Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The etiologic agents of blastomycosis belong to a group of fungi that are characterized by thermal dimorphism. Advances in genomics and phylogenetics have expanded the number of Blastomyces species to include B. dermatitidis, B. gilchristii, B. percursus , B. helicus, B. parvus, and B. silverae . Infection is primarily acquired through the inhalation of infectious conidia and hyphal fragments following the disruption of soil. Once inside the lungs, the infectious particles convert into pathogenic yeast, which causes pneumonia and can disseminate to other organs. Blastomycosis can mimic other diseases, which often delays diagnosis and initiation of therapy. Thus diagnosis requires a high degree of clinical suspicion. Antifungals with activity against Blastomyces yeast include polyenes and azoles.
Blastomycosis was first described in 1894 by Gilchrist as a protozoan infection. Subsequent research by Gilchrist and Stokes at Johns Hopkins University correctly identified the organism as a fungus, which they named Blastomyces dermatitidis . In 1907 Hamburger at Rush Medical College described thermal dimorphism in B. dermatitidis with budding yeast at 37°C and hyphal growth at ambient temperature. Colloquial names for blastomycosis coined early in the 20th century included Gilchrist disease, Chicago disease, and North American blastomycosis.
In 1951 Schwarz and Baum at the Jewish and General Hospitals in Cincinnati determined that the lung was the primary portal of entry, and involvement of other organs such as the skin was due to lymphohematogenous dissemination. In 1955 Smith and colleagues at Duke Hospital described the first recognized outbreak of blastomycosis (1953–1954) in Grifton, North Carolina. During the 1960s and 1970s the epidemiology and geographic distribution of blastomycosis were refined. In the mid-1980s B. dermatitidis was successfully cultured from soil at the site of an outbreak in Eagle River, Wisconsin. In the 1990s clinical trials under the auspices of the National Institutes of Allergy and Infectious Diseases Mycoses Study Group were conducted to assess the efficacy of fluconazole and itraconazole. The first practice guideline for treatment of blastomycosis was published by the Infectious Diseases Society of America (IDSA) in 2000 and updated in 2008. The American Thoracic Society (ATS) Fungal Working Group published their guidelines in 2011.
There have been substantial advances in the past 2 decades in understanding the molecular pathogenesis including identification and characterization of genes critical for immune evasion and morphogenesis. In addition, the Blastomyces genus has undergone taxonomic revision. In 2013 Brown and colleagues discovered a new species in North America, which they named Blastomyces gilchristii in honor of Dr. Gilchrist. B. dermatitidis and B. gilchristii are estimated to have diverged 1.9 million years ago during the Pleistocene epoch. In 2017 Dukik and colleagues identified another new species, Blastomyces percursus, when analyzing human clinical isolates from South Africa and Israel. Several Emmonsia species have been reclassified into the Blastomyces genus including Blastomyces helicus (formerly E. helicus ) and Blastomyces parvus (formerly E. parvus ). B. helicus causes blastomycosis in North America, but outside the traditional endemic region. This species chiefly infects patients with impaired immunity, and results in fungemia and high mortality. B. parvus predominantly causes infection in rodents. Blastomyces silverae is a new species named after Eleanor Silver Keeping who was a distinguished mycologist at the University of Alberta. B. silverae has been misidentified as E. parvus and can cause infection in humans. The genomic sequences of B. dermatitidis, B. gilchristii, and B. percursus are publicly available.
Blastomyces spp. belong to the Ascomycota phylum, which includes other dimorphic fungi such as Histoplasma, Coccidioides, Sporothrix, Paracoccidioides, Emmonsia, and Talaromyces marneffei (formerly Penicillium marneffei ). The biology of these fungi is characterized by the ability to reversibly convert between hyphal and yeast forms in response to temperature.
