Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Clinical ultrasound has been found to be an effective imaging modality with an excellent safety profile when used appropriately.
Ultrasound can produce physical effects, which should be understood as part of the benefit-versus-risk assessment as with any medical procedure.
The thermal index (TI) and mechanical index (MI) provide feedback to the user and should be minimized while obtaining the requisite medical benefit from the ultrasound examination.
Ultrasound exposures during clinical, research, and educational examinations should be as low as reasonably achievable (ALARA).
Users of ultrasound should be appropriately trained and familiar with the equipment operation and controls that affect ultrasound exposure.
Ultrasound has provided a wealth of knowledge in diagnostic medicine and has greatly affected medical practice, particularly in obstetrics. Millions of sonographic examinations are performed each year, and ultrasound remains one of the fastest growing imaging modalities because of its low cost, real-time interactions, portability, and apparent lack of biologic effects (bioeffects). No casual relationship has been established between clinical applications of diagnostic ultrasound and bioeffects on the patient or operator.
The U.S. Food and Drug Administration (FDA) regulates the maximum output of ultrasound devices to an established level. The marketing approval process requires devices to be equivalent in efficacy and output to those produced before 1976. This historic regulation of sonography has provided a safety margin for ultrasound while allowing clinically useful performance. The mechanism has restricted ultrasound exposure to levels that apparently produce few, if any, obvious bioeffects based on the epidemiologic evidence, although animal studies have shown some evidence for biologic effects.
In an effort to increase the efficacy of diagnostic ultrasound, the maximum acoustic output for some applications has increased through an additional FDA market approval process termed “510K Track 3.” The vast majority of ultrasound systems currently in use were approved through this process. The Track 3 process provides the potential for better imaging performance and, as discussed later, requires that additional information be reported to the operator regarding the relative potential for bioeffects. Therefore informed decision making is important concerning the possible adverse effects of ultrasound in relation to the desired diagnostic information. Current FDA regulations that limit the maximum output are still in place, but in the future, systems might allow sonographers and physicians the discretion to increase acoustic output beyond a level that might induce a biologic response.
Although the choices made during sonographic examinations may not be equivalent to the risk-versus-benefit decisions associated with imaging modalities using ionizing radiation, the operator will be increasingly responsible for determining the diagnostically required amount of ultrasound exposure. Thus the operator should know the potential bioeffects associated with ultrasound exposure. Patients also need to be reassured about the safety of a diagnostic ultrasound scan. The scientific community has identified some potential bioeffects from sonography, and although no causal relation has been established, it does not mean that no effects exist. Therefore it is important to understand the interaction of ultrasound with biologic systems.
The physical effects of sound can be divided into two principal groups: thermal and nonthermal. Most medical professionals recognize the thermal effects of elevated temperature on tissue, and the effects caused by ultrasound are similar to those of any localized heat source. With ultrasound, the heating mainly results from the absorption of the sound field as it propagates through tissue. However, “nonthermal” sources can generate heat as well.
Many nonthermal mechanisms for bioeffects exist. Acoustic fields can apply radiation forces (not ionizing radiation) on the structures within the body at both the macroscopic and the microscopic levels, resulting in exerted pressure and torque. The temporal average pressure in an acoustic field is different from the hydrostatic pressure of the fluid, and any object in the field is subject to this change in pressure. The effect is typically considered smaller than other effects because it relies on less significant factors in the formulation of the acoustic field. Acoustic fields can also cause motion of fluids. Such acoustically induced flow is called streaming.
Acoustic cavitation is the action of acoustic fields within a fluid to generate bubbles and cause volume pulsation or even collapse in response to the acoustic field. The result can be heat generation and associated free radical formation, microstreaming of fluid around the bubble, radiation forces generated by the scattered acoustic field from the bubble, and mechanical actions from bubble collapse. The interaction of acoustic fields with bubbles or “gas bodies” (as they are generally called) has been a significant area of bioeffects research for many years.
