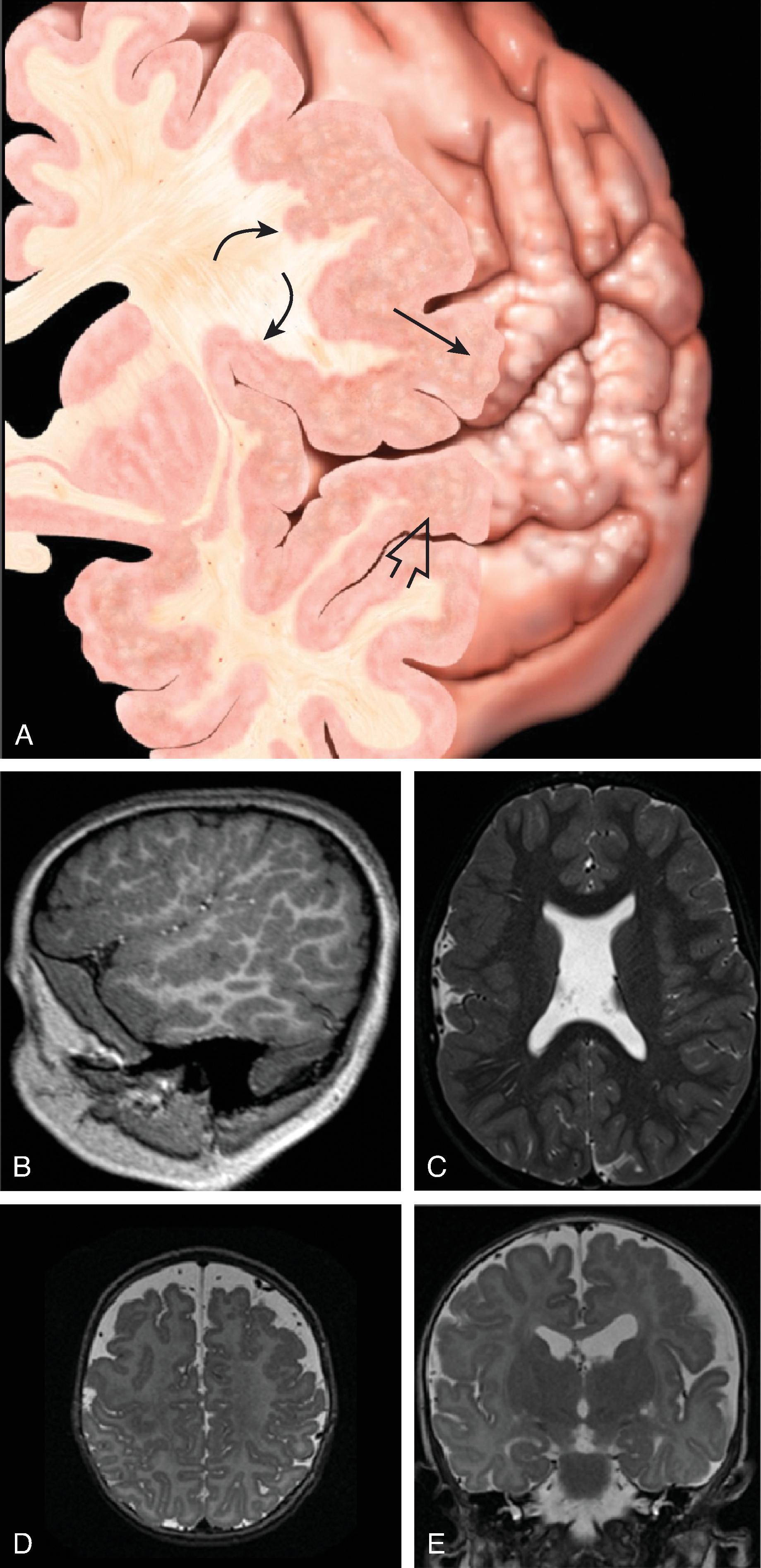

The ventricular zone gives rise to descendants of the neural plate and subventricular zone. The periventricular zone is a cell sparse layer with axons that give rise to the corpus callosum. The subventricular zone is the source of cortical and striatal neurons, astrocytes, and glial cells. The intermediate zone is a region of axonal connections. The subplate contains maturing neurons and connections important for cortical layer organization. The subplate reaches maximum thickness at 22 weeks’ gestation and disappears at 30 weeks. The cortical plate is the final destination of migrating neurons and will develop into a six-layered neocortex with the innermost layer (layer VI) forming first, and, as neurons continue to migrate, will progressively form the more outer layers. Proliferation and migration of glial progenitors continues in the postnatal period into adulthood.
Cortical malformations can be categorized as malformations due to abnormal neuronal or glial proliferation, abnormal neuronal migration, or abnormal postmigrational development ( Box 2.1 ). This section will illustrate the common cortical malformations and disorders in which these are common findings. Cortical dysplasias, which are cortical malformations resulting from abnormal neuronal/glial proliferation and postmigrational development, are discussed in Chapter 8 , Epilepsy.
I.A. Microcephaly
I.B. Megalencephalies and Hemimegalencephaly
I.C. Cortical dysgenesis with abnormal cell proliferation (FCD type 2)
II.A Heterotopia
II.B. Lissencephaly