At environmental temperature (22°C–25°C), Blastomyces grows as septated hyphae (1–2 µm in diameter) that produce small conidia (4–5 µm) attached to a short conidiophore, which resembles a lollipop ( Fig. 264.1A ). B. percursus has a slightly different morphologic appearance than B. dermatitidis or B. gilchristii at ambient temperature, with conidiophores bearing single or multiple conidia. The appearance of B. helicus is unique because the hyphae can coil into a helical morphology and fail to produce conidia under most in vitro conditions. In the laboratory, Blastomyces conidia are difficult to liberate from hyphae and require wetting the culture plate with water or phosphate-buffered saline solution followed by mechanical disruption. The morphologic appearance of mycelia is not distinctive, and definitive identification requires conversion to yeast or molecular methods.
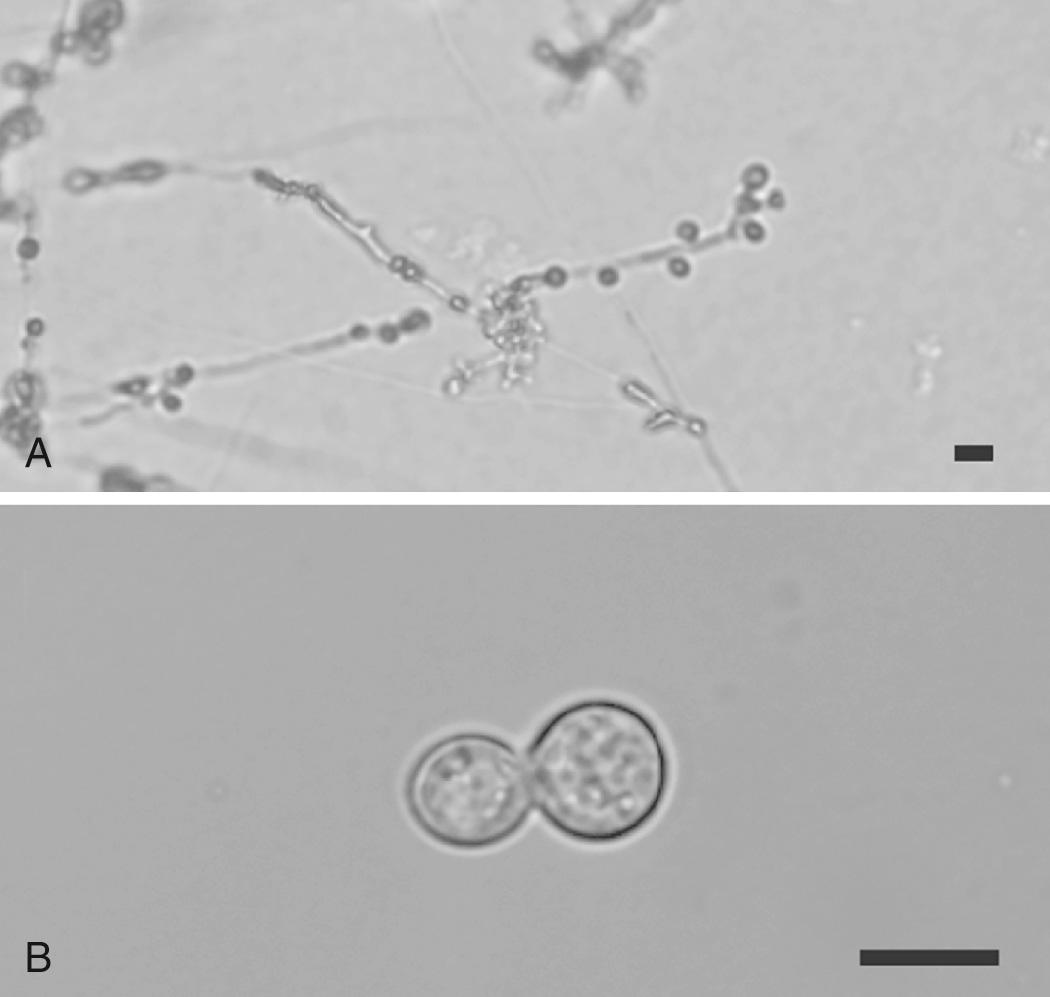
At 37°C, B. dermatitidis, B. gilchristii, and B. percursus yeast (8–20 µm) is characterized by a doubly refractile cell wall, multiple nuclei (typically three or four per cell), and a broad-based budding pattern between mother and daughter cells ( Fig. 264.1B ). B. dermatitidis and B. gilchristii have similar morphology and cannot be distinguished using microscopy. Differentiation requires DNA sequencing to assess for a single nucleotide polymorphism (C for B. gilchristii and T for B. dermatitidis ) at base-pair 19 in the untranslated region of ITS2 . B. helicus yeast (4–9 µm) also exhibit broad-based budding but can form short chains of yeast cells. Definitive identification of B. helicus requires sequencing of the ITS (internal transcribed spacer) or D1/D2 domains of the long subunit of ribosomal RNA.