As ultrasound propagates through the body, energy is lost through attenuation. Attenuation causes loss of penetration and the inability to image deeper tissues. Attenuation is the result of two processes, scattering and absorption. Scattering of the ultrasound results from the redirection of the acoustic energy by tissue encountered during propagation. With diagnostic ultrasound, some of the acoustic energy transmitted into the tissue is scattered back in the direction of the transducer, termed backscatter, which allows a signal to be detected and images to be made. Energy also is lost along the propagation path of the ultrasound by absorption. Absorption loss occurs substantially through the conversion of the ultrasound energy into heat. This heating provides a mechanism for ultrasound-induced bioeffects.
The rate of temperature increase in tissues exposed to ultrasound depends on several factors, including spatial focusing, ultrasound frequency, exposure duration, and tissue type.
Ultrasound systems use multiple techniques to concentrate or focus ultrasound energy and improve the quality of measured signals. The analogy for light is that of a magnifying glass. The glass collects all the light striking its surface and concentrates it into a small region. In sonography and acoustics in general, the term intensity is used to describe the spatial distribution of ultrasonic power (energy per unit time), where intensity = power/area and the area refers to the cross-sectional area of the ultrasound beam. Another common beam dimension is the beam width at a specified location of the field. If the same ultrasonic power is concentrated into a smaller area, the intensity will increase. Focusing occurs on both transmission of the ultrasound and when receiving the backscattered signals used to form the image. The transmit focusing is of importance in terms of potential biologic effects because this phenomenon controls the applied energy to the tissue. There are ultrasound imaging systems that use plane wave transmission or limited transmit focusing, which may reduce the local intensity, but all ultrasound systems must still operate under the FDA limits.
Focusing in an ultrasound system can be used to improve the spatial resolution of the images. The side effect is an increased potential for bioeffects caused by heating and cavitation. In general, the greatest heating potential is between the scanhead and the focus, but the exact position depends on the focal distance, tissue properties, and heat generated within the scanhead itself.
Returning to the magnifying glass analogy, most children learn that the secret to incineration is a steady hand. Movement distributes the power of the light beam over a larger area, thereby reducing its intensity. The same is true in ultrasound imaging. Thus imaging systems that scan a beam through tissue reduce the spatial average intensity. Spectral Doppler and M-mode ultrasound imaging maintain the ultrasound beam in a stationary position (both considered unscanned modes ) and therefore provide no opportunity to distribute the ultrasonic power spatially, whereas color flow Doppler, power mode Doppler, and B-mode (also called gray-scale ) ultrasound imaging require that the beam be moved to new locations (scanned modes) at a rate sufficient to produce the real-time nature of these imaging modes.
The ultrasound power is the temporal rate at which ultrasound energy is produced. Therefore controlling how ultrasound is produced in time seems a reasonable method for limiting its effects.
Ultrasound can be produced in bursts rather than continuously. Ultrasound imaging systems operate on the principle of pulse-echo, in which a burst of ultrasound is emitted, followed by a quiescent period listening for echoes to return. This pulsed wave ultrasound may be swept through the image plane numerous times during an imaging sequence. On the other hand, ultrasound may be transmitted in a continuous wave (CW) mode, in which the ultrasound transmission is not interrupted. The temporal peak intensity refers to the largest intensity at any time during ultrasound exposure ( Fig. 2.1 ). The pulse average intensity is the average value over the duration of the ultrasound pulse. The temporal average intensity is the average over the entire pulse repetition period (elapsed time between onset of ultrasound bursts). The duty factor is defined as the fraction of time the ultrasound field is “on.” With significant time “off” between pulses (small duty factor), the temporal average value will be significantly smaller. For example, a duty factor of 10% will reduce the temporal average intensity by a factor of 10 compared with the pulse average. The time-averaged quantities are the variables most related to the potential for thermal bioeffects. Combining temporal and spatial information results in common terms such as the spatial peak, temporal average intensity (I SPTA ) and spatial average, temporal average intensity (I SATA ).
