Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
ATP-binding cassette
ATPase class I type 8B member 1
bile salt export pump
bromosulfophthalein
constitutive androstane receptor
carbon monoxide
“early-labeled peak” of bilirubin
farnesoid X receptor
heme oxygenase
multidrug resistance protein 1
multidrug resistance protein 2
multidrug resistance protein 3
messenger RNA
multidrug resistance–associated protein
Na + -taurocholate–cotransporting polypeptide
organic anion–transporting polypeptide
pregnane X receptor
retinoid X receptor
small heterodimer partner
uridine diphosphoglucuronate glucuronosyltransferase
uridine diphosphoglucuronate glucuronosyltransferase family 1 member A1
Bilirubin is the degradation product of heme, and the bulk of bilirubin is derived from hemoglobin of senescent erythrocytes and hepatic hemoproteins. Bilirubin is potentially toxic but is normally rendered harmless by binding to plasma albumin, conjugation with glucuronic acid, and efficient hepatic clearance. In some disease states, severe unconjugated hyperbilirubinemia can result in encephalopathy (kernicterus).
Perhaps because of its distinctive color, bilirubin has attracted the attention of physicians since antiquity. Hippocrates considered it one of the four important humors of the body: blood, phlegm, black bile, and yellow bile. Ayurveda, the ancient Indian book of medicine, also included it among its three principal factors—gases, bile, and phlegm—the proper balance of which was considered critical for health. During the last 3 centuries, the chemistry, metabolism, and disposal of bilirubin have been investigated meticulously by generations of chemists, biologists, and clinical investigators. Excretion of bilirubin by the liver has also been studied as a model for hepatic disposal of other biologically important organic anions of limited aqueous solubility. Several inherited disorders of bilirubin metabolism and excretion have been described in humans and animals. Investigation of these inborn errors has provided important information regarding its metabolic pathways. Definitive treatment of some of these disorders continues to be a therapeutic challenge and an impetus for further research. Although bilirubin has interested physiologists mainly as a toxic metabolic product, as an antioxidant it may serve as a defense mechanism against oxidative damage.
Jaundice and hyperbilirubinemia are frequently used as indicators of liver dysfunction. In acute hepatitis, jaundice is common and usually transient. In contrast, in other hepatocellular diseases such as alcoholic or drug-induced hepatitis, and alcoholic or nonalcoholic liver cirrhosis, jaundice has a dismal prognosis. In the intensive care unit, in septic or multitrauma patients, jaundice is associated with a high mortality rate. In primary biliary cirrhosis, jaundice is a major indicator of poor prognosis, and serial serum bilirubin measurement is one of the tests used for determining the appropriate timing of liver transplant. Impairment of bile flow caused by obstruction of the intrahepatic or extrahepatic biliary tract leads to jaundice. Because this is a postconjugation event, predominantly conjugated bilirubin accumulates in the blood. After relief of bile duct obstruction, jaundice usually resolves within 1 week, although elevated plasma bilirubin levels may linger because of the covalent binding of conjugated bilirubin to albumin. Acquired causes of hyperbilirubinemia, which include hemolysis, liver disease, and biliary obstruction, need to be differentiated from inborn errors of bilirubin metabolism.
Although jaundice is a common symptom, its clinical significance varies according to the underlying disease. In some cases a simple bilirubin level determination has more clinical predictive power than a battery of expensive diagnostic tests, including invasive techniques. Therefore sound knowledge of the pathophysiology of bilirubin metabolism is required for interpretation of this simple and valuable liver function test.