Blastomyces refers to the asexual form, whereas the sexual form is known as Ajellomyces . Asexual (or clonal) reproduction involves the production of conidia by hyphae or yeast cell division by budding. In contrast, sexual reproduction occurs only in the hyphal phase. Ajellomyces undergoes heterothallic mating in which the hyphae of opposite mating types (+ and −) join together to form special structures known as cleistothecia that exchange genetic material to produce ascospores. The + mating type contains the α-box gene, whereas the − mating type contains the HMG gene. The mating type ratio (+ : −) for B. dermatitidis is 1:1, whereas it ranges from 1:1 to 1:2 for B. gilchristii . Mating typically occurs within a single species (e.g., B. dermatitidis mating with B. dermatitidis ); however, genotyping has suggested the potential for recombination between B. dermatitidis and B. gilchristii . Sexual ascospores and asexual conidia can germinate as hyphae at 22°C to 25°C or yeast at 37°C.
The genomes of B. dermatitidis and B. gilchristii range from 66.6 to 75.4 MB and contain 9180 to 10,187 genes. Although B. dermatitidis and B. gilchristii contain a similar number of genes as other thermally dimorphic fungi, the genome size is twofold larger than other closely related fungi such as Histoplasma capsulatum and, surprisingly, B. percursus (32.3 MB).
Blastomycosis is a disease primarily localized to the United States and Canada. The endemic region for Blastomyces includes the Midwest, South Central, and Southeastern United States and Canadian providences including Quebec, Ontario, Manitoba, and Saskatchewan ( Fig. 264.2A ). Blastomyces is not uniformly distributed throughout the endemic region. Rather, it is found in a specific ecologic niche characterized by sandy soil with an acidic pH and decaying vegetation that is located in forested areas near fresh water. This includes watershed areas for Mississippi, Ohio, Savannah, Saint Lawrence, and Nelson rivers as well as areas adjacent to the Great Lakes. Ecologic niche modeling incorporating vegetation indices, soil characteristics, hydrologic features, and geocoded cases of blastomycosis in Wisconsin suggests that land near waterways is favorable for Blastomyces .
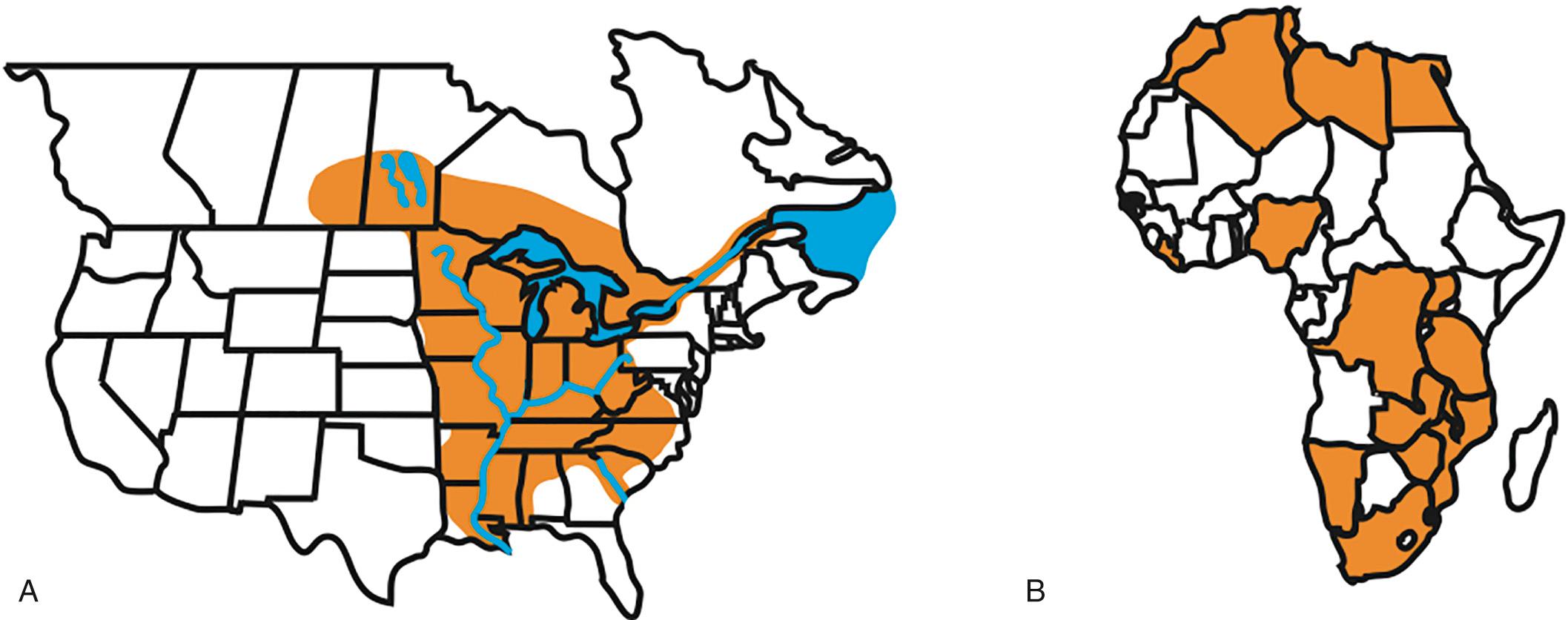