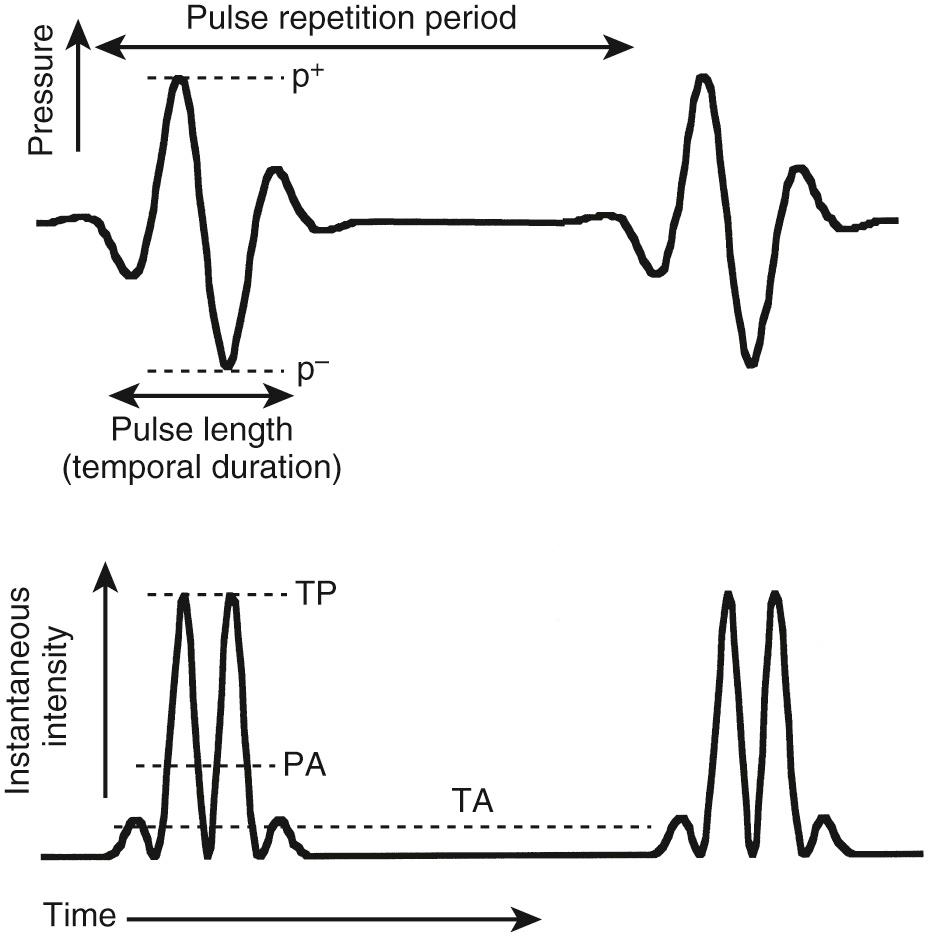
The overall duration, or dwell time, of the ultrasound exposure to a particular tissue is important because longer exposure of the tissue may increase the risk of bioeffects. The motion of the scanhead during an examination reduces the dwell time within a particular region of the body and can minimize the potential for bioeffects of ultrasound. Therefore performing an efficient scan, spending only the time required for diagnosis, is a simple way to reduce exposure.
