Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Regulation of wound healing following GFS is a complex process and involves multiple growth factors, cytokines, and proteases, and the TGF-β and CTGF systems appear to play major roles in promoting scarring and contraction that result in bleb failure. An ideal outcome of GFS is a diffuse, but healthy, bleb that filters aqueous humor without leaking. Achieving this goal is challenging, as the currently available interventions utilize broadly acting anti-metabolites that can cause early complications, such as toxicity, and delayed complications, such as bleb leaks and scleral melts. The ideal modulator of GFS would, therefore, not only be cell-specific, but also, preferably, gene-specific to selectively alter the healing of the sub-Tenon's fibroblasts without off-target or delayed effects on the surrounding tissues and the fibroblasts themselves. Understanding the molecular regulation of healing following GFS in conjunctival/Tenon's capsule tissues facilitates the design of therapies that utilize drugs which selectively interfere with genes and proteins that promote scar formation and contraction without undesirable side effects.
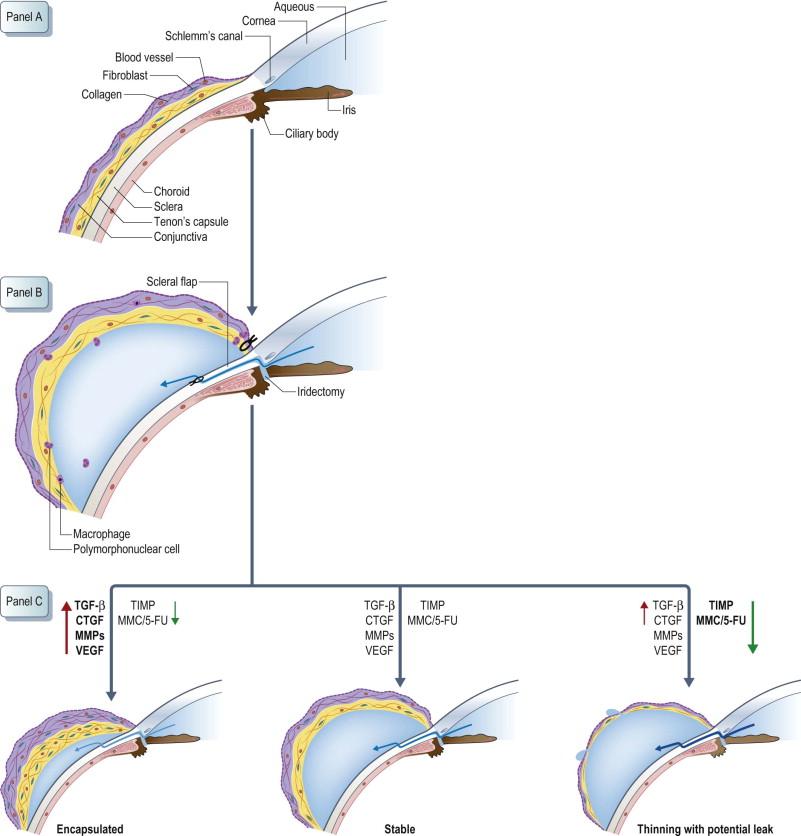
The ocular tissues relevant to glaucoma filtration surgery (GFS) consist of the ocular surface which includes the conjunctiva and the Tenon's capsule, as well as the anterior segment of the eye which includes the scleral spur, iris, and aqueous fluid ( Fig. 94-1A ). In the normal eye prior to any surgical procedures, the conjunctiva and Tenon's capsule contain quiescent fibroblasts and vascular tissue in a connective tissue matrix. GFS creates a conduit from the anterior chamber into the sub-Tenon's space resulting in aqueous fluid draining into this space for subsequent removal via the vascular tissue ( Fig. 94-1B ). Wound healing after GFS occurs in three phases – the inflammatory phase, the proliferative/repair phase, and the remodeling phase. The initial event is the extravasation of blood and formation of a fibrin clot, as well as recruitment of inflammatory cells into the area. These inflammatory cells are derived from the vessels in the ocular surface tissues as well as those leaking into the aqueous from iris trauma. These cells, as well as the ocular tissues, release growth factors and inflammatory cytokines. The next step is the migration and activation of fibroblasts that begin to lay down extracellular matrix and the formation of vascular tufts resulting in the formation of granulation tissue. The effect of these factors is counteracted by the anti-inflammatory cytokines that are also released by the ocular tissues as well as the extraneously administered anti-inflammatory and anti-metabolic drugs. The final step in the healing process is the remodeling of this tissue that occurs over months to years (for more information on the cellular basis of wound healing go to Chapter 78 ). The collagen contracts to form a dense scar. The final outcome is dependent on the relative activity of the various scar-modulating substances. If the pro-scarring cytokines and growth factors predominate, the result is a thick-walled, poorly functioning bleb ( Fig. 94-1c ). If, however, the anti-inflammatory and anti-metabolic factors predominate, the result is a thin-walled, over-filtering bleb ( Fig. 94-1C ). The holy grail of glaucoma surgery is the formation of a bleb with relatively normal-appearing conjunctiva and Tenon's capsule that allows the optimal amount of aqueous drainage from the anterior chamber to maintain ocular health ( Fig. 94-1c ).
