Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
ATP binding cassette A1;
ATP binding cassette subfamily G member 1
apical sodium-bile acid transporter
CD36 molecule
NPC1 like intracellular cholesterol transporter 1
scavenger receptor class B-type I
sterol regulatory element binding protein 2
vitamin E
The authors have declared that no conflict of interest exists.
The term vitamin E (VE) is the generic descriptor for all tocol and tocotrienol derivatives exhibiting qualitatively the biological activity of α-tocopherol. It refers to a group of eight naturally occurring fat-soluble micronutrients: four tocopherols (α, β, γ, and δ) and four tocotrienols (α, β, γ, and δ) ( Fig. 52.1 ). VE is composed of a substituted chromanol ring linked to a C16 isoprenoid side chain. Tocotrienols differ from tocopherols by the presence of three double bonds in their side chain. Tocopherol and tocotrienol isomers differ in the location of the methyl groups on the chromanol ring. Due to the presence of the asymmetric carbons in their side chains, tocopherols can form eight stereoisomers ( RRR , RRS , RSR , RSS , SRR , SRS , SSR , SSS ). Naturally occurring tocopherols exist as RRR stereoisomers (formerly known as d-tocopherol) and are not esterified. However, when supplemented to the diet, tocopherol is usually a racemic mixture of the eight possible stereoisomers (all- rac -tocopherol, formerly known as dl-tocopherol) and is esterified to protect the phenol group against oxidation (e.g., α-tocopheryl acetate) ( Fig. 52.1 ).
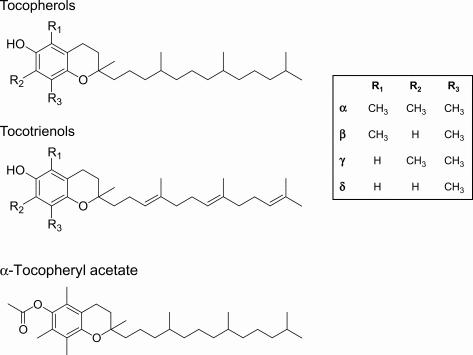
α- and γ-Tocopherol are the most abundant VE forms in Western diets and are the forms found at the highest concentrations in human blood and tissues. VE is found at relatively high concentrations in vegetable oils and nuts but it is also present in other food matrices such as wheat germs and salad. In the United States, γ-tocopherol represents ≈ 70% of VE intake, due to the high consumption of food sources rich in γ-tocopherol in the typical diet (e.g., soybean oil, corn oil). The respective contribution of α- and γ-tocopherol to VE intake in Europe is not precisely known. The current US recommended daily allowance (RDA) for healthy adults is 15 mg/day but it is estimated that > 90% of men and > 96% women in the United States do not consume the estimated average requirements (EARs). Recent data point at similar inadequacies in several European countries. A recent systematic review of global α-tocopherol status has pointed at a relatively high prevalence of VE deficiency with 13% of the subjects exhibiting serum α-tocopherol concentration < 12 μmol/L, which has been proposed as a criterion for VE deficiency, and only 21% of the subjects reaching serum α-tocopherol concentrations > 30 μmol/L, which has been proposed as a criterion for VE adequacy.
VE is quantitatively the main lipid-soluble antioxidant in mammalian tissues and blood. It acts as a chain-breaking antioxidant, especially against peroxyl radicals, and is thus essential in maintaining the integrity of long-chain polyunsaturated fatty acids found in cell membranes. Recently, it has been shown to also exert nonantioxidant activities : modulation of gene expression, inhibition of cell proliferation and regulation of bone mass. Since oxidative stress has been implicated in the etiology of several diseases, for example, cardiovascular diseases and cancers, numerous epidemiological studies have investigated the association between VE dietary intake or status (usually evaluated as the fasting blood α-tocopherol concentration) and the incidence of these diseases and reported negative associations. However, most randomized controlled trials have failed to show a benefit of VE supplementation on the incidence of these diseases. Several explanations have been put forward, such as the absence of effect of VE supplementation on these diseases, a negative effect of α-tocopherol supplementation on the bioavailability of other VE isomers and the absence of population stratification by VE status or oxidative stress in these negative studies. Recently, it has also been suggested that the high interindividual variability of α-tocopherol bioavailability may have interfered with the effects of VE supplementation and that benefit from VE supplementation depends on a subjecťs genotype.