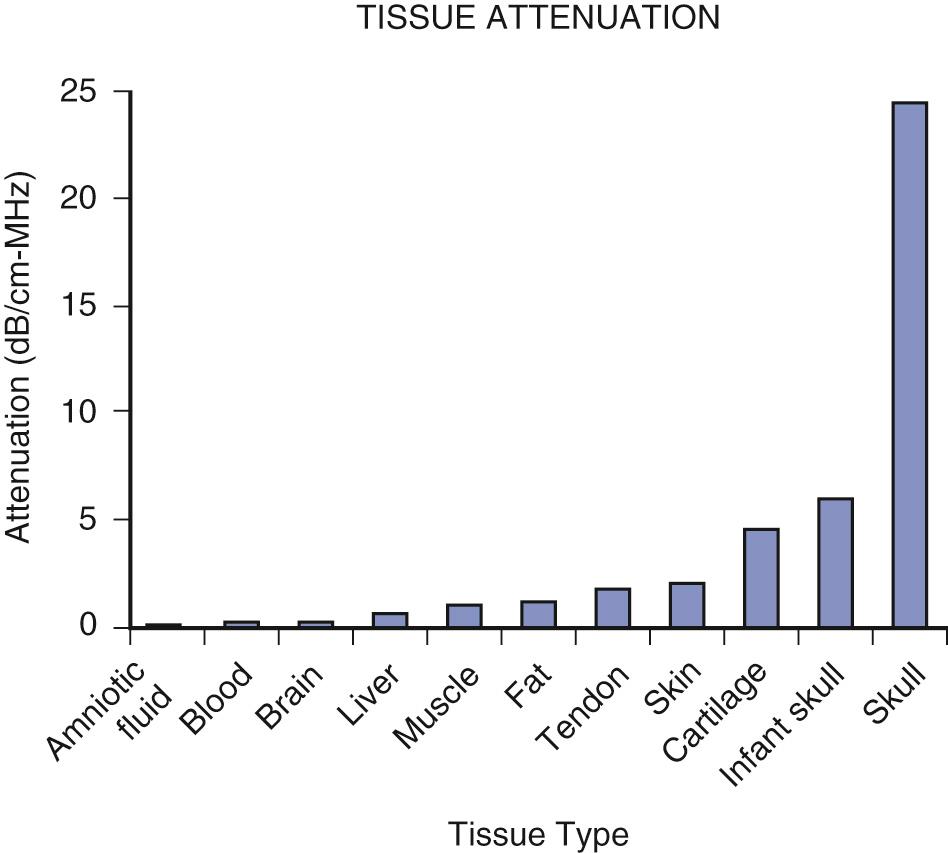
Numerous physical and biologic parameters control heating of tissues. Absorption is normally the dominant contributor to attenuation in soft tissue. The attenuation coefficient is the attenuation per unit length of sound travel and is usually given in decibels per centimeters-megahertz (dB/cm-MHz). The attenuation typically increases with increasing ultrasound frequency. The attenuation ranges from a negligible amount for fluids (e.g., amniotic fluid, blood, urine) to the highest value for bone, with some variation among different soft tissue types ( Fig. 2.2 ).
Another important factor is the body's ability to cool tissue through blood perfusion. Well-perfused tissue will more effectively regulate its temperature by carrying away the excess heat produced by ultrasound. The exception is when heat is deposited too rapidly, as in therapeutic thermal ablation.
Bone and soft tissue are two specific areas of interest based on the differences in heating phenomena. Bone has high attenuation of incident acoustic energy. In examinations during pregnancy, calcified bone is typically subjected to ultrasound, as in measurement of the biparietal diameter (BPD) of the skull. Fetal bone contains increasing degrees of mineralization as gestation progresses, thereby increasing risk of localized heating. Special heating situations relevant to obstetric ultrasound examinations may also occur in soft tissue, where overlying structures provide little attenuation of the field, such as the fluid-filled amniotic sac.