During the last 15 years, significant progress has been made in understanding the processes of ocular wound healing at the molecular level. This has made it possible to selectively modify wound healing by designing therapeutic strategies that target only certain proteins or cells. This has provided the opportunity to reduce the off-target effects of the interventions and potentially decrease complications.
The initial step in understanding the molecular and cellular regulation of normal wound healing is to identify the changes that occur in gene expression during healing. Gene microarray technology has made it possible to broadly examine the patterns of changes in gene expression that occur during glaucoma wound healing. Microarrays can rapidly evaluate the changes in expression of thousands of genes simultaneously after GFS. This allows identifying genes that are substantially up- or down-regulated during wound healing after GFS. Examining the actions of the proteins translated from these mRNAs on the healing process allows us to identify potential targets for therapy. Finally, modulating targets located farther downstream in the healing process is more likely to favorably decrease scarring with minimal off-target effects.
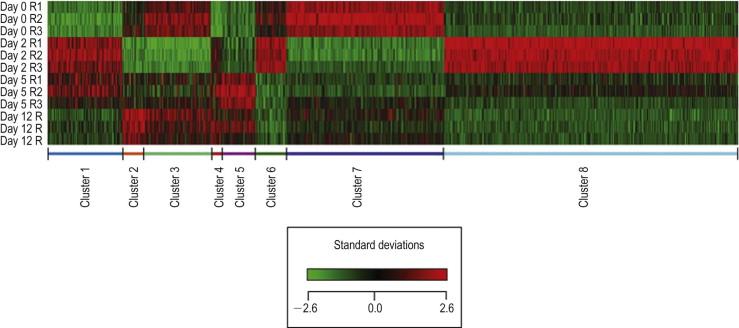
Esson et al. performed GFS on rats and harvested the bleb tissues (conjunctiva and Tenon's capsule) at 2, 5 and 12 days after surgery, extracted RNA and hybridized it to microarray chips ( Fig. 94-2 ). They found that the levels of expression of surgery number of genes significantly changed after GFS. The greatest changes in gene expression of the conjunctiva/Tenon's capsule tissues occurred between day 0 (normal non-wounded) tissue samples and days 2 and 5 after surgery. There was a five-fold or greater change in the expression levels of genes including growth factors (TGF-β, CTGF), extracellular matrix protein genes (types I, II III, V, and XVIII collagens, fibronectin, vitronectin and proteoglycans), proteases involved in cell migration and ECM remodeling (MMPs 2, 9, 11), and the tissue inhibitors of metalloproteinase inhibitors (TIMPs 1, 2, 3). The expression of these genes decreased by post-operative day 12, when the scarring in the bleb tissue was nearly complete.
Three growth factors that are thought to play key roles in regulating scarring in multiple tissues are transforming growth factor-β (TGF-β); connective tissue growth factor (CTGF) ( Table 94-1 ); and the vascular endothelial growth factor (VEGF).