VE is a lipid and it shares common transport mechanisms with other lipids: in the lumen of the human upper gastrointestinal tract, it is found in structures allowing the solubilization of lipids, that is, micelles and possibly vesicles. It is transported in the blood via lipoproteins and it is found in membranes and lipid droplets in cells. Its bioavailability, that is, the proportion of VE that is available for use or storage by the organism, has been reported to range between 10% and 81%. Numerous factors affect its bioavailability: dietary factors, such as composition of the food matrix or the presence of fat, and also host-related factors, such as pathologies or genetic factors (see Section 52.4 for an overview thereof). Its fate in the gastrointestinal tract includes emulsification, incorporation into mixed micelles, transport through the unstirred water layer, uptake by the enterocyte, incorporation into intestinal lipoproteins, and secretion from the intestinal cell into the lymph or into the portal vein. This chapter will present an overview of what is known/unknown about the fate of VE in the human upper gastrointestinal tract and then list some of the factors hypothesized to affect VE bioavailability.
Digestion of foods, or supplements, that contain VE, starts in the mouth where the diet is subjected to the action of saliva coupled with mechanical processing, leading to a partial disruption of the food matrix and an increase in the surface area. In the stomach, the food matrix is submitted to an acidic pH (between 2 and 6 during digestion) and to the action of gastric secretions, which contain several enzymes (pepsin, amylase, gastric lipase, etc.) able to further degrade the food matrix. VE, if not naturally incorporated in vegetable oils, is assumed to transfer, at least partly, from its food matrix to the oil phase of the meal. The extent of this phenomenon depends most likely on the food matrix in which VE is incorporated and on the quantity and the nature of lipids present at the same time as VE in its food matrix. It has been shown that there is no significant degradation or metabolism of α-tocopherol in the stomach during digestion. There is a lack of data on the role the gastric lipase could play on the hydrolysis of VE esters (e.g., tocopheryl acetate). This process could be significant when VE is consumed as a supplement and when pancreatic secretions are impaired (e.g., in newborns or in patients with cystic fibrosis).
In the duodenum, digestive enzyme (lipases, proteases, amylases, etc.) participate in the release of VE from the food matrix by degrading it further. The importance of the contribution of pancreatic lipase, which is responsible for the intestinal hydrolysis of triglycerides, to VE bioavailability has been recently emphasized by the association of single nucleotide polymorphisms (SNPs) in its encoding gene with the postprandial chylomicron VE concentration, an acknowledged marker of VE bioavailability, following consumption of a VE-rich meal. It is widely accepted that, to some extent, VE transfers to the lipid phase, if not naturally incorporated in vegetable oils, and then to mixed micelles. However, the possibility that some VE could be transferred to other structures that solubilize lipids in the duodenum, that is, vesicles, cannot be ruled out. Only one in vitro study has investigated VE (as α-tocopheryl acetate) distribution between the different structures present in the lumen of the small intestine during digestion, that is, mixed micelles, vesicles, oil droplets, and nonsolubilized food debris: most VE was found in matrices where its hydrolysis and its uptake by a model of human enterocytes was less efficient than in mixed micelles, suggesting this distribution could affect VE absorption efficiency. Its location within these structures, that is, at the surface or in the core of lipid droplets, or across or inside phospholipid bilayers, are not currently known and likely depend on VE interaction with the different components of these structures. Nevertheless, a computational study has suggested that, at 37°C, α-tocopherol remains in one leaflet of phospholipid