Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Preterm infants are exceptionally unstable in terms of respiratory control due to both immaturity of the central nervous system and susceptibility to disease and infection. As a result, they exhibit a high incidence of apnea, bradycardia, and desaturation events during the first few months of life. Respiratory support including supplemental oxygen, continuous positive airway pressure (CPAP), and mechanical ventilation are ubiquitous in the neonatal intensive care unit (NICU) but with a goal of decreasing duration and level of support to minimize the incidence of chronic lung disease. These treatment decisions are most often based on nursing documentation of apnea, heart rate, and desaturation alarms making bedside cardiorespiratory monitoring an integral part of NICU clinical care.
Because of their small stature and fragile skin, extremely low birth weight (ELBW) infants continue to be a challenge in monitor design with a focus on continuous, accurate, artifact-free recordings obtained in a safe and noninvasive manner. More often than not, monitors are developed for adult patients or older children followed by application in newborns with minimal data demonstrating accuracy and safety. Nevertheless, even a well-designed monitor is only as good as the end user. Therefore, a thorough understanding of bedside monitor functionality is also needed to maintain optimal settings to reduce both nuisance alarms and signal distortion of real events. This chapter includes a description of the principles of operation of cardiorespiratory monitoring modalities, including current and future directions of hemodynamic, blood gas, and respiratory waveform acquisition in the NICU setting.
During each heartbeat, an electrical impulse originates in the sinoatrial node, and is propagated among the muscles of the atrium, through the atrioventricular node, followed by dispersion throughout the ventricles. In summary, the heart can be viewed as a dipole, as excited myocardium is negatively charged with respect to the myocardium at rest. The small alterations in voltage generated by the heart can be measured at the body surface with electrodes placed on the chest. The most common type of electrode used in the NICU is the silver-silver chloride, foil-based, recessed, or floating electrode. Each electrode contains a highly conductive electrolyte gel with a composition that varies by manufacturer. Electrode location is important in acquiring a signal of adequate resolution, especially in neonates with a limited surface area. Multiple probe applications should be avoided to optimize adhesiveness of the electrodes and ECG signal integrity and decrease the chance of skin damage, including high transepidermal water losses, in extremely preterm infants with fragile skin.
The ability of the electrodes to detect small electrical changes with each heartbeat requires the application of a current to the chest wall. The recommendations of acceptable current limits by the American Heart Association (AHA) cover two aspects of electrical safety. The first entails the amount of current allowable in a patient-connected lead that can flow through the myocardium without inducing ventricular fibrillation. The second aspect pertains to the allowable chassis leakage current that flows through the patient to ground. The AHA recommends that currents be limited to 10 µA through patient leads and less than 100 µA, with an optimal level of 10 µA for chassis leakage current.
The initial goal of simple heart rate measurements has expanded to include identification of specific arrhythmias and more complex algorithms of heart rate variability. For example, alterations in spectral indices and prolongation of QT interval have been shown to be associated with prematurity and sudden infant death. In addition, longitudinal regression-based models of abnormal heart rate characteristics associated with systemic infection and inflammation have been shown to reduce infant mortality in VLBW infants. Although sophisticated mathematical analyses have been predominantly limited to the research arena, development of the ability to detect alterations in heart rate variability in an automated fashion may play a role in future clinical care.
Application of the Fick principle is considered the gold standard of cardiac output monitoring in a research setting. This method states
where VO 2 = oxygen consumption, Ca = oxygen concentration of arterial blood, and Cv = oxygen concentration of mixed venous blood. Although the Fick principle may be accurate, especially in low flow states, precision may be limited by air leakage, cardiopulmonary disease, and enhanced pulmonary oxygen consumption.
Noninvasive methods that are validated in neonates include transcutaneous Doppler, transthoracic echocardiography (TTE), and thoracic electrical impedance. Doppler ultrasound uses an ultrasound beam to measure blood flow velocity. A velocity-time waveform is then produced from spectral analysis of Doppler shifts, caused by moving erythrocytes. The stroke distance can be calculated by the area under the velocity-time waveform. If the cross-sectional area of the vessel is known, cardiac output can be calculated by:
Doppler-based cardiac output measurements can vary widely and should be limited to trend monitoring.
During TTE, measures of left ventricular output, right ventricular output, or superior vena cava flow can be obtained. Validation data with TTE have been limited to transthoracic left ventricular output measures, which have been shown to be comparable with pulmonary artery thermodilution and O 2 -Fich methods. A variation of thoracic electrical impedance, electrical velocimetry, includes surface ECG electrodes placed on the forehead, left side of the neck, left mid-axillary line at the level of xiphoid process, and left thigh. During application of a small alternating electrical current through the thorax, changes in voltage are measured during periods of systole and diastole. Stroke volume (SV) is then determined using the following equation:
where V ept (mL) = volume of electrically participating tissue derived from body mass and height, ν LVET (s −1 ) I = ohmic equivalent of mean aortic blood velocity during left ventricular ejection, and LVET (s) = left ventricular ejection time. Data have shown electrical velocimetry as a comparable mode of measuring left ventricular output in neonates when compared with echocardiography, although variation among individuals was seen using both techniques.