| MMP Common Name | Designation | Substrates and Actions |
|---|---|---|
| Fibroblast collagenase | (MMP-1) | Cleaves single bond in native types I, II and III collagens |
| 72-kDa gelatinase | (MMP-2) | Degrades types IV, V and VII collagens, gelatin, fibronectin; synthesized by fibroblasts and macrophages |
| 92-kDa gelatinase | (MMP-9) | Degrades types IV and V collagen, gelatin; synthesized by epithelial cells, macrophages, PMNs |
| Stromelysin | (MMP-3) | Degrades proteoglycans, fibronectin, laminin, gelatin and types III, IV and V collagen |
| Neutrophil collagenase | (MMP-8) | Similar to MMP-1, degrades type I, II and III collagens |
The TGF-β system has become well-established as playing a pivotal role in promoting scar formation in multiple tissues. The three TGF-β isoforms that are found in mammals (TGF-β1, TGF-β2, and TGF-β3) produce similar actions on cultured fibroblasts, including stimulating synthesis of ECM proteins, such as collagen I, collagen III, proteoglycans, elastin, the collagen cross-linking enzyme, lysyl oxidase, and CTGF. In addition, TGF-β decreases synthesis of MMPs and increases synthesis of tissue inhibitors of metalloproteinases (TIMPs) by fibroblasts ( Fig. 94-3 ). Results of animal experiments support the fibrotic actions of TGF-β observed with cultured cells. For example, treatment of skin incisions in normal rats or impaired healing rats with TGF-β increased scar formation and improved tensile strength. In addition, elevated levels of TGF-β were reported in many human pathological fibrotic diseases, including kidney glomerulonephritis, liver cirrhosis, lung fibrosis, skin sclerosis, vascular restenosis, and hypertrophic burn scar. The TGF-β system also appears to regulate key aspects of corneal wound healing. TGF-β protein was immunolocalized in corneal cells and lacrimal gland cells, and TGF-β levels in tears increased after PRK in patients. Levels of mRNAs for all three isoforms of TGF-β, as well as its receptors, increased and remained elevated for 90 days in rat corneas during scarring following PRK ablation. As described above, the levels of TGF-β and CTGF also increased in bleb tissue during healing of GFS wounds in rats. Thus, substantial data from multiple systems demonstrate that the combined effects of the TGF-β system are to promote scar deposition.
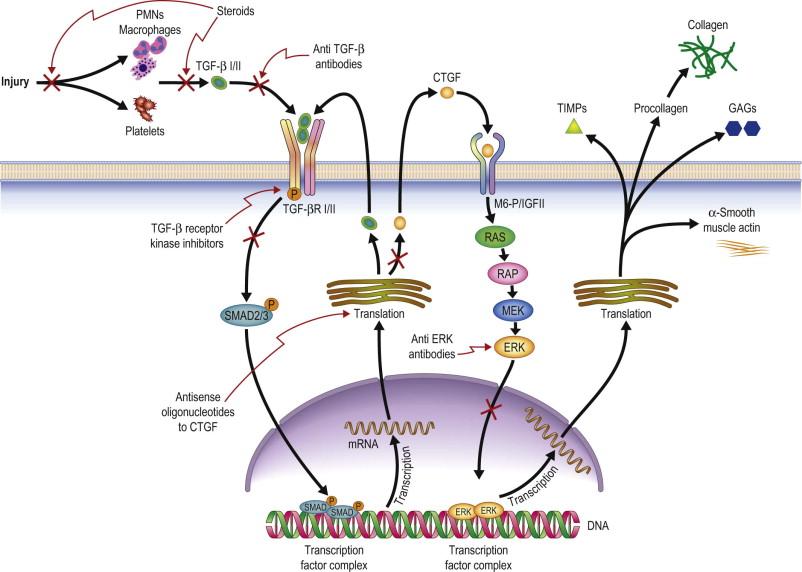
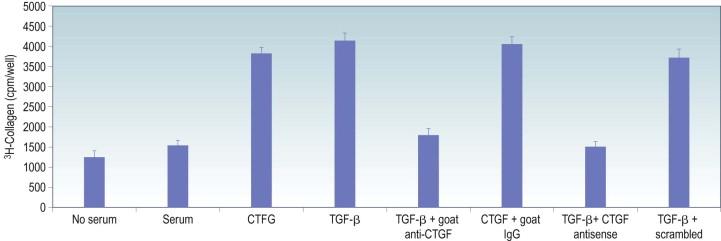
Connective tissue growth factor (CTGF), has emerged as another key regulator of scar formation. CTGF was first isolated from conditioned medium of human vascular endothelial cells, and CTGF has been found to be both a mitogenic and chemotactic for fibroblasts, and to stimulate the synthesis of ECM proteins by cultured fibroblasts, including type I collagen, fibronectin, and elastin. Levels of CTGF protein are elevated in many fibrotic diseases, but more importantly, there appears to be a key link between the TGF-β and CTGF systems. Specifically, TGF-β induces synthesis of CTGF, and antibodies or antisense oligonucleotides to CTGF block TGF-β-induced proliferation of fibroblasts and increase of ECM synthesis ( Fig. 94-4 ). Thus, CTGF appears to be a downstream mediator for TGF-β-induced fibroblast proliferation and ECM synthesis. The role of CTGF in ocular wound healing has not been fully established, but CTGF protein was immunolocalized in corneal, conjunctival, scleral and uveal tissues following GFS, and CTGF mRNA increased in rat bleb tissue following GFS. These findings suggest that excess deposition of ECM in wound healing may be mediated more directly by CTGF rather than TGF-β. Therefore, targeted inhibition of CTGF may be a key to reducing scarring after GFS.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here