II.C. Subcortical heterotopia and sublobar dysplasia
II.D. Cobblestone malformations
III.A. Polymicrogyria and schizencephaly
III.B. Polymicrogyria without schizencephalic clefts
III.C. Focal cortical dysplasias (FCD type 1)
III.D. Postmigrational microcephaly
Microcephaly is defined as a head circumference at least 3 standard deviations below age-matched normative values.
Primary or genetic mediated microcephaly is believed to result from early exhaustion of the neuronal precursors or accelerated neuronal apoptosis.
Primary microcephaly must be differentiated from secondary, acquired microcephaly due to brain injury (e.g., after a hypoxic ischemic injury or TORCH infection).
Multiple classifications are available of which the significance is being debated.
Prognosis varies but often is associated with epilepsy, motor deficits, and intellectual disability. Patients with microcephaly primary hereditary typically have mild to moderate intellectual disability and speech and motor delay. Patients with microlissencephaly often have severe hypertonia or hypotonia, seizures, and early death.
On sagittal imaging the cranial vault/brain appears disproportionally small compared to the maxillofacial skeleton.
Microcephaly may be associated with several patterns of the cerebral cortex and remainder of the brain:
Microcephaly primary hereditary (MCPH) demonstrates microcephaly with architecturally normal cortical thickness and folding.
Microcephaly with simplified gyral pattern (MSG) demonstrates absent tertiary sulcation, shallow sulci, delayed/impaired myelination, and normal cortical thickness. The corpus callosum may be thin but is typically completely formed from anterior to posterior.