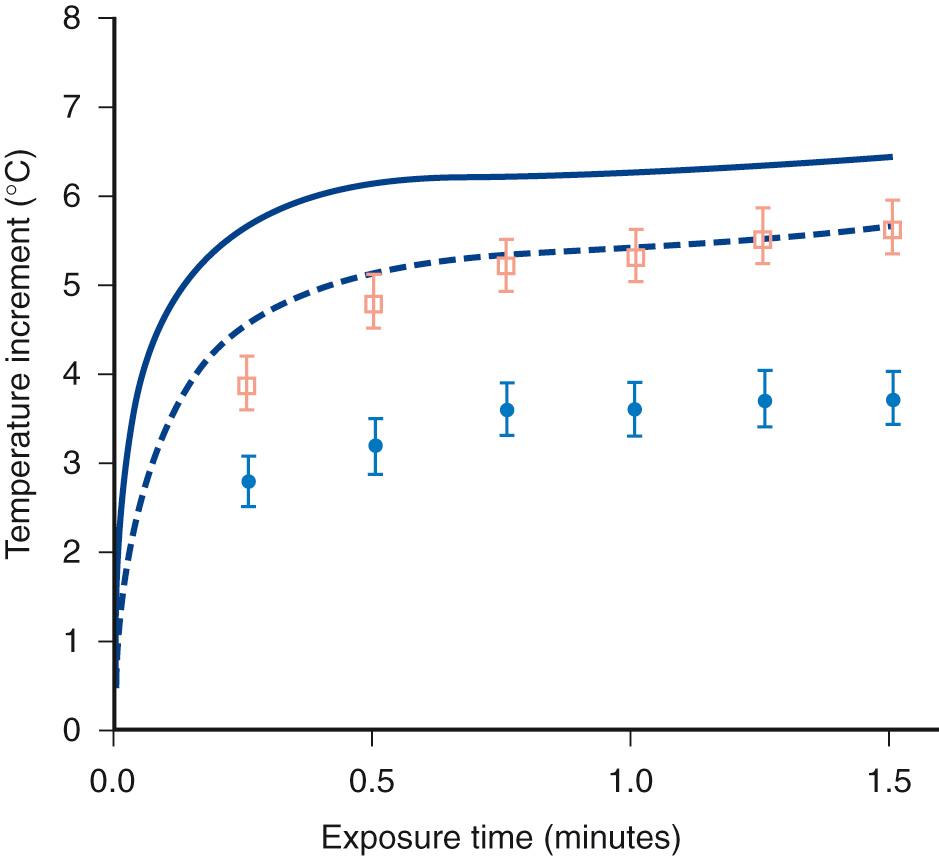
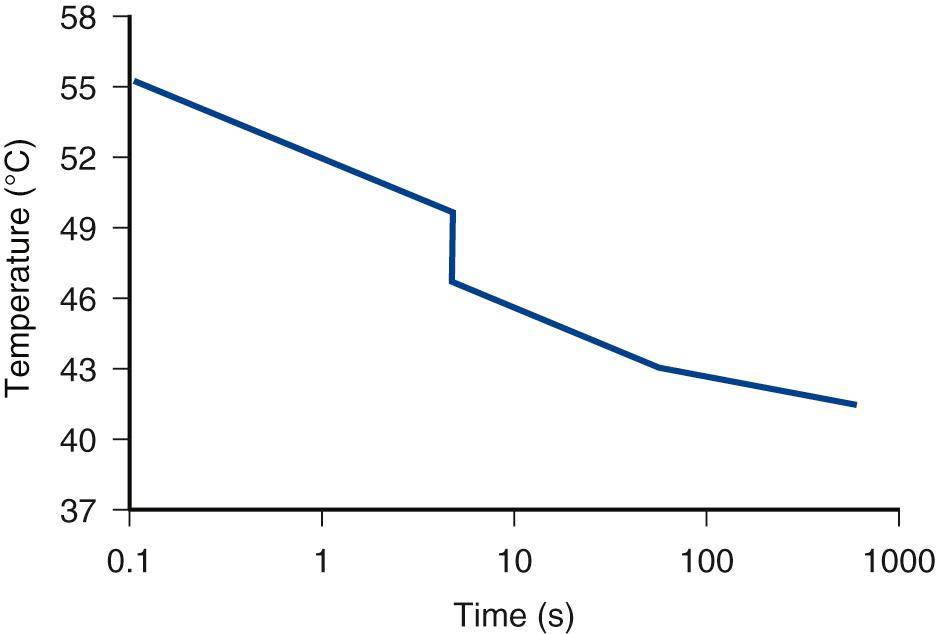
The absorption of ultrasound at the bone surface allows for rapid deposition of energy from the field into a limited volume of tissue. The result can be a significant temperature rise. For example, Carstensen and colleagues combined an analytic approach and experimental measurements of the temperature rise in mouse skull exposed to CW ultrasound to estimate the temperature increments in bone exposures. Because bone has a large absorption coefficient, the incident ultrasonic energy is assumed to be absorbed in a thin planar sheet at the bone surface. The temperature rise of mouse skull has been studied in a 3.6-MHz focused beam with a beam width of 2.75 mm ( Fig. 2.3 ). The temporal average intensity in the focal region was 1.5 W/cm. One of two models (upper curve in Fig. 2.3 ) in common use predicts values for the temperature rise about 20% greater than that actually measured in this experiment. Thus the theoretical model is conservative in nature.
Similarly for the fetal femur, Drewniak and colleagues indicated that the size and calcification state of the bone contributed to the ex vivo heating of bone ( Table 2.1 ). To put this in perspective and to illustrate the operator's role in controlling potential heating, consider the following scenario. By reducing the output power of an ultrasound scanner by 10 dB, the predicted temperature rise would be reduced by a factor of 10, making the increase of 3°C seen by these researchers (see Table 2.1 ) virtually nonexistent. This strongly suggests the use of maximum receive gain and reduction in output power during ultrasound examinations (see section on considerations for controlling ultrasound output ). In fetal examinations an attempt should be made to maximize receive gain because this comes at no cost to the patient in terms of exposure. Distinctions are often made between bone positioned deep to the skin at the focal plane of the transducer and bone near the skin surface, as when considering transcranial applications. This distinction is discussed later with regard to the thermal index (TI).
| Gestational Age (days) | Diameter (mm) | Temperature Increments (°C) |
|---|---|---|