bilayers, with its hydroxyl group located between the third and the fifth carbon atom in the sn-2 acyl chains of the lipids. As it is accepted that only free VE is taken up by enterocytes, VE esters need to undergo hydrolysis to be absorbed. This hydrolysis is carried out, at least partly, by cholesteryl ester hydrolase (CEH; also known as carboxyl ester lipase, CEL; bile salt-dependent lipase, BSDL; bile salt-stimulated lipase, BSSL), secreted by the exocrine pancreas and whose activity requires the presence of bile salts. Contrarily to what is observed with retinyl esters, we have shown that pancreatic lipase and pancreatic lipase-related protein 2 are not involved in this process. Nonetheless, other luminal candidate enzymes exist, for example, phospholipase B, and the participation of brush border enzyme has been suggested as well. Actually, Nagy et al. have recently shown that α-tocopherol and α-tocopheryl acetate exhibited the same bioavailability in the absence of digestive enzymes and bile salts in healthy subjects, even at supplemental doses. This study thus stressed the high potential contribution of brush border enzymes, enzymes released after cellular damage, or enzymes on the exterior of shed cells to the cleavage of α-tocopheryl acetate. These processes are summarized in Fig. 52.2 . Interestingly, a recent study has revealed the existence of a VE-ω-hydroxylase activity in human intestinal mucosa with the following rank order of activity on tocopherol isomers: γ = δ ≫ α. The ω-hydroxylase activity in human intestinal mucosa was 20%–30% of that found in the liver. In mice, it was found that only one enzyme, namely cytochrome P450, family 4, subfamily f, polypeptide 14 (Cyp4f14), was responsible for this activity. The quantitative, and qualitative (i.e., isomer specificity), contribution of this pathway to VE bioavailability warrants further investigation.
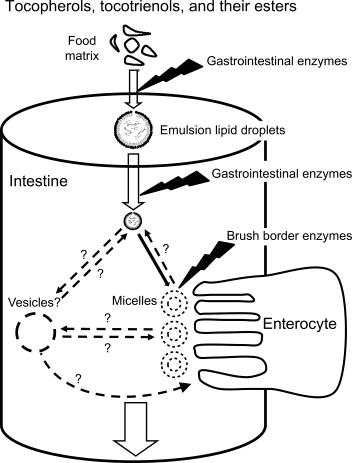
After its extraction from the food matrix and incorporation into mixed micelles, or vesicles, bioaccessible VE can be taken up by enterocytes. It should be stressed here that this extraction is only partial and as a consequence, VE bioaccessibility can be relatively low and is highly variable between food matrices (see Section 52.4 for more details). VE is then transported across the enterocyte before its incorporation into chylomicrons, and also intestinal high-density lipoprotein (HDL), and secretion in the blood circulation via the lymph and the portal vein. These processes are summarized in Fig. 52.3 .
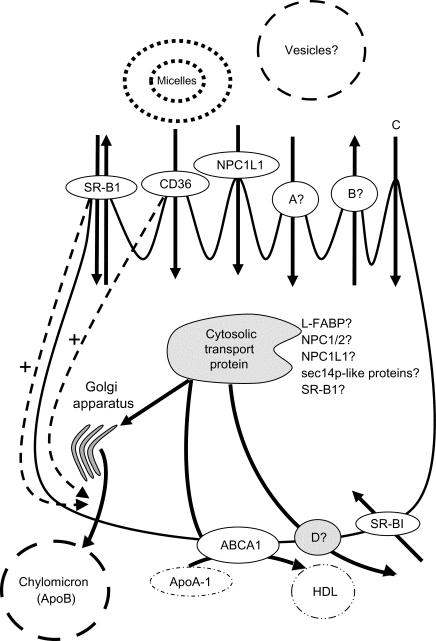
VE uptake by the enterocyte was long thought to occur only by simple passive diffusion but several proteins, which are temporarily present at the apical membrane, have been shown to facilitate the uptake of VE. NPC1 like intracellular cholesterol transporter 1 (NPC1L1), which was first identified as an intestinal protein involved in cholesterol absorption, has been shown to be involved in the uptake of α-tocopherol into the enterocyte. Moreover, SNPs in the gene encoding sterol regulatory element binding protein 2 (SREBP2), a modulator of NPC1L1 expression, have been shown to be associated with the postprandial chylomicron VE concentration following consumption of a VE-rich meal. Scavenger receptor class B type I (SR-B1) facilitates the uptake of several molecules with fairly different chemical structures, for example, cholesterol, vitamin K, carotenoids, and α-tocopherol. Since mixed micelles are assumed to dissociate in the unstirred water layer bordering the apical membrane of the enterocyte, VE incorporated in mixed micelles is supposed to reach the apical membrane as free molecules. Given that SR-B1 facilitates the uptake of fairly different molecules and that it has been shown to act as a sensor of mixed micelles promoting the assembly and secretion of chylomicrons, we hypothesize that a possible mechanism may be that this protein, by increasing the basolateral secretion of these molecules, increases their gradient between the intestinal lumen and the enterocyte, thereby promoting their uptake at the apical membrane. Another hypothesis might be that this