Within the ecologic niche, B. dermatitidis and B. gilchristii exhibit geographic restriction and overlap. On the basis of phylogeographic mapping of soil and species determination of clinical isolates, B. gilchristii appears to be restricted to Canada, Minnesota, Wisconsin, and New York. In contrast, B. dermatitidis has a wider geographic distribution including Canada and Midwest, South Central, and Southeastern United States along with states along the St. Lawrence River. B. dermatitidis and B. gilchristii overlap in Canada, Minnesota, and Wisconsin. Genetic diversity within B. dermatitidis and B. gilchristii is influenced by geography that corresponds to freshwater drainage basins (e.g., Nelson River, Mississippi River). McTaggart and colleagues hypothesized that glaciation during the Pleistocene epoch, when B. dermatitidis and B. gilchristii diverged 1.9 million years ago, may have influenced the geographic distribution of B. gilchristii because it is restricted to formerly glaciated areas.
Human and veterinary cases of B. helicus infection occur outside the endemic region of B. dermatitidis and B. gilchristii in North America. All clinical cases occurred in the western United States and Canada. The majority of B. silverae isolates have been from the United States or Canada; however, the geographic range of this species has yet to be fully elucidated. Outside of North America, blastomycosis is a rare cause of invasive fungal infection. Autochthonous culture-proven cases of blastomycosis have been reported from Africa and India ( Fig. 264.2B ). African Blastomyces strains have a decreased ability to convert to yeast at 37°C in vitro, unique media requirements for growth in the laboratory, and a cell surface that is less antigenically complex. B. percursus has been isolated from patients living in Israel and South Africa.
The epidemiology of blastomycosis is largely based on passive surveillance data, small-scale retrospective studies, and analysis of outbreaks. Mandatory reporting of blastomycosis is required in Minnesota, Wisconsin, Michigan, Arkansas, Louisiana, and Manitoba. In Ontario, laboratory-confirmed cases are tracked by the Northwestern Health Unit jurisdiction. The lack of reliable skin and serologic tests for assessing exposure to Blastomyces has hindered the epidemiologic understanding of blastomycosis. Thus epidemiologic data are limited to reports of patients with clinically apparent infection. However, an estimated 50% of patients with blastomycosis may have asymptomatic or subclinical infection. Collectively, these limitations result in underestimation of the true incidence of blastomycosis.