Microlissencephaly with increased cerebral cortical thickness, agenesis or dysgenesis of the corpus callosum, and hypoplasia of the brainstem and cerebellum.
Microcephaly with polymicrogyria or cortical dysplasia.
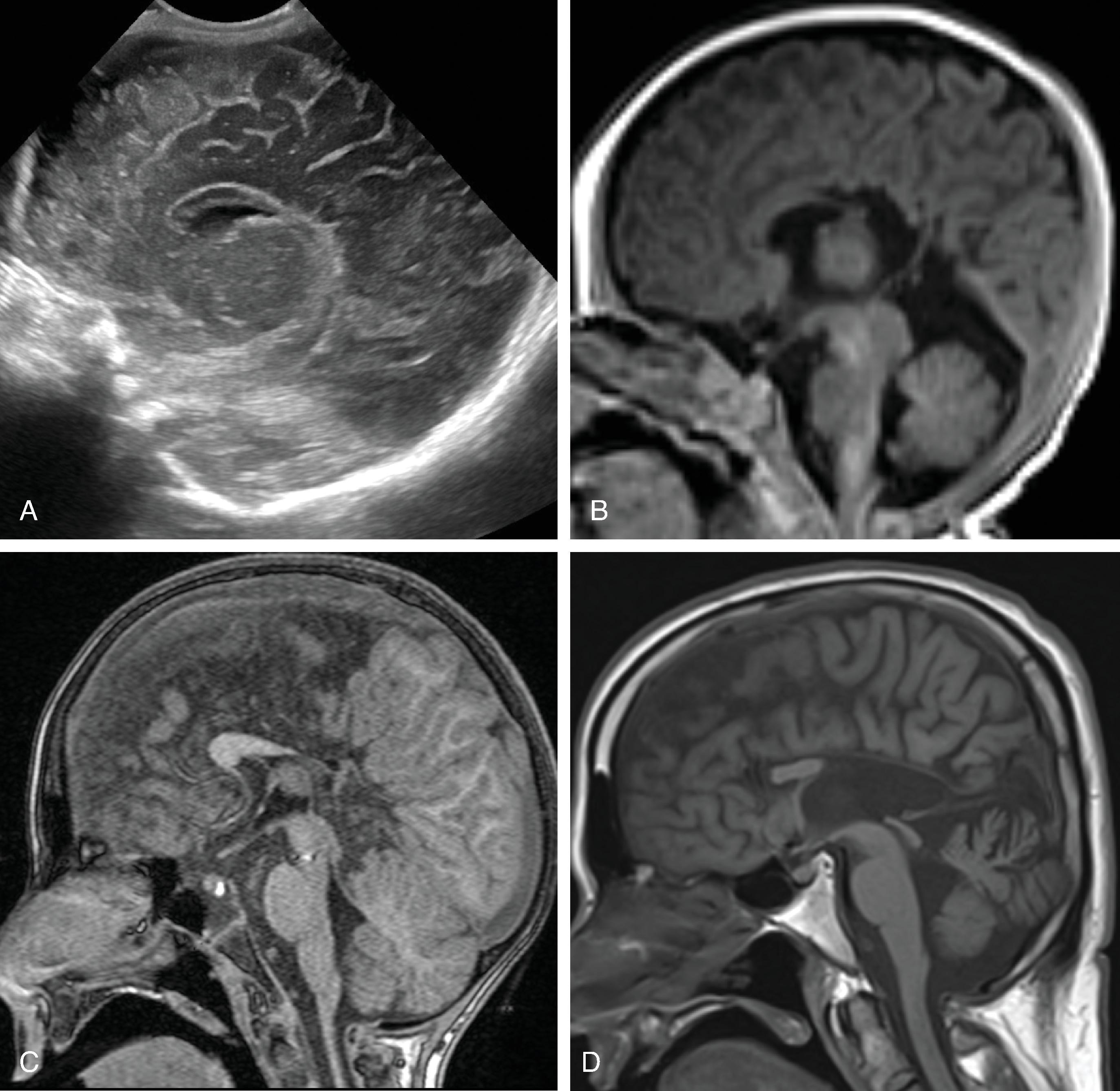
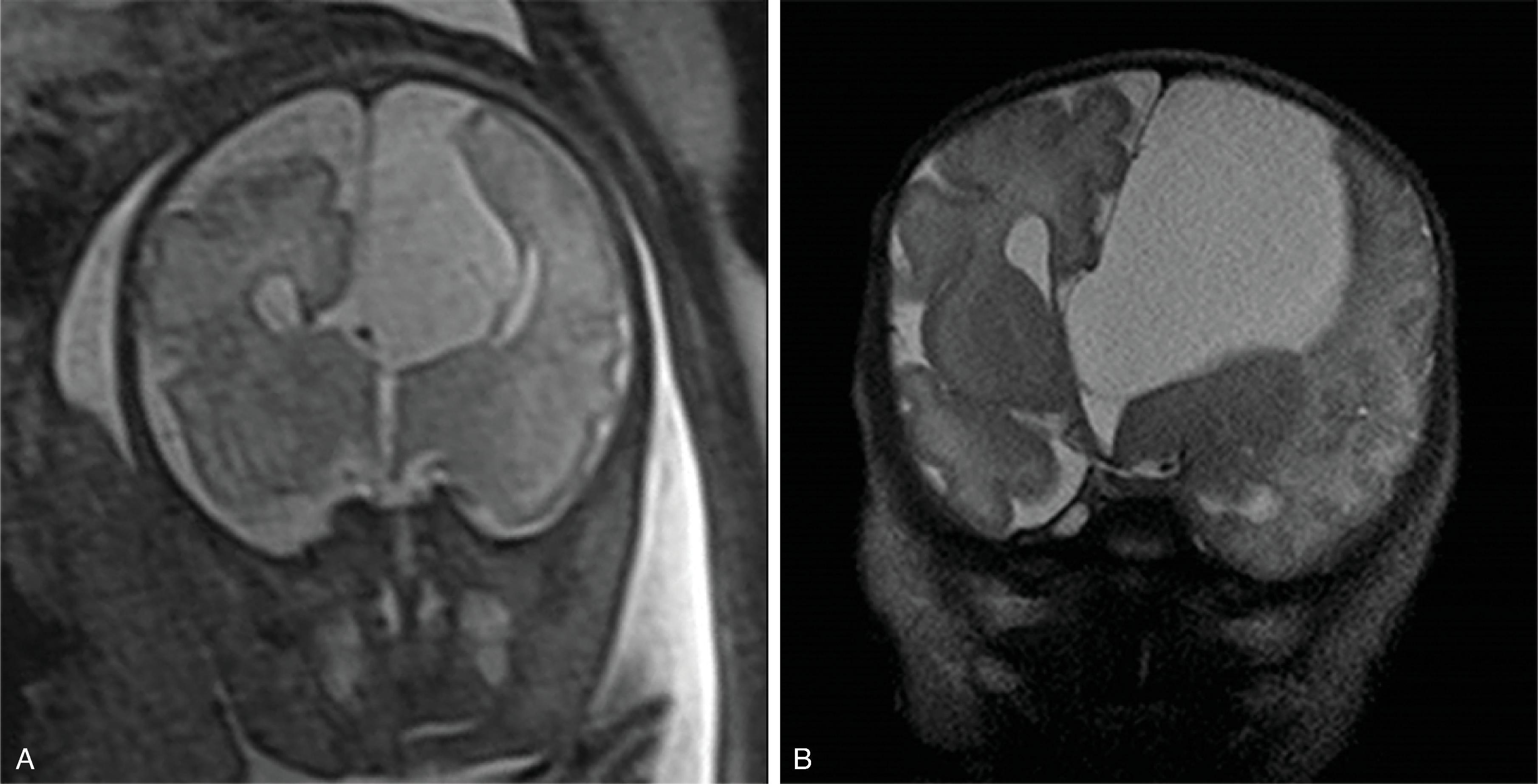
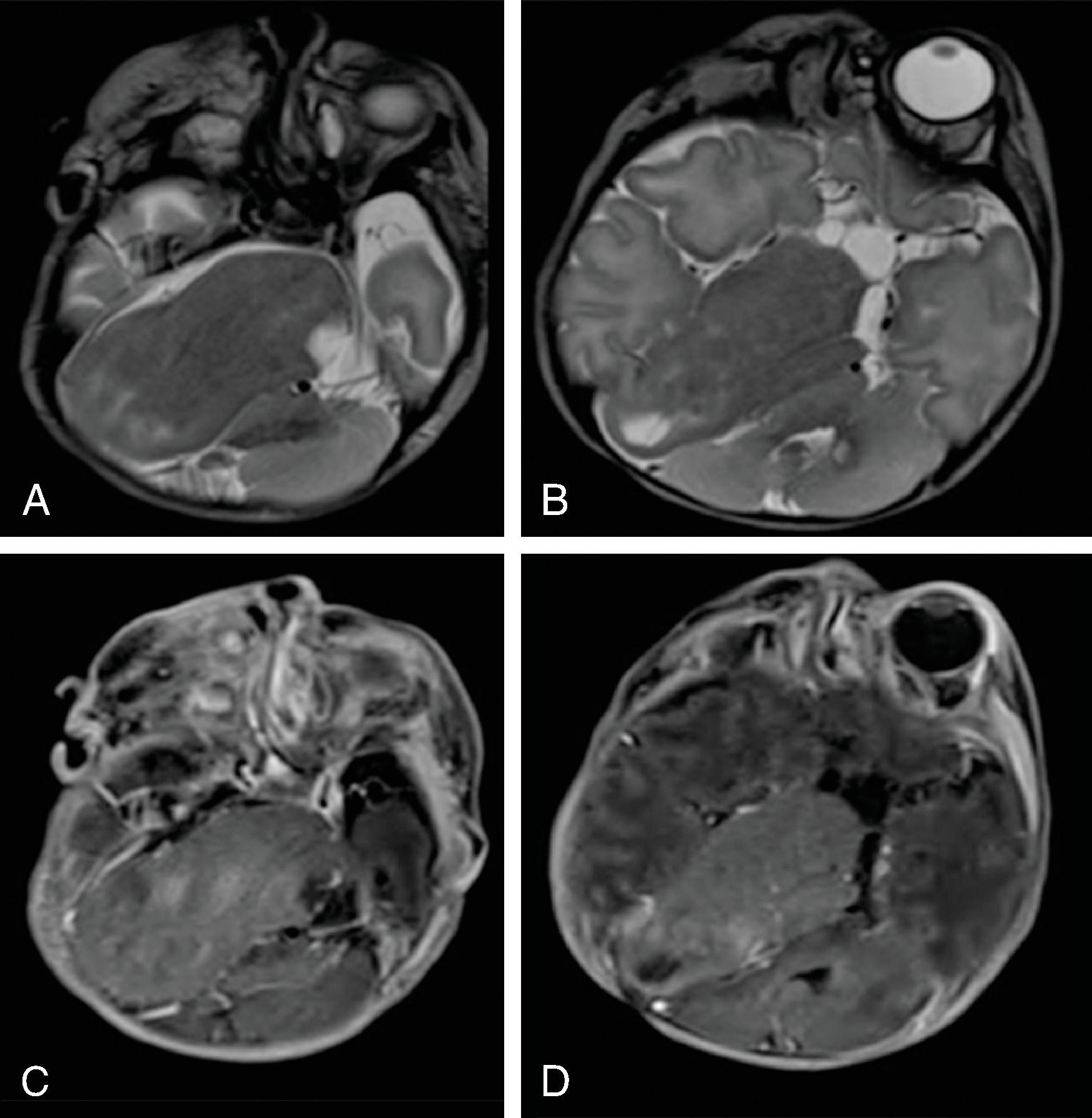
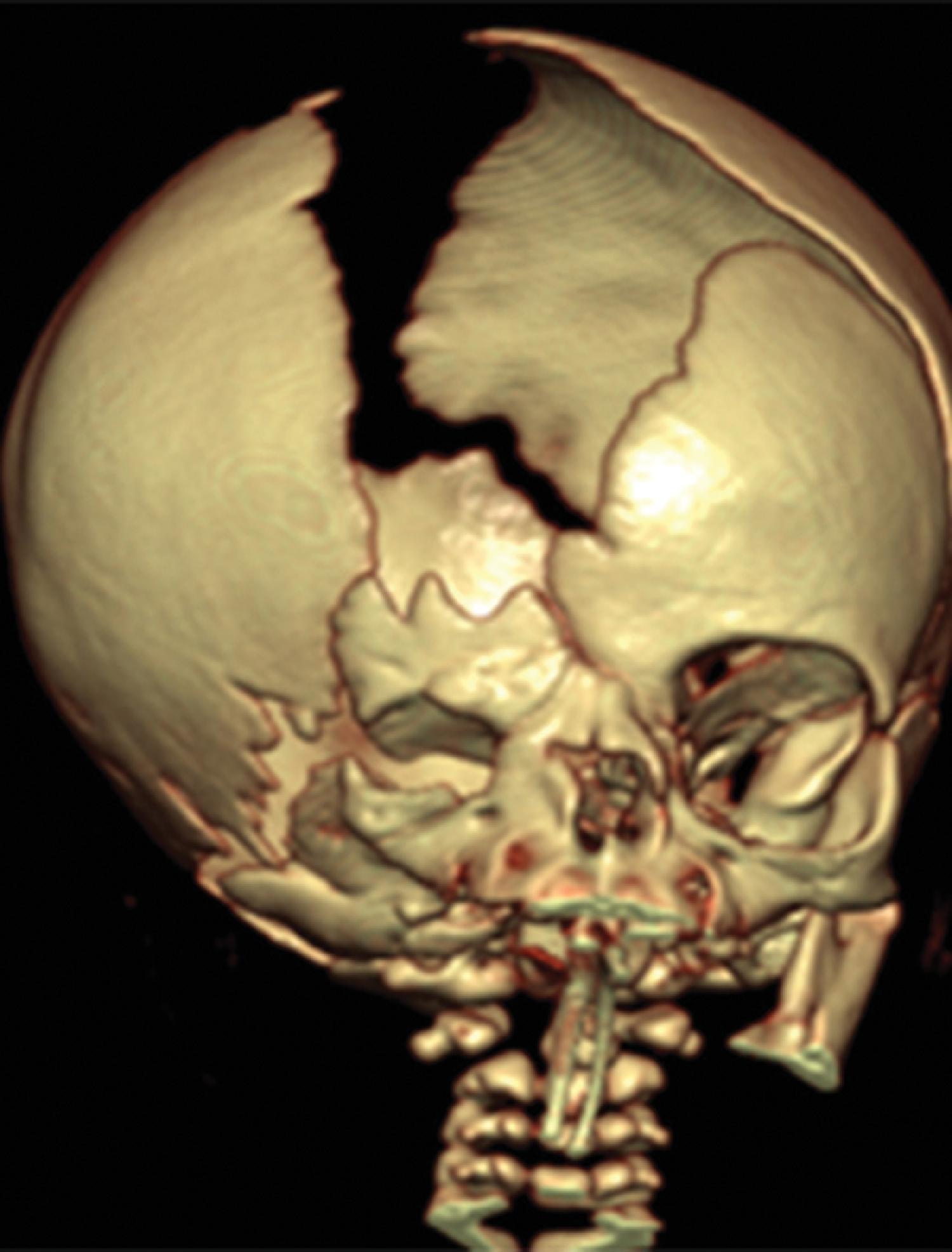
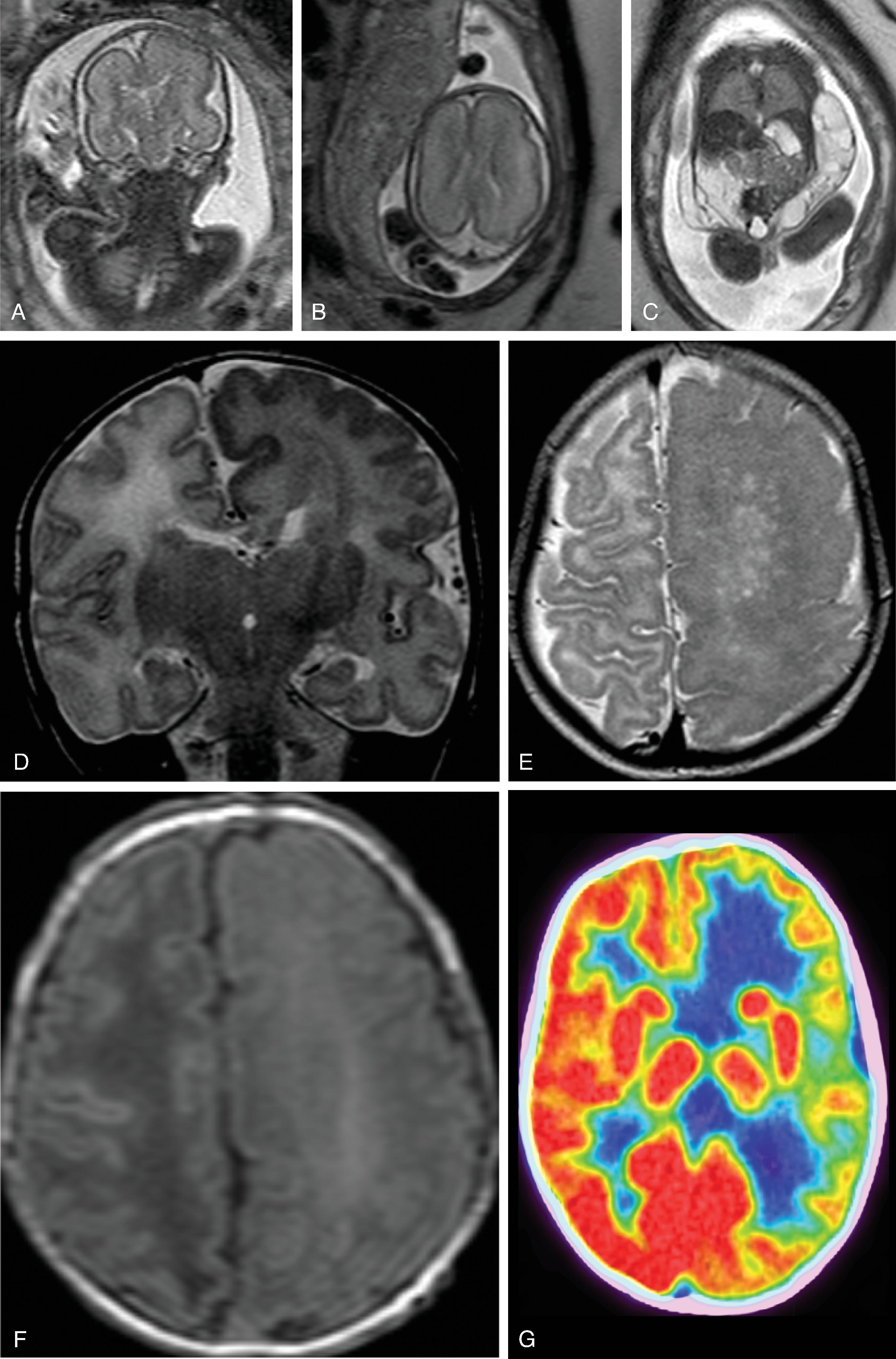
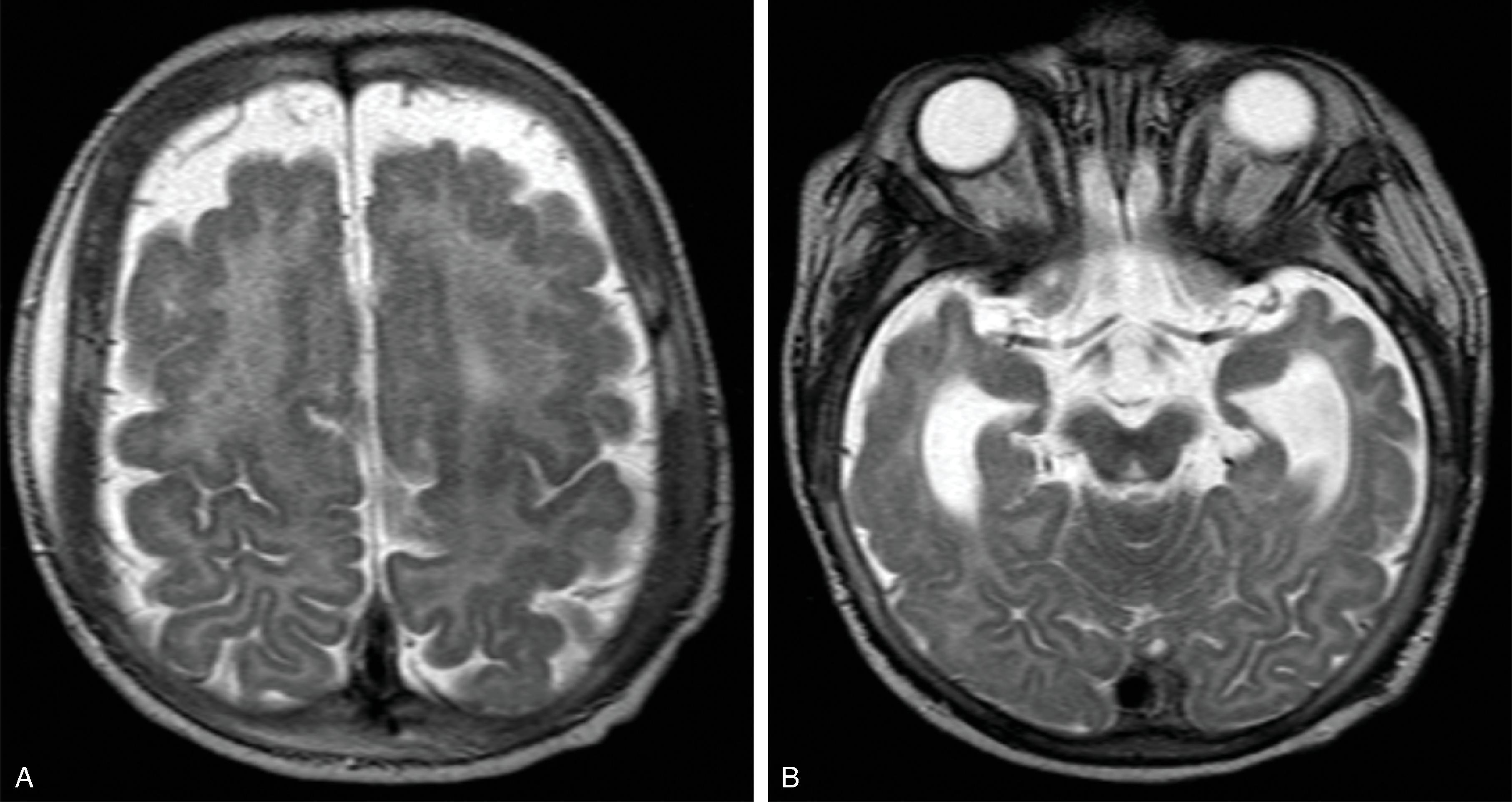
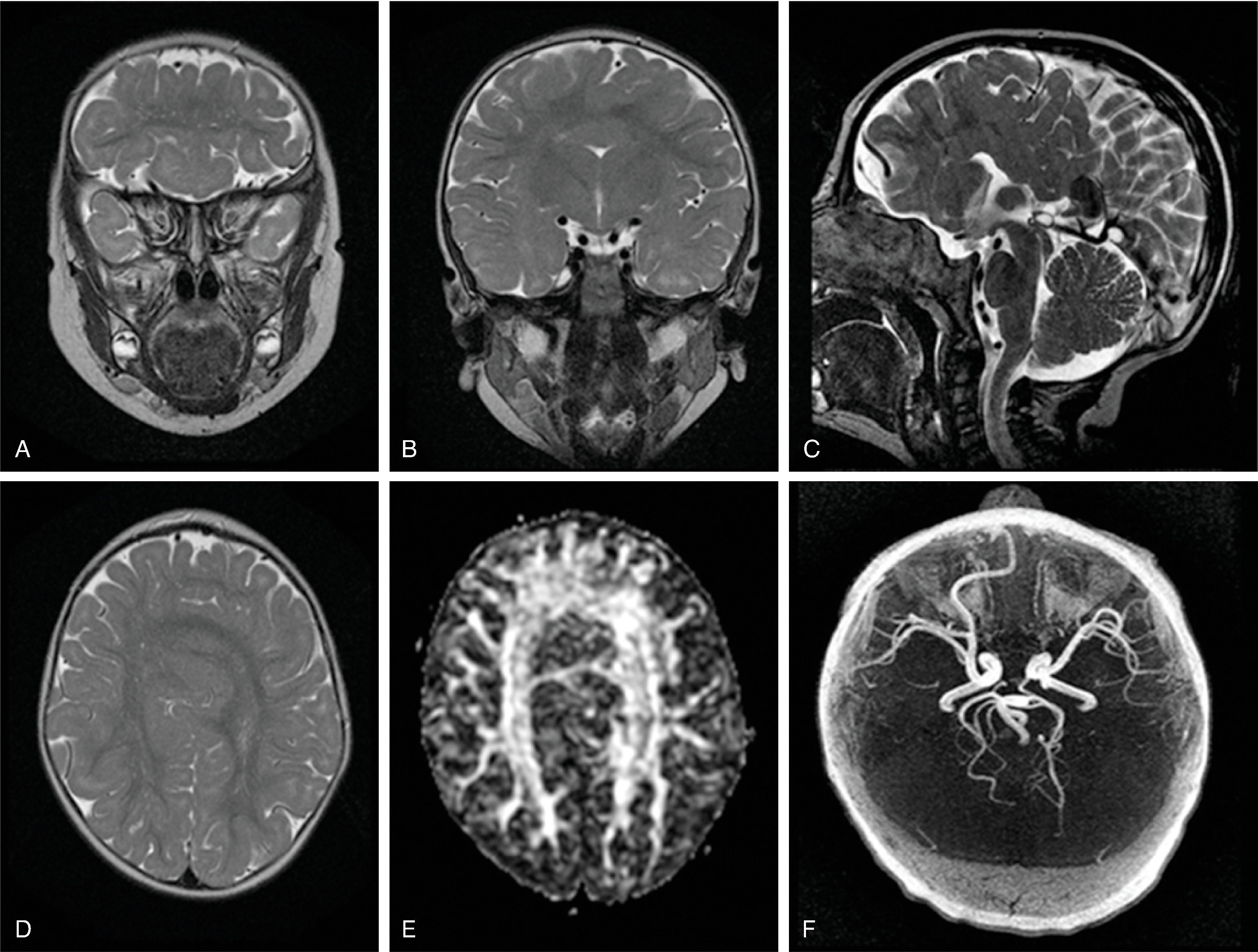
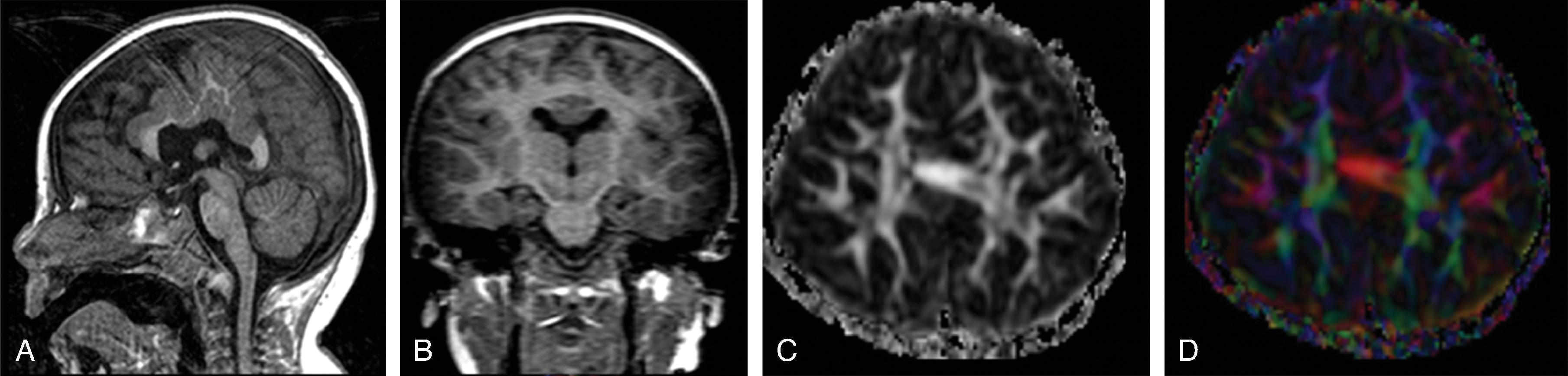
Hemimegalencephaly is characterized by a diffuse hamartomatous overgrowth of part or entirety of a cerebral hemisphere. The ipsilateral cerebellum may also be enlarged (total hemimegalencephaly).
The exact etiology remains unclear, but this severe congenital malformation is presumed to be secondary to a combination of an accelerated, excessive neuronal proliferation and impaired apoptosis .
Most children present with intractable seizures, developmental delay, and hemiplegia.
Hemimegalencephaly is seen as an isolated finding but may also be part of various syndromes including PIK3/akt/mTOR disorders, hypomelanosis of Ito, neurofibromatosis type 1, Klippel-Trenaunay syndrome, and Proteus syndrome.
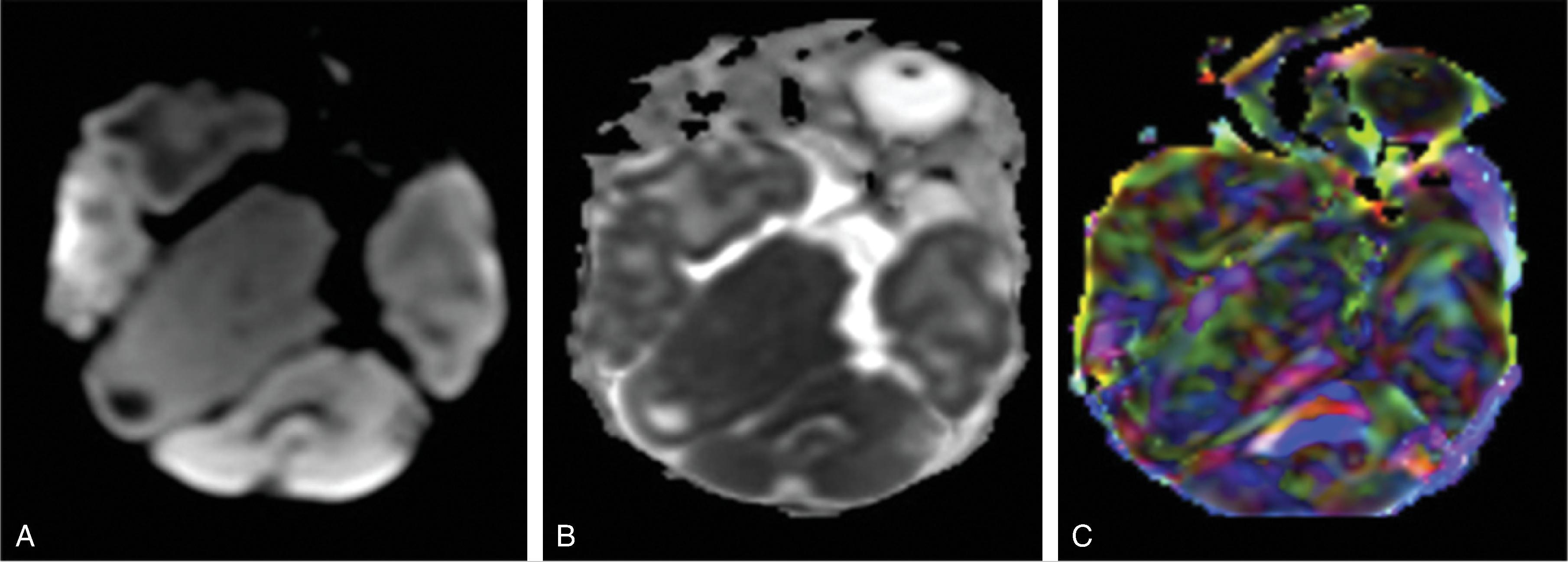
The enlarged cerebral hemisphere shows a thickened, dysmorphic cortical ribbon with simplified gyration and shallow sulci. The adjacent white matter is abnormal with features consistent with dysmyelination.