protein interacts with mixed micelles rather than with free VE molecules and that micelle components and VE then diffuse toward the apical membrane. The mechanisms by which these molecules then cross this membrane and are secreted into the cytoplasm are not known. CD36 molecule (CD36) has also been associated with the uptake of α- and γ-tocopherol in vitro but since it has also been shown to promote the assembly and secretion of chylomicrons, a mechanism similar to the one we propose for SR-B1 could explain this association. Finally, the apical sodium-bile acid transporter (ASBT) has also been suggested to be involved in VE uptake following the association of SNPs in its encoding gene ( solute carrier family 10 member 2 , SLC10A2 ) with the postprandial chylomicron VE concentration following consumption of a VE-rich meal. Other membrane proteins might be involved but they are yet to be discovered. To summarize, both simple and facilitated diffusion mechanisms are involved in the apical uptake of VE by the enterocyte. Future studies will clarify if facilitated diffusion of VE is directly or indirectly mediated by proteins such as SR-B1 and CD36, that is, by promoting basolateral secretion. We hypothesize that their respective contribution probably depends on VE concentration in mixed micelles, which depends to some extent on the amount of VE ingested with the diet: at pharmalogical doses (e.g., when VE is provided by a supplement), simple diffusion is likely to be preponderant while at nutritional doses, the contribution of facilitated diffusion is likely to increase. In addition, SR-B1 has been shown in vitro in Caco-2 cells (a cell line derived from a human colon adenocarcinoma used as a model for enterocytes) to be involved in the efflux of VE from the enterocyte toward the apical side, a process greatly enhanced by the presence of VE-free mixed micelles. The in vivo contribution of this pathway to VE absorption efficiency is unknown.
Data are lacking on the trafficking of VE from the apical membrane of the enterocyte to the Golgi apparatus (where chylomicrons are assembled, in which a fraction of VE is incorporated). VE is a hydrophobic molecule and it is thus unlikely to cross the aqueous intracellular compartment without being bound to (a) transport protein(s). Although no protein has been clearly identified, several candidates exist. Liver-fatty acid binding protein (L-FABP), which can transport large molecules in its hydrophobic pocket, and which has been implicated in cholesterol trafficking in the enterocyte, could participate in VE intracellular transport. If VE does indeed bind to SR-B1, it is also possible for SR-B1-associated VE to be transported from the apical membrane toward cytoplasmic lipid droplets since it has been shown that during fat absorption, SR-B1 is internalized via clathrin-coated vesicles. A similar mechanism could occur with NPC1L1-associated VE, again if VE does indeed bind to NPC1L1. A protein called supernatant protein factor (SPF) or tocopherol-associated protein (TAP) has been found to bind VE in bovine and human tissues. Its mRNA is ubiquitously expressed, although there are no data regarding its expression in the small intestine. However, a systematic study of substrate specificity has shown that it had a weak nonselective affinity toward tocopherols, thus questioning its implication in intracellular VE transport. Other candidates for intracellular transport of VE within the enterocyte could be the sec14p-like proteins TAP1, 2, 3 (which are encoded by TAP1 , 2 , and 3 in humans). The proteins are expressed ubiquitously, including in the small intestine, and they have been shown in vitro to improve the transport of α-tocopherol to mitochondria with the same efficiency as α-tocopherol transfer protein (α-TTP), the main tocopherol binding protein in hepatocytes. Finally, the Niemann-Pick disease, type C1 and C2 proteins (NPC1/2) have been shown to be involved in the intracellular transport of tocopherol in fibroblasts and hepatocytes and neurons. However, it is not known whether these proteins are expressed in enterocytes or whether they are involved in VE absorption.