| 59 | 0.5 | 0.10 |
| 78 | 1.2 | 0.69 |
| 108 | 3.3 | 2.92 |
a Temperature increments in human fetal femur exposed for 20 seconds were found to be approximately proportional to incident intensity.
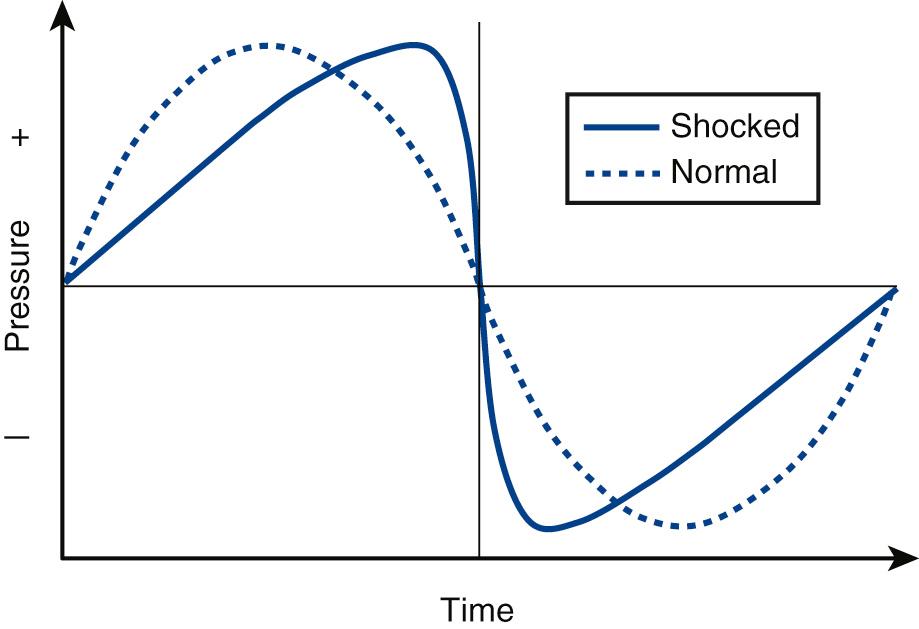
Two clinical situations for ultrasound exposure in soft tissue are particularly relevant to obstetric and gynecologic applications. First, a common scenario involves scanning through a full bladder. The urine is a fluid with a relatively low ultrasound attenuation coefficient. The reduced attenuation allows larger acoustic amplitudes to be applied deeper within the body. Second, the propagating wave may experience finite amplitude distortion, resulting in energy being shifted by a nonlinear process from lower to higher frequencies. The result is a shockwave —a gradual wave steepening results in a waveform composed of higher frequency components ( Fig. 2.4 ). Attenuation increases with increasing frequency; therefore the absorption of a large portion of the energy in such a wave occurs over a much shorter distance, concentrating the energy deposition in the first tissue encountered, which may include the fetus.
Ultrasound imaging systems include specific modalities that rely on nonlinear effects. In tissue harmonic imaging, or native harmonic imaging, the image is created using the backscatter of harmonic components induced by nonlinear propagation of the ultrasound field. This has distinct advantages in terms of reducing image artifacts and improving lateral resolution in particular. In these nonlinear imaging modes the acoustic output must be sufficiently high to produce the effect. The acoustic power currently used is still within the FDA limits, but improvements in image quality using such modes may create the need to modify or relax the regulatory restrictions. Similarly, in elasticity or shear wave imaging, the acoustic pressure may need to be increased to provide sufficient acoustic radiation force for imaging tissue stiffness. This detail will be discussed in the section on considerations for increasing acoustic output .