Within the endemic region, the annual incidence of blastomycosis ranges from 0.11 to 1.94 cases per 100,000 persons ( Table 264.1 ), and the overall mortality rate is 0.21 per 1 million person-years. A study analyzing a sample of Medicare beneficiaries (1999–2008) reported a nationwide incidence of 0.7 per 100,000 with the highest rates in the Midwest and Southern United States. Within the endemic region, certain areas are considered hyperendemic including Kenora (Ontario), Eagle River (Wisconsin), Vilas County (Wisconsin), and south-central Mississippi (see Table 264.1 ). In the mid-1980s, Washington Parish in Louisiana was recognized as a hyperendemic area with 6.8 cases per 100,000 persons (vs. a statewide incidence of 0.23/100,000). Subsequent analyses demonstrated that the incidence in Washington Parish is lower at 1.29 per 100,000 but remains higher than the statewide incidence of 0.11 per 100,000.
| STATE OR PROVINCE | YEARS | INCIDENCE PER 100,000 PERSONS | AGE (Y) | MALE:FEMALE | REFERENCE |
|---|---|---|---|---|---|
| Wisconsin | 1973–1982 | 0.5 (0.32–0.72) | 43.7 a | 1.8:1 | |
| 1986–1995 | 1.4 | 46 b | 1.5:1 | ||
| 1984–1990 | 40.4 (Vilas County); 101.3 (Eagle River) | 42 b | 1.6:1 | ||
| 2002–2006 | 2.0 | — | — | ||
| 2011–2015 | 1.61 (sporadic only) | 48 b | 2.1:1 | ||
| Minnesota | 2002–2006 | 0.5 | — | — | |
| 2012–2016 | 0.6 | 46 b | 2.9:1 | ||
| Illinois | 1981–1989 | 1.94 (Rockford) | 40 b | 2.2:1 | |
| 2001–2007 | 0.4–1.1 c | 41–47 b | 1.4–2.4:1 | ||
| Michigan | 2012–2016 | 0.14 (0.11–0.20) | — | 4.4:1 | |
| Arkansas | 2007–2016 | 0.39 (0.2–0.74) | — | 1.97:1 | |
| Missouri | 1992–1999 | 0.2 d | 46.7 a | 2.1:1 | |
| Alabama | 1992–1994 | 0.6 | — | — | |
| Mississippi | 1979–1988 | 1.3 e | 46 a | 1.7:1 | |
| Louisiana | 1987–2016 | 0.11 | 48 b | 1.4:1 | |
| Tennessee | 1988–1995 | 1.23 f | 52 a | 2.3:1 | |
| 1996–2005 | 1.29 f | 59 a | 2.5:1 | ||
| Northwest Ontario | 1989–2005 | 17.0 | — | — | |
| Kenora, Ontario | 1997–1999 | 117.2 | 41.9 a | 1.1:1 | |
| Manitoba | 1988–1999 | 0.62 | 38 a | 1.9:1 | |
| Ontario, Quebec, Manitoba, Saskatchewan | — | 0.62 | — | — |
c Highest incidence of infection occurred in northeastern Illinois (Cook, Lake, Kane, and Will counties).
d Highest incidence was in southeastern Missouri with the highest incidence in Mississippi County (12/100,000).
e Highest incidence was in central and south-central Mississippi (>5/100,000) and accounted for 63% of blastomycosis from 1979–1988.
f For Carter, Cocke, Greene, Hawkins, Johnson, Sullivan, Unicoi, and Washington counties, which are located in northeastern Tennessee.