VE follows the fate of other newly absorbed lipid molecules (fatty acids, monoglycerides, cholesterol, etc.) but contrarily to what is observed for retinol or cholesterol, VE is not reesterified before its basolateral secretion from the enterocyte. Most VE is incorporated into chylomicrons in the Golgi apparatus before secretion in the lymph (apolipoprotein B-dependent route). Patients with abetalipoproteinemia, homozygous hypobetalipoproteinemia, or chylomicron retention disease, which are caused by mutations in microsomal triglyceride transfer protein ( MTTP ), apolipoprotein B ( APOB ), and secretion-associated Ras-related GTPase 1B ( SAR1B ), respectively, three genes critically involved in the assembly and secretion of chylomicrons, exhibit dramatically impaired VE absorption efficiency and need to be aggressively supplemented with VE to prevent occurrence of neurologic abnormalities. This highlights the major contribution of the apolipoprotein B-dependent route to VE absorption. However, it has been suggested in mice that the small intestine is able to secrete HDL directly into the portal vein via the basolateral membrane protein ATP binding cassette A1 (ABCA1), although earlier studies in rats had suggested intestinal secretion of HDL occurred into the mesenteric lymph. ABCA1 has been shown to participate in the basolateral secretion of VE toward apolipoprotein A1-containing HDL (apolipoprotein A1-dependent route). Whether this secretion occurs in the lymph or in the portal vein remains to be determined. Recently, it has been suggested that the basolateral membrane protein ATP binding cassette subfamily G member 1 (ABCG1) could also be involved in VE basolateral efflux to HDL and a SNP in its encoding gene has been shown to be associated with the postprandial chylomicron VE concentration following consumption of a VE-rich meal. The qualitative or quantitative contribution of nonapolipoprotein B-dependent routes to VE absorption efficiency are not known and further investigations are warranted. In addition, it has been suggested that SR-B1 present at the basolateral membrane could participate in HDL-VE uptake from the basolateral side.
The precise localization of the main VE absorption sites in humans is not known. A study in everted small intestine sacs in rats showed that VE absorption rates were as follows: jejunum > duodenum > ileum. A more recent study in mice showed that 2 h after oral administration of an emulsion containing 5 mg γ-tocopherol, the highest γ-tocopherol concentration was found in the distal part of the jejunum. VE absorption efficiency along the intestine likely depends on bioaccessible VE luminal concentration (which is probably not constant along the intestine as absorption occurs), the rate of apical uptake, intracellular transport, and basolateral secretion. Several of these processes are, at least partly, mediated by proteins. Interestingly, the distribution of some of these proteins is not homogeneous. A study of postmortem intestinal samples from 11 subjects revealed that jejunal and ileal levels of NPC1L1, CD36, and ABCA1 were higher than those found in the duodenum or colon. Another study in mice showed a gradual decrease in SR-B1 levels along the gastrocolic axis of the intestine. Interestingly, the SR-B1 apical/basolateral localization ratio was not constant along this axis with SR-B1 present mostly in the apical membrane in the duodenum, in both apical and basolateral membranes in the duodenum, and almost exclusively present in the basolateral membrane in the ileum.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here