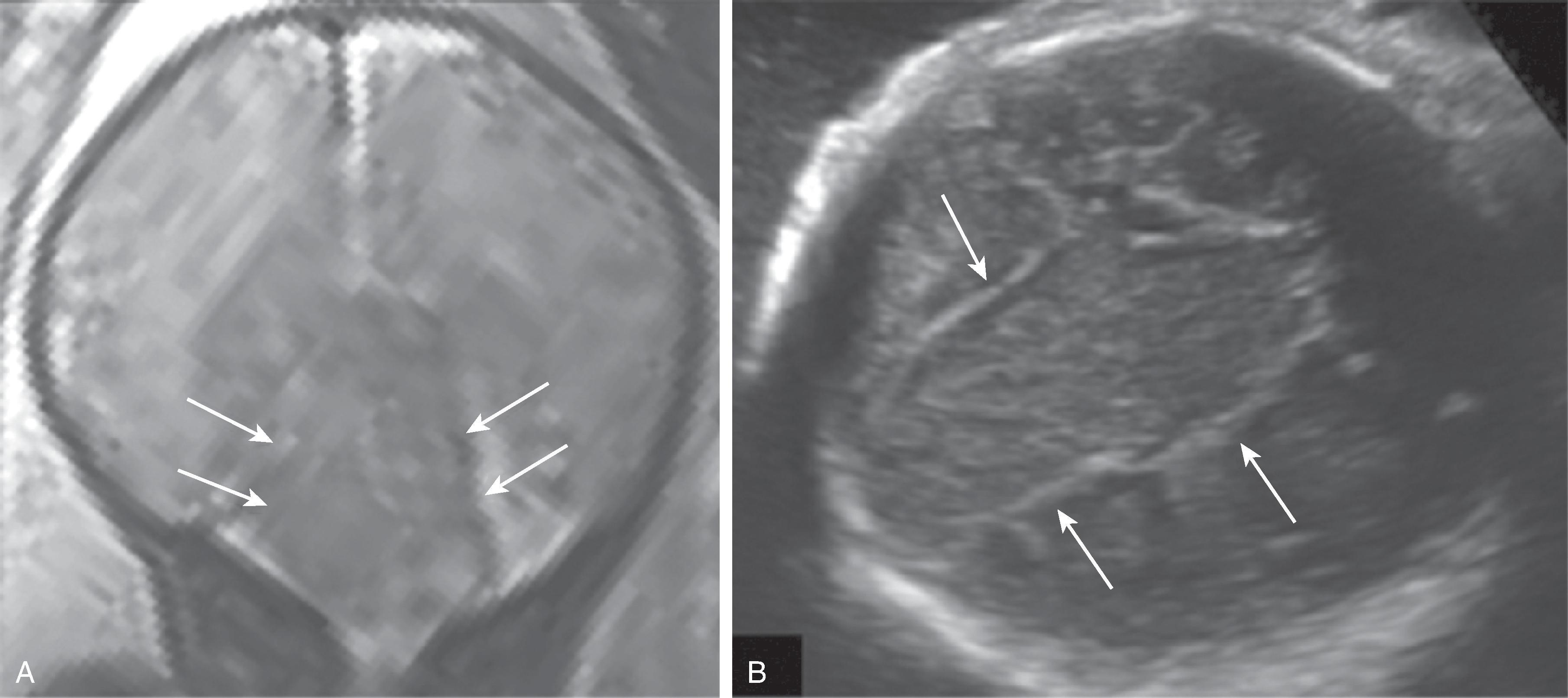
Color-coded fractional anisotropy maps confirm the disorganization of the cortical ribbon and the subcortical, hemispheric white matter.
Characteristically, the ipsilateral lateral ventricle is enlarged compared to the contralateral side, often commensurate with the enlarged affected hemisphere. In addition, the shape of the enlarged ventricle is abnormal. The midline is in most cases preserved.
The hemicranium containing the enlarged hemisphere is typically enlarged, resulting in an increased head circumference/macrocephaly.
Lissencephaly and pachygyria are cortical malformations due to abnormal neuronal migration often occurring as a spectrum in patients.
Lissencephaly results from an arrested neuronal migration resulting in a four-layer cortical ribbon.
Lissencephaly may be secondary to a LIS1 mutation (classic lissencephaly), DCX mutation also known as X-linked (XLIS), TUBA1A gene mutation, or secondary to infections (cytomegalovirus). LIS1 and DCX mutations account for 40% to 75% of patients with classic lissencephaly.
Pachygyria is likely caused by abnormal regulation of microtubule activities.
Pachygyria can be caused by infections or be part of overgrowth syndromes, including hemimegalencephaly.
Lissencephaly is characterized by the appearance of a smooth brain surface with little or no gyri (agyria) and sulci generally resembling a figure-8 shape . Pachygyria is characterized by broad/wide gyri, shallow or absent sulci, and abnormal thickened cortical ribbon with a smooth inner and outer contour of the cortex. Many cases of occurring as a spectrum in patients have a combination of agyria and pachygyria.
Pattern of agyria-pachygyria can indicate underlying genetic mutation:
LIS1 mutations often have greater agyria of the parietal and occipital lobes and pachygyria of the frontal lobes.
DCX mutations have more severe involvement of the frontal lobes compared to the occipital lobes.
TUBA1A gene mutations often demonstrate classic lissencephaly as well as malformation of the corpus callosum, rounded hippocampi, and pontocerebellar hypoplasia.
Heterotopia refers to an abnormal collection of neurons in an anomalous location other than the cortical gray matter.
Heterotopia is believed to be secondary to an arrested migration of neurons from the germinal matrix toward the cortical ribbon along the radial glial path.
Heterotopia may occur isolated or be part of complex brain malformations or syndromes including Chiari II malformation, commissural anomalies, Joubert syndrome, fragile X syndrome, and FLNA gene mutation.
Clinical symptoms are variable. Mild degrees of periventricular heterotopia may be incidentally identified. Other patients may have developmental delay, seizures, and motor dysfunction.
Heterotopias are isointense to cortical gray matter on all MRI sequences and do not show contrast enhancement.
On (color-coded) fractional anisotropy maps, heterotopia have low FA values due to the high degree of isotropic diffusion, similar to cortical and central gray matter.
Heterotopia may be subcortical or periventricular.
Heterotopia may be focal/nodular, multifocal, linear/band-like, unilateral, or bilateral. Band heterotopia is typically seen in females with LIS1 or DCX mutation.
Large heterotopia may exert a mass effect on adjacent structures.
Overlying cortical ribbon may be affected/malformed.
Also known as neuroglial hamartoma. A focal mass lesion/malformation composed of differentiated derivatives of neuroectodermal tissue, including disorganized mature neuronal and glial cells.
Occurs between the fifth and sixth week of gestation, when the forebrain vesicle is divided into the telencephalon and diencephalon.
Most are large at presentation, supporting that the etiologic event occurs early in gestation.
Glioneuronal heterotopia is clinically and pathologically benign and must be differentiated from true neoplasms.
On neuroimaging the lesions are typically extraaxial with lack of infiltration of adjacent brain structures. The CT and MRI characteristics follow gray and white matter densities/signal intensities on all sequences. No directional diffusion seen on fractional anisotropy maps. Faint or absent contrast enhancement. Lack of growth on serial imaging supports diagnosis.
Glioneuronal heterotopias are typically located extracranially, including the orbit, pharynx, middle ear, neck, and thorax. The nasal cavity represents the most common location.
Rarely, glioneuronal heterotopias may be located intracranially. Intracranial glioneuronal heterotopias are frequently associated with congenital craniofacial anomalies.
Prenatal ultrasound or fetal MRI allows diagnosis of the lesion but is challenging.
Cobblestone malformations are malformations of neuronal migration .
Multiple genes (autosomal recessive inheritance) have been identified linked to alpha-dystroglycan mutations. Consequently, these disorders are also known as alpha-dystroglycanopathies.
Abnormal glycosylation of the cell-surface glycoprotein alpha-dystroglycan results in a deficient binding of extracellular matrix proteins containing laminin domains. This binding is essential for muscle fibers to attach to the muscle basal lamina, and deficiency prevents normal muscle contraction ( muscular dystrophy ). Proper function of glycosylated alpha-dystroglycan is also relevant for the linkage of radial glial cells to the basement membrane during development of the cortical ribbon, and for the glial guide cells to the retinal limiting membrane. The lack of a proper attachment of the radial glial cells to the pial basement membrane allows for overmigration of neurons through the pial basement membrane into the subpial and subarachnoid space, resulting in the typical cobblestone appearance. Consequently, in dystroglycanopathies additional neuronal cerebral and cerebellar migrational abnormalities and retinal abnormalities including colobomas may accompany the muscular dystrophy.
Clinically, they are subclassified into merosin-positive or -negative muscular dystrophy (CMD1), Fukuyama congenital muscular dystrophy (CMD2), muscle eye brain disease (CMD3), and Walker-Warburg syndrome (CMD4).
Muscle biopsy typically confirms diagnosis.
Neonates present at birth with hypotonia, early onset progressive muscular weakness, delayed motor development, impaired vision, and seizures.
Cortical anomalies, including cobblestone lissencephaly, polymicrogyria, and/or pachygyria
Z-shaped or kinked, thin brainstem, and cervicomedullary kinking
Cortical/subcortical cerebellar inclusion cysts, hypoplastic vermis and cerebellum, and tectal plate deformities
Abnormal myelination
Ventriculomegaly
Malformed corpus callosum
Retinal abnormalities as well as colobomas
Occipital encephaloceles may be seen in CMD4
Imaging findings typically become more prominent on postnatal imaging, in particular the dysmyelination and cortical anomalies
Polymicrogyria results from abnormal late neuronal migration/early cortical organization impairing the normal lamination of the cortical ribbon. This occurs in the second half of the second trimester.
Polymicrogyria may be secondary to intrauterine infections, ischemia, toxins (e.g., alcohol) or genetic/syndrome related (e.g. Aicardi syndrome, Zellweger syndrome, tubulinopathy, mTORopathy).
Polymicrogyria may be focal or multifocal, unilateral or bilateral.
If the polymicrogyria is bilateral and symmetrical, evaluation for a genetic disorder or syndrome should be performed.
Prognosis is variable and depends on extent and location, associated anomalies. Unilateral polymicrogyria can be found in otherwise healthy children who present in the first or second decade with seizures.
Abnormal cortical ribbon characterized by multiple small and irregular-shaped gyri with small, shallow or fused intervening sulci.
The surface of the brain may appear “smooth” due to the obliterated or fused sulci.
Dysplastic, abnormal leptomeningeal vessels may overlay the abnormal cortical ribbon.
On fetal MRI, polymicrogyria can appear as either early appearance of a sulcus or irregular cortical sulcation.
Polymicrogyria typically lines a schizencephalic cleft.
Associations: CMV infection, septo-optic dysplasia, callosal anomalies, gray matter heterotopia, MPPH syndrome (macrocephaly, polymicrogyria, polydactyly, hydrocephalus), tubulinopathy, Zellweger syndrome.