Transvaginal ultrasound is important to note because of the proximity of the transducer to sensitive tissues such as the ovaries. As discussed later, temperature increases near the transducer may provide a heat source at sites other than the focus of the transducer. In addition, the transducer face itself may be a significant heat source because of inefficiencies in its conversion of electric to acoustic energy. Such factors must be considered in the estimation of potential thermal effects in transvaginal ultrasound and other endocavitary applications.
Knowledge of the bioeffects of ultrasound heating is in part based on the experience available from other, more common forms of hyperthermia, which serve as a basis for safety criteria. Extensive data exist on the effects of short-term and extended temperature increases, or hyperthermia. Teratogenic effects from hyperthermia have been demonstrated in birds, all the common laboratory animals, farm animals, and nonhuman primates. The wide range of observed bioeffects, from subcellular chemical alterations to gross congenital abnormalities and fetal death, is an indication of the effectiveness or universality of hyperthermic conditions for perturbing living systems.
The National Council on Radiation Protection and Measurements (NCRP) Scientific Committee on Biological Effects of Ultrasound compiled a comprehensive list of the lowest reported thermal exposures producing teratogenic effects. Examination of these data indicated a lower boundary for observed thermally induced bioeffects. Questions remain, however, about the relevance of this analysis of hyperthermia to the application of diagnostic ultrasound. After a careful literature review, O’Brien and colleagues suggested a more detailed consideration of thermal effects with regard to short-duration exposures. Fig. 2.5 shows the recommended approach to addressing the combination of temperature and duration of exposure. Note that the tolerance of shorter durations and higher temperatures suggests a substantial safety margin for diagnostic ultrasound. Regardless, it is beneficial to provide feedback to the ultrasound operator as to the relative potential for a temperature rise in a given acoustic field under conditions associated with a particular examination. This will allow an informed decision as to the exposure needed to obtain diagnostically relevant information.