Although most cases of blastomycosis are sporadic, outbreaks have been reported from seven states, with multiple outbreaks occurring in Wisconsin, North Carolina, and Minnesota ( Table 264.2 ). These outbreaks have often been associated with activities that disrupt soil, such as construction or recreation along riverbanks (see Table 264.2 ). Although exposure to Blastomyces most commonly occurs in rural areas, soil disruption in urban areas can lead to infection (sporadic cases and outbreaks) (see Table 264.2 ). Similar to rural areas, urban blastomycosis has been associated with waterways such as lakes, rivers, and streams; use of a community compost pile; and highway construction.
| YEAR | STATE | LOCATION | NO. CASES | RURAL OR URBAN | SOURCE OF OUTBREAK | REFERENCE |
|---|---|---|---|---|---|---|
| 1953–1954 | North Carolina | Pitt County | 11 | Rural | Not identified | |
| 1972 | Minnesota | Big Fork | 12 | Rural | Cabin construction | |
| 1975–1976 | North Carolina | Enfield | 5 | Rural | Peanut harvest | |
| 1974–1975 | Illinois | Westmont | 5 | Urban | Construction | |
| 1979 | Wisconsin | Namekegon River | 8 | Rural | Canoeing | |
| 1984 | Wisconsin | Eagle River | 48 | Rural | Beaver lodge | |
| 1984 | Virginia | Southampton County | 4 | Rural | Raccoon hunting | |
| 1985 | Wisconsin | Tomorrow River | 7 | Rural | Fishing on river bank | |
| 1985 | Wisconsin | Crystal River | 7 | Rural | Underground fort | |
| 1988 | Wisconsin | Watersmeet Lake | 32 a | Rural | Hotel construction | |
| 1989 | Tennessee | Elizabethton | 3 | Urban | Factory construction | |
| 1989–1990 | Wisconsin | Oconto County | 8 | Rural | Not identified | |
| 1998 | Colorado | Boulder | 2 | Rural | Prairie dog relocation | |
| 1998–2000 | Wisconsin | Indian reservation | 9 | Rural | Likely excavation | |
| 1999 | Minnesota | Mountain Iron | 18 | Urban | Excavation | |
| 2001–2002 | North Carolina | Duplin County | 8 | Rural | Likely construction or excavation | |
| 2005–2008 | Indiana | Indianapolis and surrounding counties | 59 | Urban | Highway construction | |
| 2006 | Wisconsin | Lincoln County | 21 | Urban | Pine needle yard waste | |
| 2009–2010 | Wisconsin | Marathon County | 55 | Urban | Not identified | |
| 2015 | Wisconsin | Little Wolf River | 90 | Rural | Tubing on the river |
Outbreak investigations have advanced the clinical understanding of blastomycosis and uncovered novel areas for further study. Analysis of outbreaks along the Eagle River, Tomorrow River, and Crystal River in Wisconsin helped define the ecologic niche and established that the incubation period of blastomycosis ranges from 3 weeks to 3 months. Moreover, these investigations demonstrated that approximately 50% of people exposed to blastomycosis develop symptomatic disease, whereas the remaining 50% have asymptomatic or subclinical disease. Analysis of blastomycosis outbreaks in Marathon County, Wisconsin, in 2006 and 2009–2010 found a high rate of blastomycosis in people of Hmong ethnicity (adjusted odds ratio of 12.1 [95% confidence interval 1.3–611.9; P = .019] by multivariable logistic regression). The high rate of blastomycosis did not appear to be related to environmental exposure. This led to the hypothesis that increased susceptibility to blastomycosis in people of Hmong ethnicity may be related to genetic predisposition. In Ontario and Manitoba, people of Aboriginal ethnicity have an increased incidence of blastomycosis.
Fungal traits that contribute to invasive disease include thermotolerance (e.g., growth at 37°C), intracellular survival, and upregulation of yeast phase–specific virulence factors that subvert host defenses. Hyphal growth at ambient temperature promotes environment survival, genetic diversity through mating, and transmission to mammalian hosts via spores and hyphal fragments. Temperature is the major stimulus that drives the reversible conversion between hyphae (22°C–25°C) and yeast (37°C). The uptake of exogenous cysteine, which restarts mitochondrial respiration during the morphologic switch, is required to complete the conversion to yeast. Compared with Coccidioides and Paracoccidioides , carbon dioxide tension and estradiol do not appear to affect the morphologic switch or growth of Blastomyces . Following inhalation of conidia into the lungs, resident pulmonary macrophages engulf these infectious particles, of which a subpopulation will survive and convert to yeast. Within macrophage, the morphologic switch from conidia to yeast is accelerated. The capacity for intracellular survival as well as extracellular replication is a shared biologic feature among the endemic dimorphic fungi.