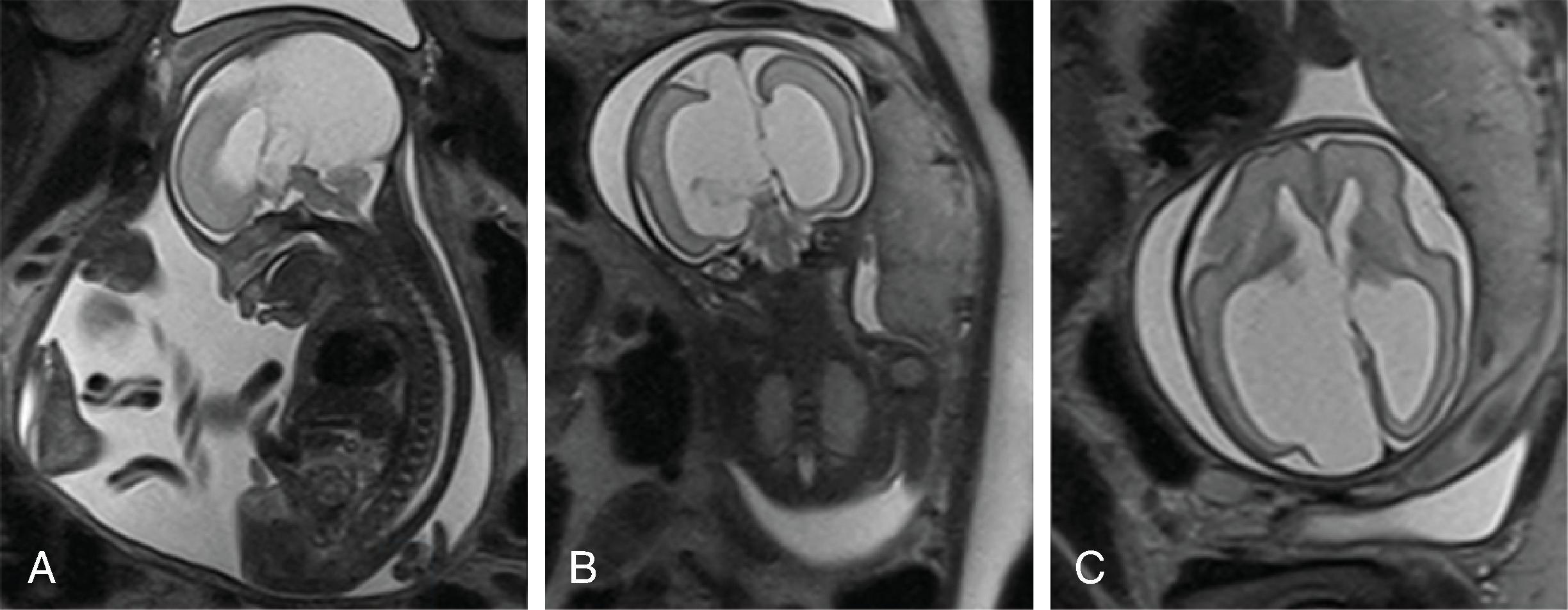
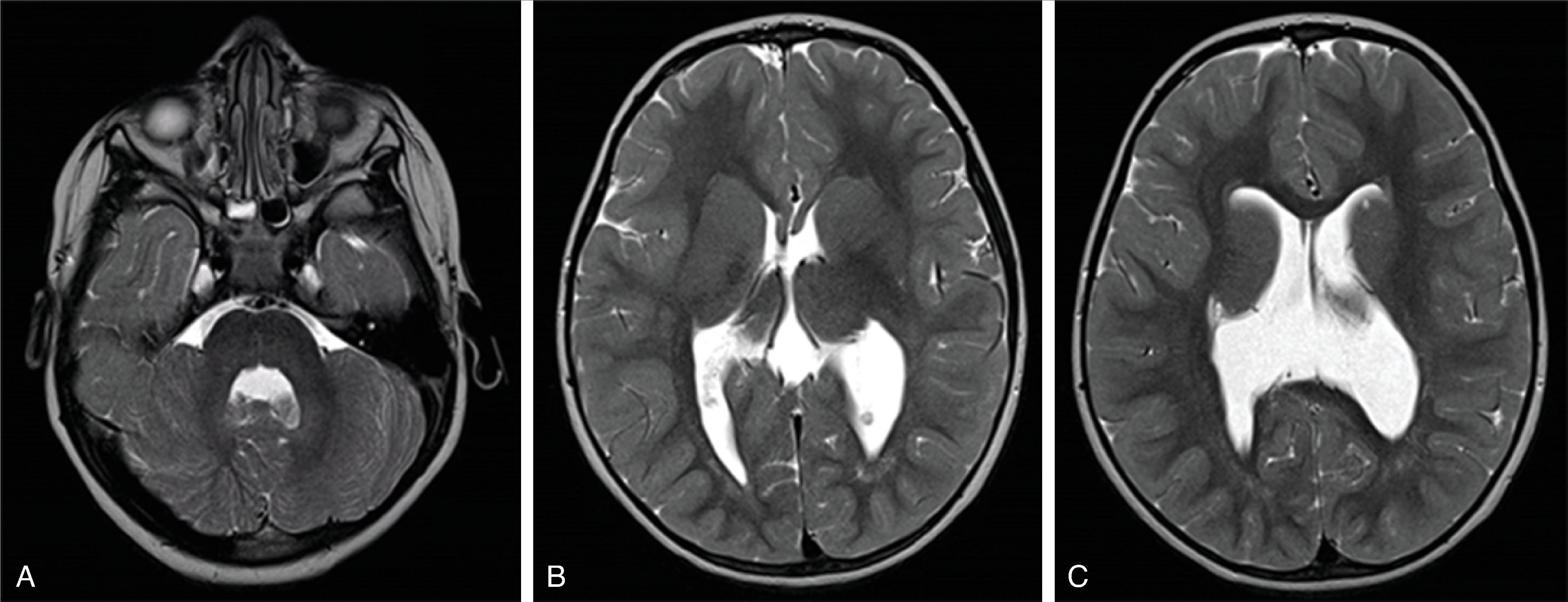
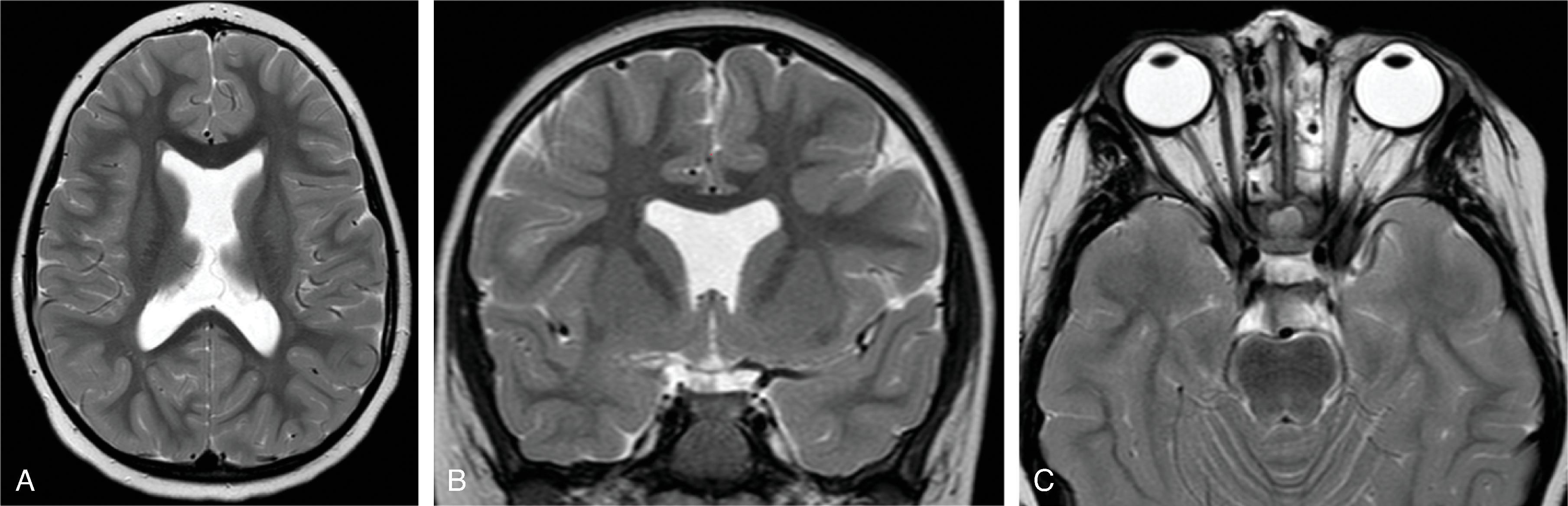
Schizencephaly is a malformation of cortical migration resulting in a gray matter–lined cleft in the cerebral hemisphere communicating from the subpial space to the ventricle.
The most likely etiology of a schizencephaly is an early, intrauterine vascular insult during neuronal migration and cortical organization occurring at ∼16 to 21 weeks’ gestation, similar to polymicrogyria.
Open-lip schizencephaly is more frequently symptomatic compared to closed-lip schizencephaly. Bilateral schizencephaly is associated with more severe neurocognitive impairment compared to unilateral. 90% of patients have motor dysfunction, 78% have cognitive dysfunction, and 68% have seizures.
Schizencephaly is defined as a cleft extending from the surface of the brain toward the ventricle lined by malformed gray matter (transmantle clefting). The cortical gray matter lining the cleft is dysplastic with abnormal lamination and may include areas of pachygyria and polymicrogyria.
Schizencephaly is subdivided into open-lip and closed-lip schizencephaly.
In open-lip schizencephaly, the lips/borders of the cleft are separated by cerebrospinal fluid, while in closed-lip schizencephaly the lips/borders of the cleft are “touching” each other with minimal or no cerebrospinal fluid within the cleft.
In closed-lip schizencephaly, a focal dimple may be seen along the ventricular wall at the “insertion site” of the cleft.
Dysplastic vessels may extend into the cleft.
Schizencephaly may be unilateral (∼60%) or bilateral (∼40%). In bilateral schizencephaly the leaves of the septum pellucidum are nearly always absent; in unilateral schizencephaly they are absent in most cases.
On (color-coded) fractional anisotropy maps the aberrant course or lack of affected white matter tracts are easily identified.
Schizencephaly can be diagnosed on fetal MRI in most cases.
Additional findings include: agenesis or hypogenesis of the corpus callosum (38%), septo-optic dysplasia (17%), hydrocephalus (24%), and microcephaly (42%).
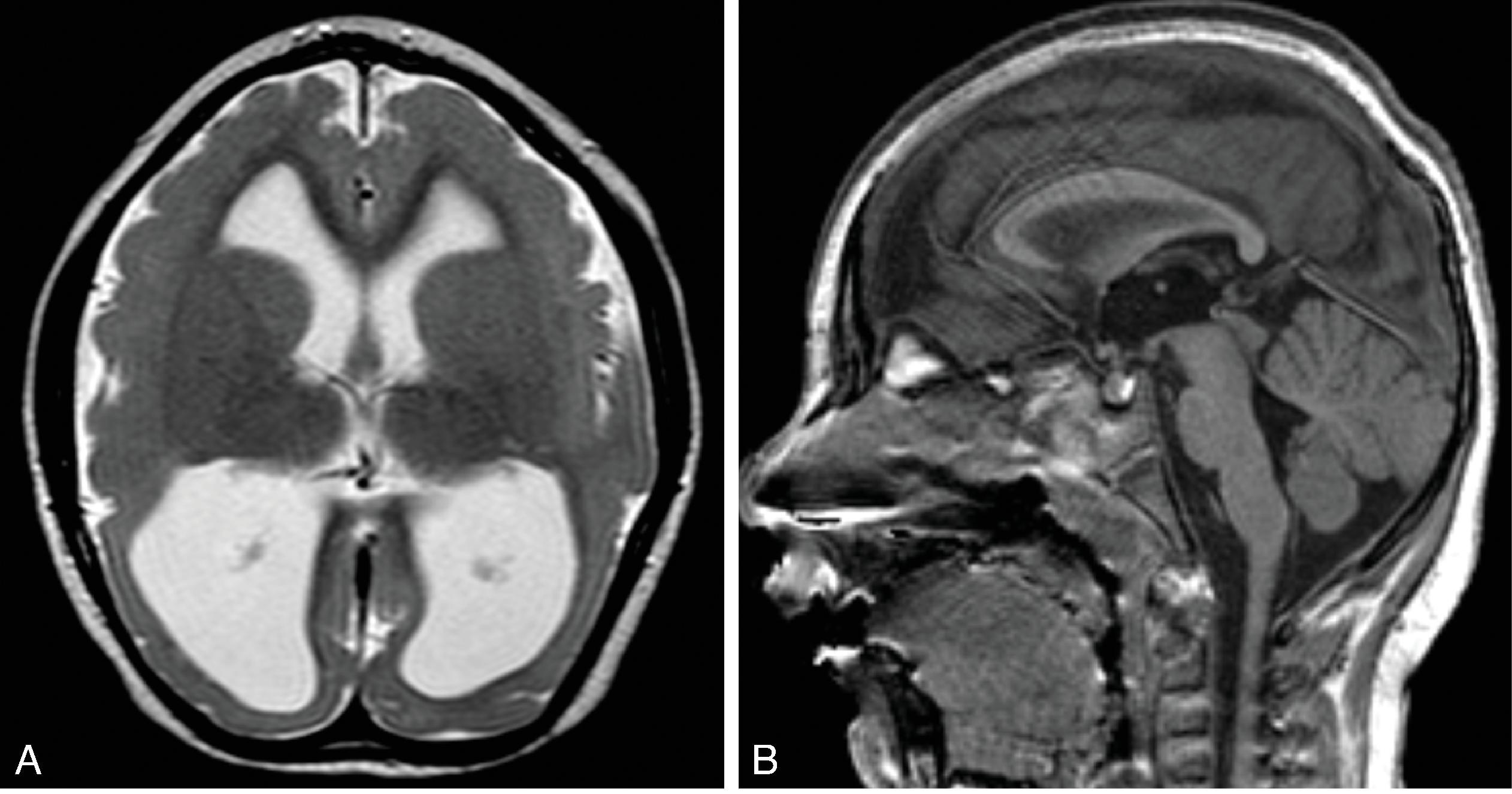
Porencephalic cysts are focal brain defects filled with cerebrospinal fluid. These are not classified as malformations due to the destructive etiology but are presented here in this section because of the imaging pattern.
In most cases they are chronic sequelae of remote injury, which may include a focal arterial or venous stroke, focal intraparenchymal hemorrhage, trauma, surgery, or infection.
Porencephalic cysts are frequent complications of hemorrhagic periventricular venous infarctions secondary to a germinal matrix hemorrhage.