Based on analysis of hyperthermia data, the NCRP proposed a general statement concerning the safety of ultrasound examinations in which no temperature rise greater than 1°C is expected. In an afebrile patient within this limit, the NCRP concluded that there was no basis for expecting an adverse effect. In cases where the temperature rise might be greater, the operator should weigh the benefit against the potential risk. To assist in this decision, given the range of different imaging conditions seen in practice, a thermal index (TI) was approved as part of the Standard for Real-Time Display of Thermal and Mechanical Acoustical Output Indices on Diagnostic Ultrasound Equipment of the American Institute of Ultrasound in Medicine (AIUM). This standard provides the operator with an indication of the relative potential risk of heating tissue, with calculations based on the imaging conditions and an on-screen display showing the TI. This standard was subsequently adopted as an international standard through the International Electrotechnical Commission (IEC).
To more easily inform the physician of the operating conditions that could, in some cases, lead to a temperature elevation of 1°C, a thermal index is defined as
where W deg is the ultrasonic source power (in watts) calculated as capable of producing a 1°C temperature elevation under specific conditions. W 0 is the ultrasonic source power (in watts) being used during the current exam.
The NCRP ultrasound committee introduced the TI concept. The purpose of the TI is to provide an indication of the relative potential for increasing tissue temperature, but it is not meant to provide the actual temperature rise. The NCRP recommended two tissue models to aid in the calculation of the ultrasound power that could raise the temperature in tissue by 1°C: (1) a homogeneous model in which the attenuation coefficient is uniform throughout the region of interest, and (2) a fixed-attenuation model in which the minimum attenuation along the path from transducer to a distant anatomic structure is independent of the distance because of a low-attenuation fluid path (e.g., amniotic fluid). Because of concern for the patient, it was recommended that “reasonable worst case” assumptions be made with respect to estimation of temperature elevations in vivo . The FDA, AIUM, and National Electrical Manufacturers Association (NEMA) adopted the TI as part of the output display standard. They advocate estimating the effect of attenuation in the body by reducing the acoustic power/output of the scanner (W 0 ) by a derating factor equal to 0.3 dB/cm-MHz for the soft tissue model.
The AIUM Thermal Index Working Group considered three tissue models: (1) the homogeneous tissue or soft tissue model, (2) a tissue model with bone at the focus, and (3) a tissue model with bone at the surface, or transcranial model. The TI takes on three different forms for these tissue models.
The assumption of homogeneity helps simplify the determination of the effects of acoustic propagation and attenuation, as well as the heat transfer characteristics of the tissue. Providing one of the most common applications for ultrasound imaging, this model applies to situations where bone is not present and can generally be used for fetal examinations during the first trimester (low calcification in bone). In the estimation of potential heating, many assumptions and compromises had to be made to calculate a single quantity that would guide the operator. Calculations of the temperature rise along the axis of a focused beam for a simple, spherically curved, single-element transducer result in two thermal peaks. The first is in the near field (between the transducer and the focus), and the second appears close to the focal region. The first thermal peak occurs in a region with low ultrasound intensity and wide beam width. When the beam width is large, cooling will occur mainly because of perfusion. In the near field the magnitude of the local intensity is the chief determinant of the degree of heating. The second thermal peak occurs at the location of high intensity and narrow beam width at or near the focal plane. Here the cooling is dominated by conduction, and the total acoustic power is the chief determinant of the degree of heating.
Given the thermal “twin peaks” dilemma, the AIUM Thermal Index Working Group compromised in creating a TI that included contributions from both heating domains. Their rationale was based on the need to minimize the acoustic measurement load for manufacturers of ultrasound systems. In addition, adjustments had to be made to compensate for effects of the large range of potential apertures. The result is a complicated series of calculations and measurements that must be performed, and to the credit of the many manufacturers, there has been considerable effort in implementing a display standard to provide user feedback. Different approaches to these calculations were considered, but changes will require that the currently accepted implementation be reexamined and approved for use by the FDA and considered by the IEC.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here