Once in the yeast phase, B. dermatitidis and B. gilchristii upregulate Blastomyces -adhesin-1 (BAD1, formerly known as WI-1), an essential virulence factor that is specific to the yeast phase. In a murine model of pulmonary infection, BAD1 was the most upregulated gene in the Blastomyces transcriptome. BAD1 is secreted by yeast and can either bind back to the yeast cell surface via interactions with chitin in the cell wall or remain soluble in the extracellular environment. BAD1 functions as an adhesin and an immune evasin. It facilitates binding of yeast cells to host tissue via interactions with heparan sulfate and adherence to host immune cells via interactions with complement receptors (CR3) and CD14. BAD1 on the yeast cell wall inhibits the production of tumor necrosis factor-α (TNF-α) by macrophages and neutrophils in a transforming growth factor-β–dependent manner. Soluble BAD1 also inhibits TNF-α production, but independent of transforming growth factor-β. In addition to blocking TNF-α, BAD1 inhibits CD4 + T-lymphocyte activation, which results in reduced production of interleukin-17 (IL-17) and interferon-γ. Importantly, deletion of BAD1 severely attenuated the pathogenicity of Blastomyces yeast in a murine model of pulmonary infection, which indicates that this gene is essential for virulence.
Other mechanisms that contribute to virulence include secretion of dipeptidyl peptidase IVA (DppIVA), relative resistance to oxidative and nitrosative killing, and alteration of yeast cell wall carbohydrates. DppIVA is a serine protease that inactivates granulocyte-macrophage colony-stimulating factor, which in turn impairs recruitment of innate immune cells into the lungs and inhibits macrophage and neutrophil activation. Silencing of DppIVA using RNA interference results in yeast cells with attenuated virulence in vivo and reduced survival when cocultivated with activated immune cells. Compared with conidia, yeast is more difficult for macrophages to kill and is less susceptible to reactive oxygen species. The resistance to oxidative damage may be due to upregulation of superoxide dismutases and catalase in yeast during infection. Blastomyces yeast suppresses the production of nitric oxide by macrophages; however, the mechanism underlying this defense strategy remains to be elucidated. During the temperature-dependent morphologic switch, the amount of β-(1,3)-glucan in the cell wall decreases from 40% in hyphae to less than 5% in yeast. This reduction has the potential to limit recognition of yeast by the dectin-1 and mannose receptors on host immune cells. In addition, the reduction of β-(1,3)-glucan impairs the ability of echinocandin antifungals to effectively kill Blastomyces yeast.
To uncover novel virulence factors, in vivo transcriptional profiling using RNA sequencing has been performed on RNA isolated from Blastomyces yeast during murine pulmonary infection. Compared with controls (yeast and mycelia grown in medium alone or yeast cocultivated with macrophages in vitro), 72 genes were significantly upregulated more than twofold during pulmonary infection, independent of temperature, culture media, and interaction with macrophages. A subset of these 72 genes contributed metal uptake (zinc, nickel) and amino acid metabolism (tyrosine, tryptophan, cysteine). Exogenous uptake of zinc and nickel contributes to the virulence of Candida albicans and Cryptococcus neoformans, respectively. The catabolism of L-cysteine influenced virulence of C. albicans in a murine model of infection, and sulfite, a breakdown product of L-cysteine, promoted keratin breakdown by dermatophytes such as Arthroderma benhamiae . Ongoing research is deciphering the role of these processes in the pathogenesis of Blastomyces infection.