Depending on the timing of the injury, prenatal versus postnatal, additional malformations (e.g., cortical dysplasia) may coexist secondary to a disruption of normal intrauterine brain development.
May be isolated within the white matter or may communicate with the adjacent ventricular system or subarachnoid space.
Depending on the etiology, timing of injury, size, and location of the cyst in relation to functional centers, symptoms may vary, including minimal to severe functional deficits, motor and cognitive developmental delays, and seizure activity.
Unilateral large focal porencephalic cysts are often accompanied with hemiatrophy of the brainstem, including Wallerian degeneration, in particular if the corona radiata or perirolandic area is involved.
Typically, marginated by white matter with or without adjacent white matter gliosis; the overlying cortical ribbon may be thinned.
On color-coded fractional anisotropy maps, a reduction in size or occasional complete lack of white matter tracts may be observed.
Prenatal or early postnatal destructive lesions typically show little to no gliosis on follow-up because of the immature response of the brain to injury.
Occasionally porencephalic cysts may exert mass effect on adjacent brain tissue, even when communicating with ventricular system, and may require fenestration or drainage.
On imaging the cysts follow the imaging characteristics of cerebrospinal fluid. If the cyst is not communicating with the ventricular system or subarachnoid space an increased protein content within the cyst may result in an increased signal intensity on T1W/FLAIR imaging.
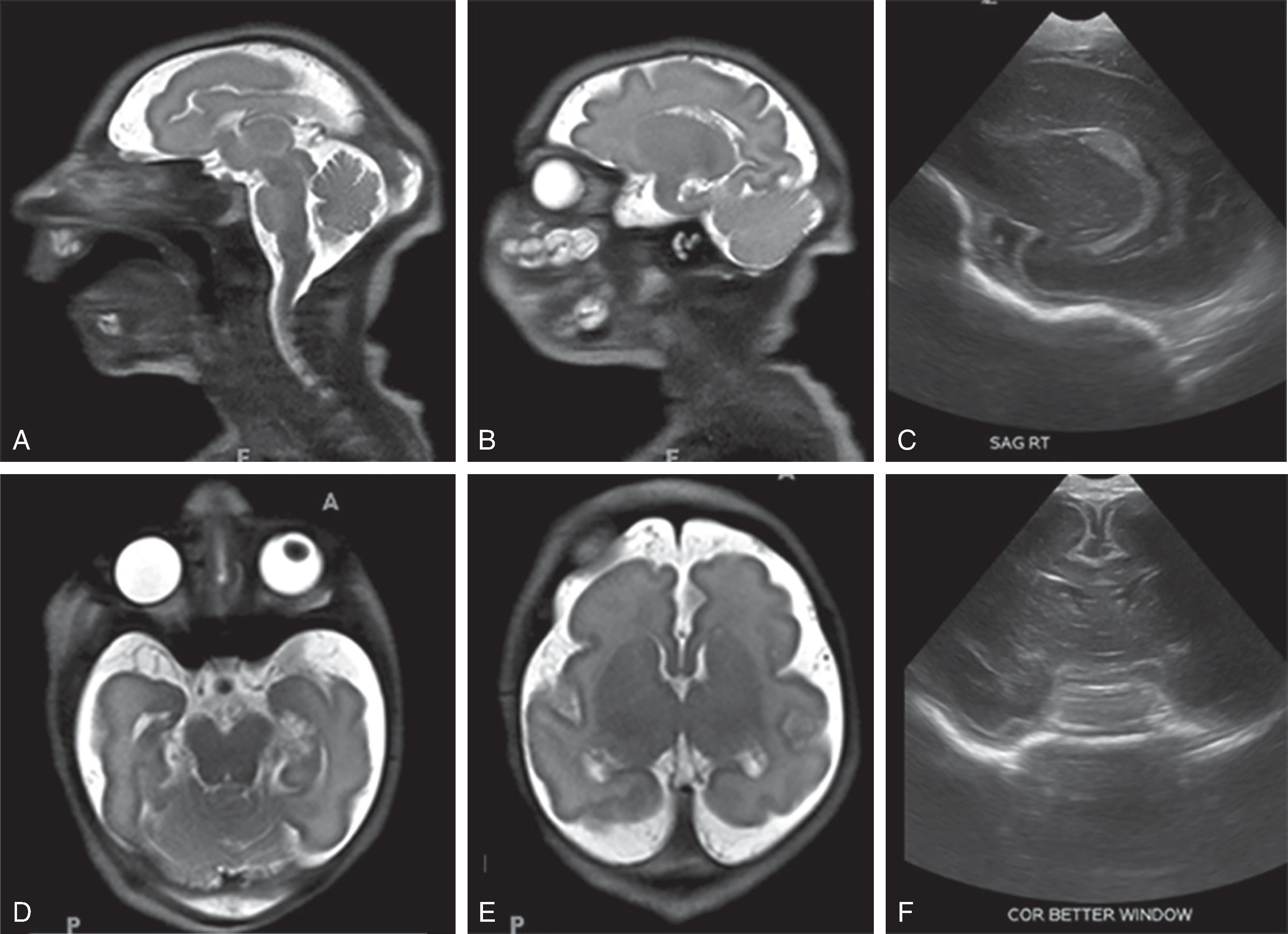
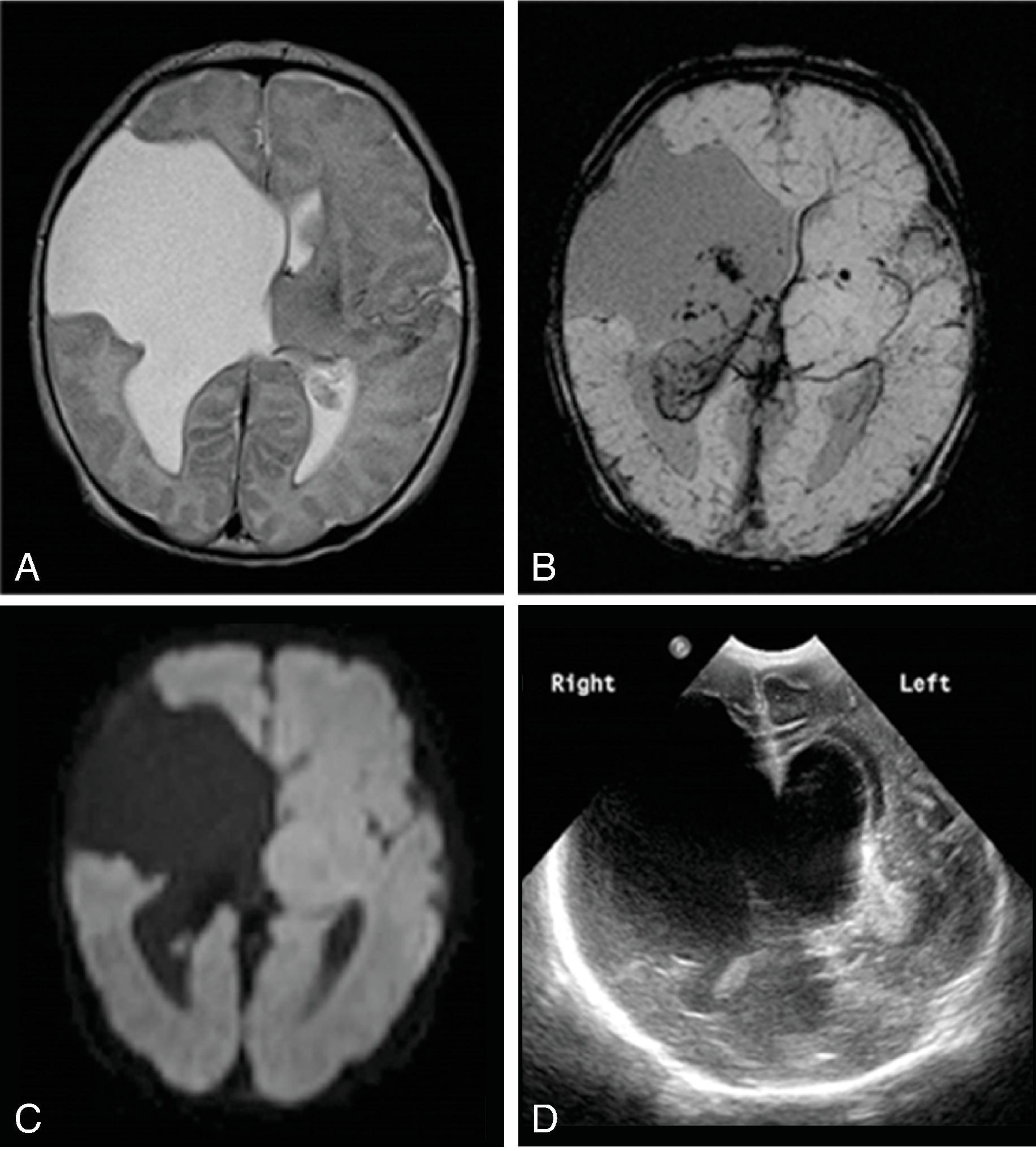
Complete or near complete absence of the supratentorial brain (hemispheric white matter and cortical gray matter) likely secondary to occlusion or atresia of the supraclinoid internal carotid arteries occurring in the second trimester. Causes can include in utero TORCH infections, twin-twin transfusion, maternal toxins, thrombotic causes, and COL4A1 mutation.
Hydranencephaly is not a malformation because it is a destructive process, but it is presented in this section due to the imaging pattern.
Initially infants have microcephaly that progresses to macrocephaly due to poor CSF regulation. Shunting of the supratentorial CSF may be indicated to assist management of macrocephaly.
Prognosis is poor, with function limited to the brainstem.
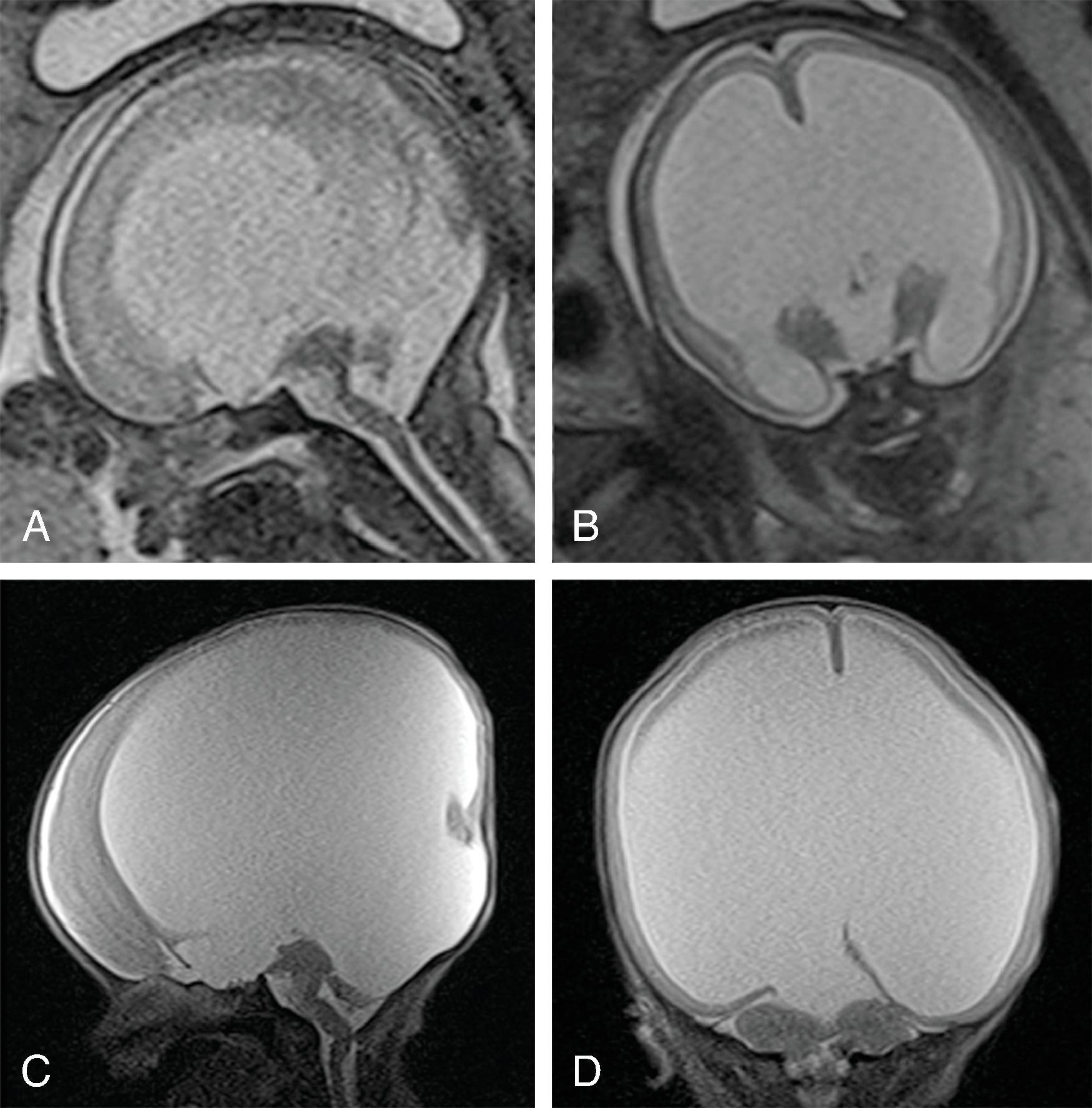
Absent cerebral hemispheres (some remaining medial temporal and occipital lobes can be seen) and CSF-filled cranium.