Direct continuous readings from an indwelling arterial line are considered the gold standard for blood pressure monitoring in the neonate. Arterial and venous pressures are usually accessed by a catheter placed in the umbilical vessels. The catheter is then attached to a transducer. The transducer works on the principle that fluid exerts a force on the diaphragm. This force can be converted to a change in voltage and calibrated to a given pressure. The dome must be flushed, making sure there are no bubbles in the circuit; the transducer must be placed at the level of the distal tip of the catheter; and a zero pressure should be established before patient data are acquired.
Indirect blood pressure readings can be acquired through a cuff or inflatable bladder placed around the upper arm or calf. The cuff is inflated to a pressure adequate to cause occlusion of the arterial flow. During deflation of the cuff, measurements of diastolic and systolic blood pressure can be obtained. Mean blood pressure is then defined as the integrated area under the arterial pressure waveform.
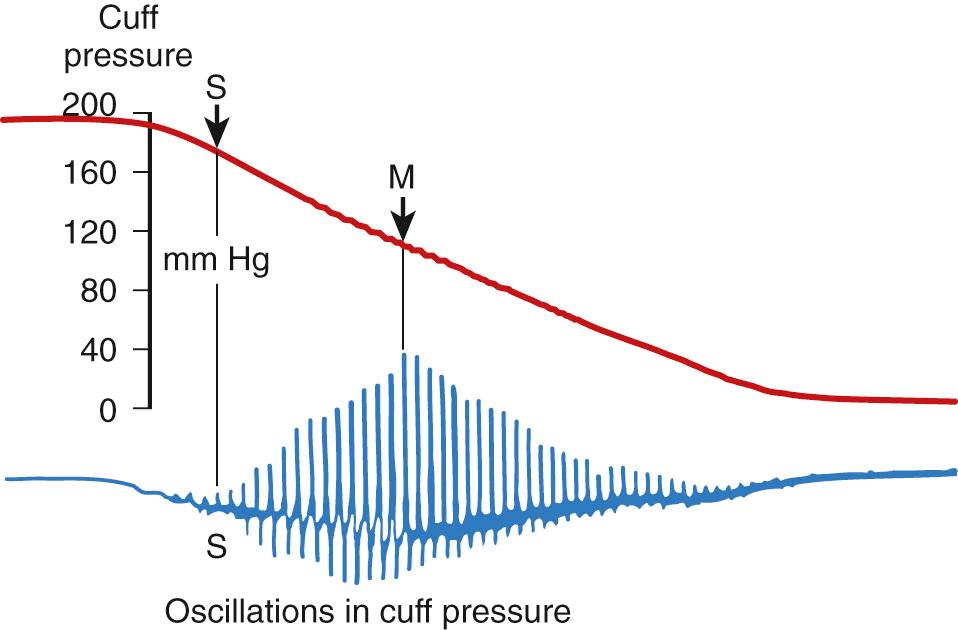
The size and placement of the cuff can affect accurate measurements of blood pressure. A cuff that is too narrow or applied loosely may result in falsely high readings. The American Heart Association recommends a cuff width of approximately 40% of the limb circumference. The two modes of indirect blood pressure monitoring include the auscultatory and oscillometry methods. The auscultatory method entails rapid inflation of the cuff, followed by slow deflation while listening for distal Korotkoff sounds with a stethoscope. This method, most commonly used in adults, is limited by the inaudible frequency range of arterial sounds in neonates, intra-observer variability, and disturbance to the patient. The oscillometry method is more often used in newborn intensive care units. In this method, cuff pressure is rapidly inflated to above systolic pressure. As the pressure is slowly released, small pulsations can be detected as the cuff approaches systolic pressure. When the cuff pressure decreases to below systolic pressure the oscillations increase in magnitude because of blood flowing into the artery. Ultimately a maximum oscillation point will be reached, corresponding to mean arterial pressure, followed by a decline as the cuff pressure decreases to baseline ( Fig. 37.1 ). In critically ill premature infants, oscillometric blood pressure measurements have been shown to have good agreement with arterial catheter values, although accuracy is greatly diminished in infants with a mean arterial pressure less than or equal to 30 mm Hg. Although the use of a cuff does not allow for continuous monitoring of blood pressure, many systems have the ability to provide automated transient readings.
Arterial blood gas sample measurements provide the most accurate estimate of arterial oxygen and carbon dioxide status. However, blood gases are limited by painful and time-consuming procedures, inherent risks including infection and vascular events, and intermittent short-term monitoring of PaO 2 and Pa co 2 . Continuous monitoring of oxygenation and carbon dioxide modalities offer an improved alternative by providing noninvasive, easy-to-use, portable, high-resolution, and fast-response options to alert the clinician to rapid decompensations that often occur in this high-risk infant cohort. Ideally, implementation of these devices in the NICU setting would lead to the ultimate goal of ventilation of the neonate—to stabilize respiration and minimize the use of supplemental oxygen and invasive respiratory support.
The multitude of diseases associated with prematurity frequently necessitates oxygen therapy as a component of clinical care. Even during periods of supplemental oxygen, severity of illness compounded with immature respiratory control quite often leads to respiratory instability presenting as rapid intermittent hypoxemia events. Therefore, blood gas measurements are useful for estimating baseline levels of oxygenation but cannot quantify short desaturation events that commonly occur in this patient population. As a result, intermittent blood gas sampling is often accompanied by noninvasive continuous pulse oximetry or transcutaneous monitoring.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here