The application of molecular techniques to genetically manipulate fungi has led to the identification of genes that govern the temperature-dependent morphologic switch. The discovery of DRK1 (dimorphism regulating kinase-1), which encodes a group III hybrid histidine kinase, provided the first genetic proof that the temperature-dependent morphologic switch from hyphae or conidia to yeast is essential for virulence. Homologues of DRK1 participate in the high-osmolarity glycerol signaling pathway, which promotes adaptation to osmotic stress, oxidative stress, and temperature. Silencing or deleting DRK1 in Blastomyces or Histoplasma prevents the conversion of mycelia to yeast in response to an increase in temperature. Thus Blastomyces and Histoplasma cells remain locked in the mold form at 22°C and 37°C, respectively. Moreover, they are unable to upregulate yeast phase–specific virulence genes ( BAD1 in Blastomyces, CBP1 in Histoplasma ) and exhibit defects in the cell wall. DRK1 -silenced strains of Blastomyces and Histoplasma are avirulent in murine models of pulmonary infection. Subsequent work in T. marneffei highlighted the conserved nature of DRK1 on the phase transition. Deletion of the DRK1 homologue in T. marneffei hindered the ability of conidia to germinate to yeast in macrophages.
The phase transition in the other direction, from yeast to hyphae, following a drop in temperature is regulated in part by a GATA transcription factor encoded by SREB (siderophore biosynthesis repressor in Blastomyces ). Deletion of SREB results in (1) the failure of yeast to convert to hyphae at ambient temperature without affecting viability and (2) the inability to suppress the biosynthesis and uptake of iron-gathering molecules known as siderophores. The defect in the morphologic switch is associated with a quantitative reduction in lipid droplets and decreased neutral lipid biosynthesis (triacylglycerol, ergosterol). The addition of exogenous saturated fatty acids to SREB null mutants partially corrects the defect in lipid droplets and hyphal development. The function of SREB appears to be conserved in other endemic dimorphic fungi. Targeted gene knockdown of SRE1, an SREB homologue in H. capsulatum, impairs hyphal conversion and siderophore biosynthesis. In addition to SREB and SRE1, N -acetylglucosamine (GlcNAc) transporters NGT1 and NGT2 promote hyphal development in Blastomyces and Histoplasma spp.
Although Blastomyces uses multiple strategies to subvert and evade the immune system, the host can mount a successful defense with innate and adaptive immune cells. Macrophages and neutrophils are capable of killing a large percentage of conidia inhaled into the lungs. T lymphocytes coordinate the adaptive immune response to control infection by activating macrophages to become fungicidal against yeast. This requires Th1 cytokines such as TNF-α and interferon-γ as well as the production of IL-17 by Th17 T lymphocytes. Cell-mediated immunity following recovery from blastomycosis can last for at least 2 years.
Cell-mediated immune defenses have the potential to be exploited to prevent invasive fungal infections. When injected subcutaneously, BAD1 null ( BAD1 Δ) yeast induces sterilizing immunity in mice, which provides 100% protection against experimental pulmonary infection. Thus BAD1 Δ yeast can serve as a live, attenuated fungal vaccine. Following subcutaneous injection, BAD1 Δ yeast is transported intracellularly by inflammatory monocytes to draining lymph nodes for antigen presentation to lymph node resident dendritic cells. This results in priming of naïve CD4 + T lymphocytes to differentiate into Th17 cells via dectin-2/FcRγ/Syk/Card9, dectin-3 (also known as MCL), and mannan receptor signaling pathways. Once differentiated, Th17 cells migrate to the lungs in response to infection to mediate vaccine immunity by secreting IL-17 to recruit and activate phagocytes (neutrophils, macrophages). In the absence of CD4 + T cells, BAD1 Δ-vaccinated mice use IL-17 CD8 + T lymphocytes (Tc17 cells) to mediate protective vaccine immunity against lethal pulmonary infection. Proliferation and activation of IL-17 CD8 + T cells occurs through Myd88-Akt1-mTOR signaling. The antigenic component of the BAD1 Δ vaccine that induces T cell–based immunity is a 13–amino acid sequence in calnexin that is conserved among ascomycetes including H. capsulatum, Coccidioides posadasii, Aspergillus fumigatus, Fonsecaea pedrosoi (chromoblastomycosis), and Pseudogymnoascus destructans (white nose syndrome in bats). This has led to the development of a calnexin-based vaccine. The addition of endoglucanase II as an adjuvant enhances the immunogenicity of the calnexin vaccine and protects mice against pneumonia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here