Brainstem, cerebellum, thalami, and choroid plexus, which are supplied by the vertebral arteries, are typically intact. Parts of the basal ganglia and medial occipital lobes may also be present. The preserved tissue survives via an intact posterior circulation.
The falx cerebri is present .
Residual leptomeninges may be seen following the inner table of the skull.
No circle of Willis is seen; no supraclinoid internal carotid arteries visible.
Differential Diagnosis: Alobar holoprosencephaly: absent falx Severe hydrocephalus: thin mantle of cortex present
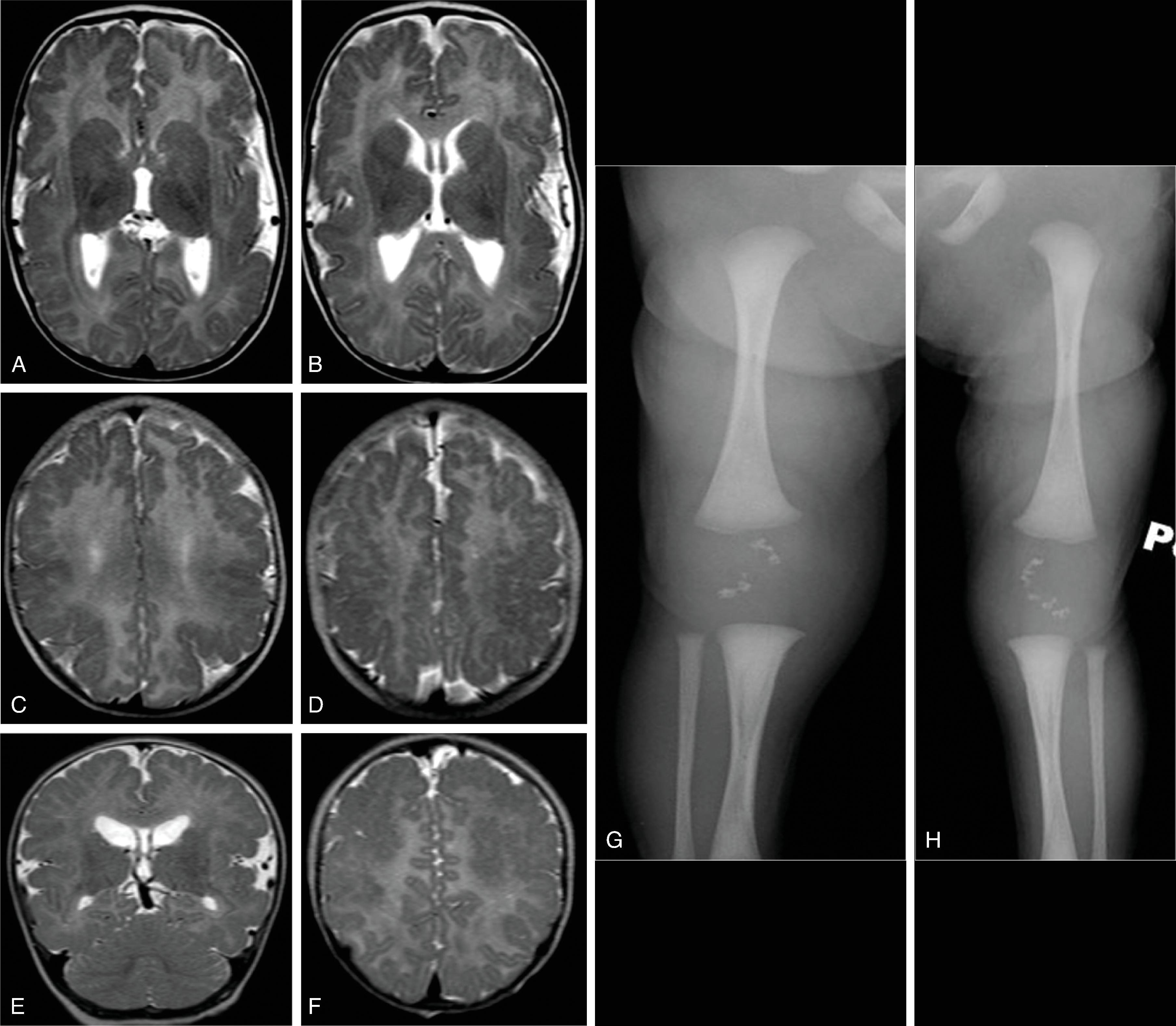
Autosomal recessive peroxisome disorder that can result from multiple gene mutations
Congenital anomalies (craniofacial, eyes, liver, bone, brain), hypotonia, psychomotor retardation, facial dysmorphism, seizures, periarticular calcifications, and hepatomegaly; death frequently within 1 year
Bilateral perisylvian polymicrogyria ; small gyri in the anterior frontal and temporal lobes
Caudothalamic groove germinolytic cysts
Hypomyelination
MRS: elevated lipid and lactate on short echo
PI3K/AKT/mTOR signaling pathway ( Fig. 2.54) involved in multiple disorders.
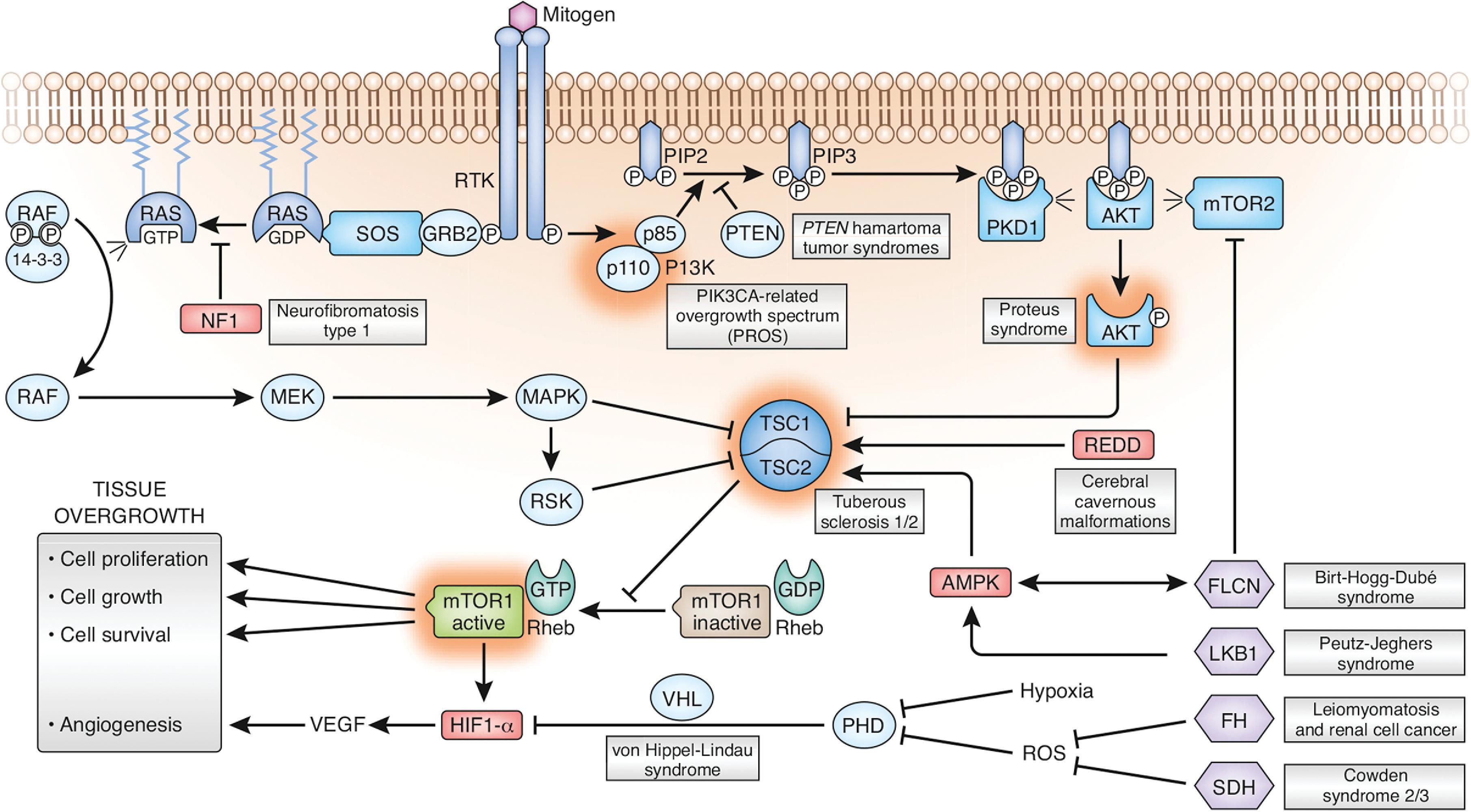


Tuberous sclerosis: TSC1 and TSC2 genes result in dysregulation of mammalian target of rapamycin (mTOR) and lead to cortical dysplasias.
PTEN hamartoma tumor syndrome (also known as Cowden and Bannayan-Riley-Ruvalcaba syndromes): the PTEN mutation leads to dysregulated AKT activity and the PIK3CA-related overgrowth spectrum, which includes epidermal nevi, CLOVES syndrome, megalencephaly-capillary-malformation, congenital lipomatous overgrowth, fibroadipose hyperplasia, and hemihyperplasia multiple lipomatosis.
Brain and extracranial findings in PI3K3CA-related overgrowth spectrum include the following:
Hemimegalencephaly, megalencephaly, and polymicrogyria
Focal cortical dysplasia
Ventriculomegaly
Tonsillar ectopia/Chiari I malformation
Large, isolated lymphatic malformation
Tubulinopathies result from mutations of the genes TUBA , TUBB , or TUBG encoding for the different isotypes of alpha-, beta- or gamma-tubulin, proteins that form the components of microtubules. The isotypes are the basis for the microtubule cytoskeleton that are assembled from the polymers of these tubulin heterodimers. The microtubule cytoskeleton is essential for the normal nervous system development.
Mutations of these essential genes consequently result in complex malformations affecting the neuronal proliferation, migration, and axonal pathfinding.
Depending on the genetic mutation, various brain malformations are present, including the following:
Basal ganglia malformation: A relatively characteristic imaging findings is that the b asal ganglia may appear malrotated , in particular the caudate nucleus, putamen, and globus pallidus are difficult to separate from each other on imaging. This is partially secondary to hypoplasia of the anterior limbs of the internal capsule (absence of the ALIC sign).
Cortical malformations: lissencephaly, pachygyria, polymicrogyria-like cortical dysplasia, simplified gyral pattern, and microlissencephaly
Cerebellar hypoplasia
Brainstem malformation: Pontine thinning or clefting, asymmetric cerebral peduncles
Malformation of the corpus callosum
GPR56: Autosomal recessive disorder; involves a protein involved in cell-cell and cell-extracellular matrix interactions; clinically manifests with developmental delay, seizures, dysconjugate gaze, pyramidal and cerebellar signs. Imaging: bilateral frontoparietal polymicrogyria with decreasing anterior-posterior gradient of severity , cerebellar cysts, thick corpus callosum, hypoplastic pons, and diffusion tensor imaging (DTI) demonstrate reduced transverse pontine fibers and middle cerebellar peduncle fibers and some patients with aberrant fibers from the corticospinal tract crossing the corpus callosum.
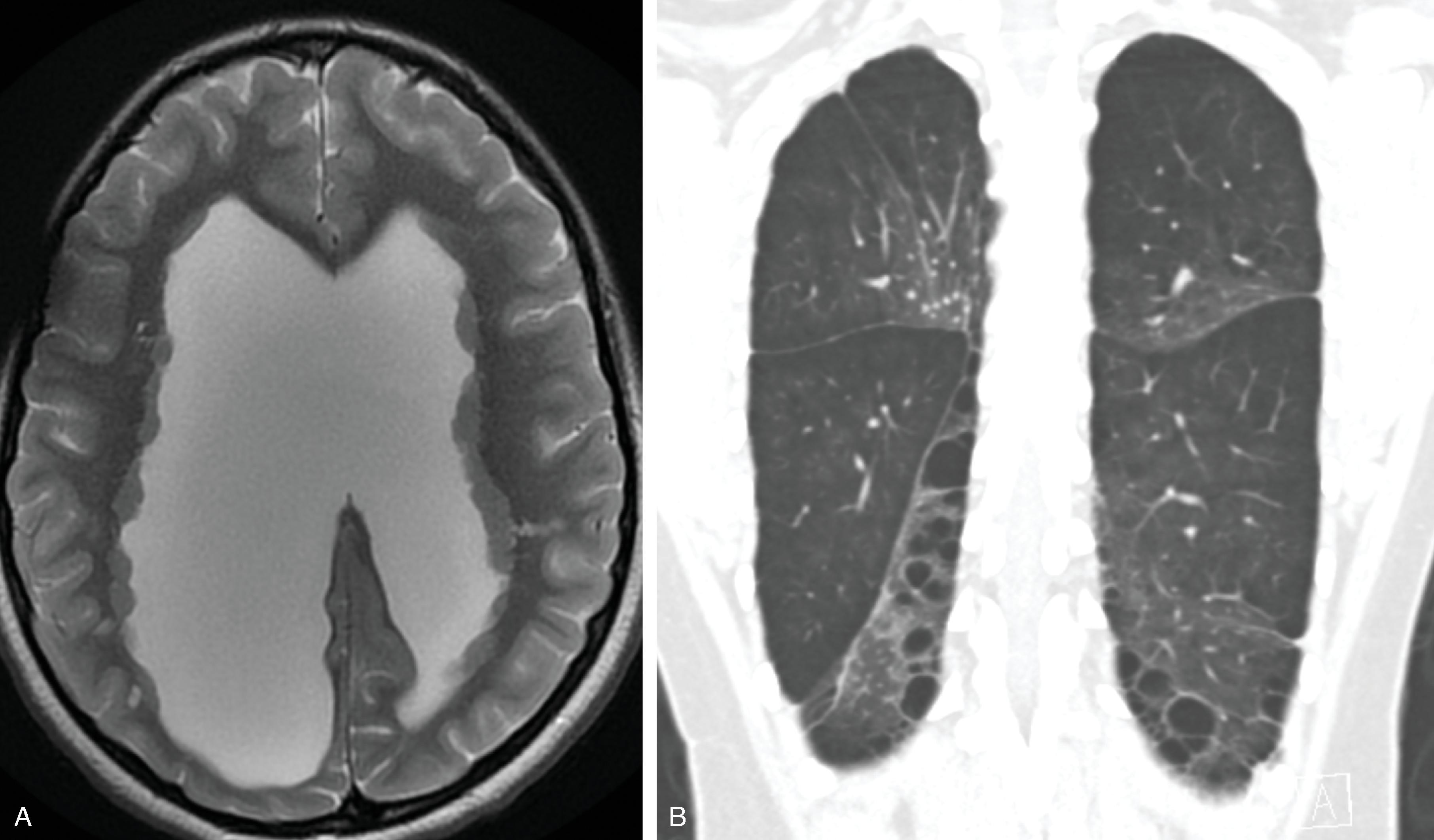
Filamin A deficiency: X-linked disorder; filamin A is an actin-binding protein that regulates cell shape and migration. Imaging demonstrates gray matter heterotopia , cerebellar hypoplasia, bilateral perisylvian or temporo-parieto-occipital polymicrogyria, and hydrocephalus. Lungs demonstrate emphysema , which manifests with respiratory distress in infancy.
Chudley-McCullough syndrome (GPSM2): autosomal recessive; clinical features include severe to profound sensorineural hearing loss; imaging findings include frontal polymicrogyria , heterotopia, callosal hypogenesis, dysplastic inferior cerebellar fissures.
Reelin mutation: Imaging findings include pachygyria/lissencephaly and bilateral cerebellar hypoplasia.
VLDRL: Imaging findings include pachygyria/lissencephaly and bilateral cerebellar hypoplasia.
CASK: Imaging findings include simplified gyral pattern and cerebellar hypoplasia.
MPPH syndrome: Macrocephaly, polymicrogyria, polydactyly, hydrocephalus.
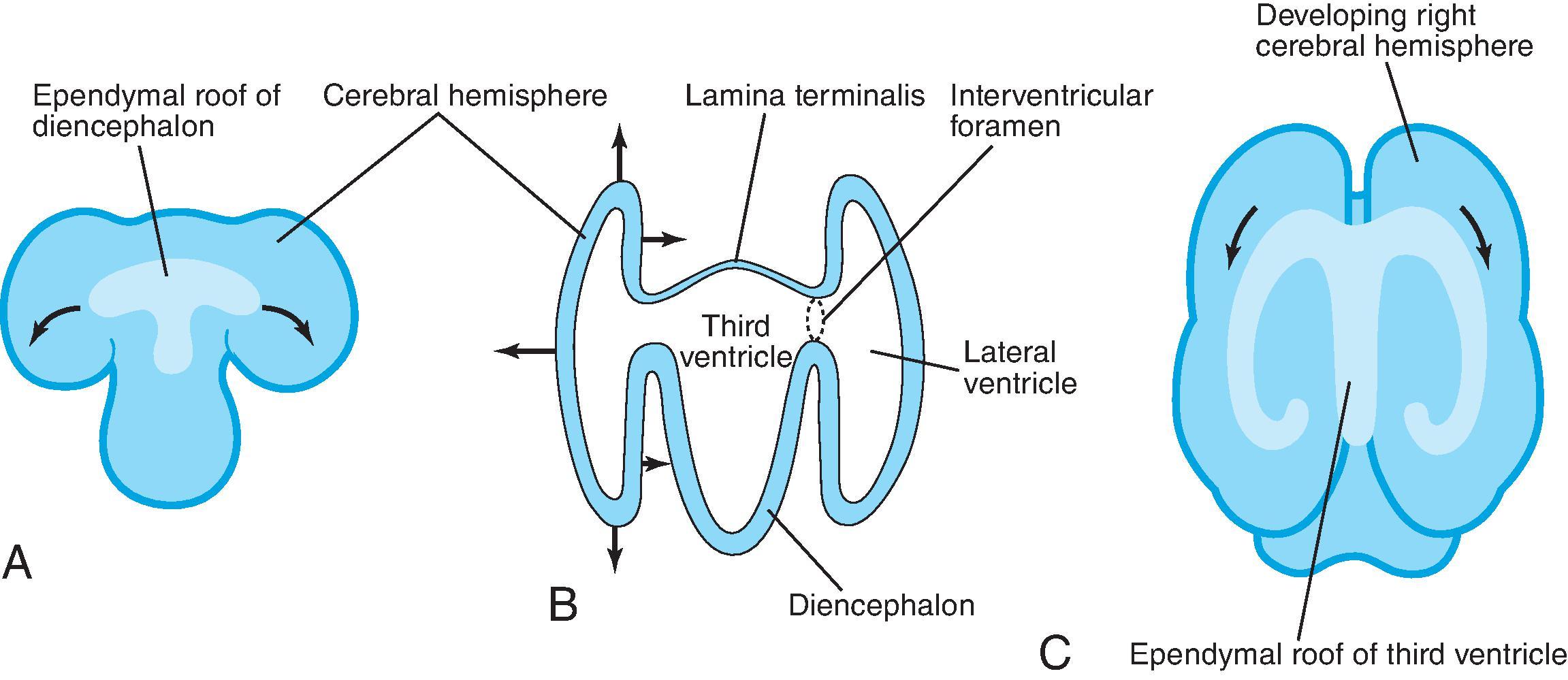
At approximately 35 days of gestation, the prosencephalon divides into the telencephalon and diencephalon. The telencephalon, which forms the cerebral hemispheres, separates as outpouchings in the region of the foramen of Monro. The thin-walled cerebral hemisphere vesicles expand and cover the diencephalon. The telencephalon vesicle will partition in the midline along the roof of the telencephalon, leading to the interhemispheric fissure and differentiation of the falx. Separation of the embryonic prosencephalic vesicle depends on a balance between ventral and dorsal midline induction. The segmentation has a posterior → anterior, caudal → rostral, and dorsal → ventral pattern, which means the inferior frontal lobes are the last region to separate.
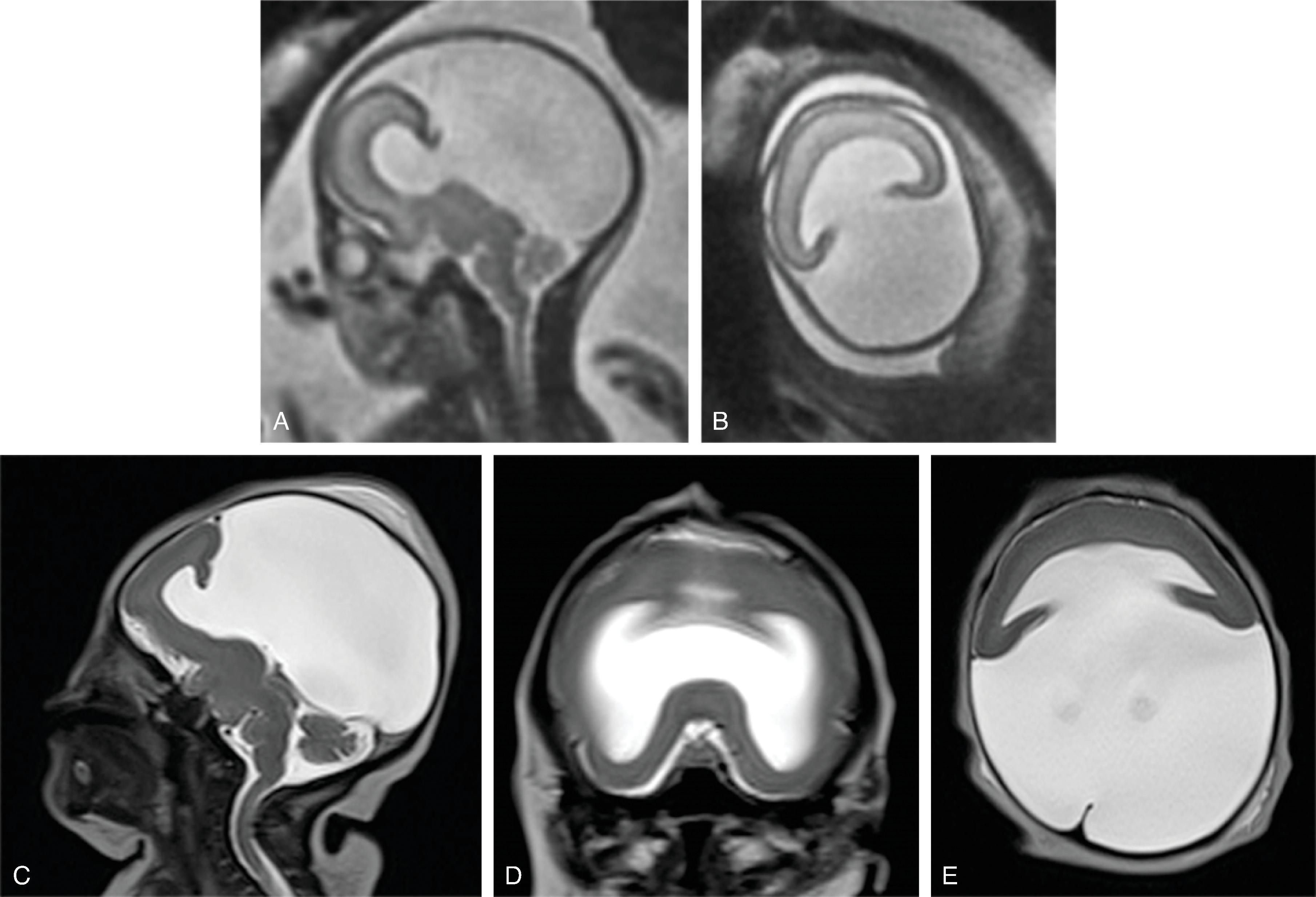
Holoprosencephalies are a result of abnormal ventral induction occurring ∼fifth to sixth week of gestation. The interhemispheric fissure fails to form, and the prosencephalic vesicle is not split into the two telencephalic vesicles, resulting in varying degrees of midline fusion. Etiology of holoprosencephaly is heterogeneous and includes teratogens (alcohol, retinoic acid), environmental factors (maternal diabetes), and genetic causes (∼25% to 50% of cases), including trisomy 13 and at least 14 gene mutations, of which sonic hedgehog gene (30% to 40%) and ZIC1 (5%) are the most common. Additional maxillofacial malformations may exist, including hypotelorism, midline clefts, single central incisor, absent metopic suture, and cyclopia (the face may predict the brain). Additional midline structures that may be affected include the rhinencephalon (hypoplastic olfactory bulbs), hypothalamus/pituitary gland, septum pellucidum, and corpus callosum.
Holoprosencephaly is subdivided in alobar, semilobar, and lobar forms, depending on the severity and extent of the midline anomaly ( Table 2.2 ). Holoprosencephaly and similar malformations are discussed in this section.
| Finding | Craniofacial Anomalies | Ventricles | Septum Pellucidum | Falx Cerebri | Interhemispheric Fissure | Thalami, Basal Ganglia |
|---|---|---|---|---|---|---|
Alobar  |
Severe | Monoventricle | Absent | Absent | Absent | Fused |
Semi-lobar  |
Variable | Rudimentary temporal and occipital horns | Absent | Present posteriorly | Present Posteriorly | Partial fusion |
Lobar  |
Absent or mild | Squared-off frontal horns | Absent | Well-formed | Present | Separated |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here