Breakdown of heme results in the daily production of 250 mg to 400 mg of bilirubin in humans. Normally, approximately 80% of bilirubin originates from the hemoglobin of senescent erythrocytes, and the remainder is derived from heme-containing enzymes (e.g., tissue cytochromes, catalase, peroxidase, tryptophan pyrrolase) and from myoglobin. A fraction of bilirubin is also derived from free heme. After radiolabeled heme precursors glycine and δ-aminolevulinic acid are injected into humans or rats, radioactivity is incorporated into bile pigments in two phases. The “early-labeled peak” of bilirubin (ELB) contains 20% of the radiolabel and is excreted in bile within 3 days. The initial “fast” component of ELB comprises two thirds of the ELB in humans and is largely derived from hepatic hemoproteins, such as cytochromes, catalase, peroxidase, and tryptophan pyrrolase, and from a rapidly turning over pool of free heme in the cytosol of hepatocytes, a fraction of which may be degraded without incorporation into hemoproteins. Induction of hepatic cytochrome P-450 increases the amount of ELB. Because δ-aminolevulinic acid is preferentially incorporated into hepatic hemoproteins when labeled δ-aminolevulinic acid is used as a precursor, only the initial component of the ELB incorporates radioactivity. The relatively “slower” phase of the ELB, which normally comprises one third of the area under the peak, is derived from erythroid and nonerythroid sources. This slower phase is enhanced in conditions associated with ineffective erythropoiesis, such as congenital dyserythropoietic anemias, megaloblastic anemias, iron-deficiency anemia, lead poisoning, and erythropoietic porphyria. This phase is also increased during accelerated erythropoiesis, probably because of intramedullary breakdown of normoblasts, destruction of reticulocytes in the peripheral circulation, and trimming of reticulocytes during maturation. The “late-labeled peak,” normally comprising 80% of the radiolabel, is derived from the hemoglobin of senescent erythrocytes and is associated with the life span of erythrocytes (approximately 50 days in rats and 110 days in humans). When the erythrocyte life span is reduced, as in hemolytic syndromes or intravascular or extravascular hemolysis, the late-labeled peak appears earlier.
Heme (ferroprotoporphyrin IX) is a ring of four tetrapyrroles connected by methene bridges ( Fig. 58-1 ). The ring is opened by cleavage of the α-methene bridge, catalyzed by microsomal heme oxygenases (HOs). Initially, an electrophilic attack at Fe(II) by a reducing agent, such as the reduced form of nicotinamide adenine dinucleotide phosphate, and oxygen results in the formation of α-oxyheme (see Fig. 58-1 ). Subsequently, the α-methene bridge carbon is eliminated as carbon monoxide (CO) and the porphyrin ring carbons that flank the α-methene bridge are oxidized by two additional oxygen molecules, resulting in the two lactam oxygens of biliverdin and bilirubin. Iron is released from the open tetrapyrrole after addition of electrons, suggesting that conversion of ferric iron to ferrous iron is required. Only a minute fraction of heme is opened at the β, γ, or δ bridges, resulting in the excretion of traces of bilirubin IXβ, IXγ, or IXδ, respectively, in bile.
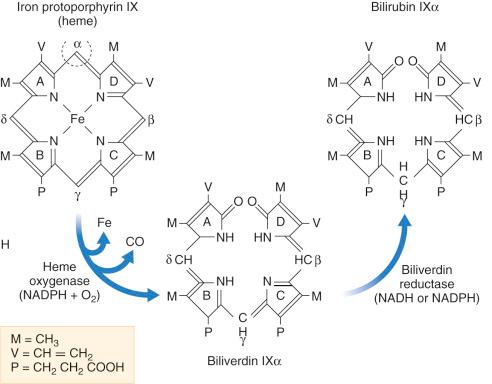
HO catalyzes physiologic heme degradation. It consists of three structurally related isozymes: HO-1, HO-2, and HO-3. HO-1 is the inducible form, HO-2 is a constitutive isoform, and HO-3 is a minor isoform present in spleen, liver, thymus, heart, kidney, brain, and testis. HO-2 is the isoform in hepatocytes and spleen responsible for biliverdin and CO production under normal physiologic conditions. High levels of HO-2 activity are present in cells involved in the breakdown of hemoproteins, such as cells in the spleen, where senescent erythrocytes are sequestered. In the liver, both hepatocytes and Kupffer cells have HO activity; the activity in the Kupffer cells is as high as that in the spleen. Apart from breakdown of heme from circulating hemoglobin, constitutive HO-2 is important for cellular hemoprotein homeostasis. HO-2 also functions as an oxygen sensor in the lungs. HO-2-deficient mice are severely hypoxic, and data suggest that HO-2 is responsible for matching the ventilation to perfusion. HO-1 is a 32-kDa protein that is induced by lipopolysaccharides, cytokines, heavy metals, reactive oxygen species, protoheme IX, oxidized low-density lipoprotein, hypoxia, and probably also shear stress in endothelial cells in the cirrhotic liver. Nuclear factor κB and p38 mitogen-activated protein kinase signaling pathways mediate the lipopolysaccharide-dependent induction of HO-1 gene expression via DNA sequences in the proximal promoter region. HO-1 acts as a stress-response protein and, by converting prooxidant heme to antioxidant biliverdin and bilirubin, it plays a role in the cellular defense against oxidative injury. A prerequisite for this antioxidant action is that toxic ferrous iron that is released on cleavage of the porphyrin ring is efficiently scavenged by ferritin. HO-1 deficiency in humans is associated with growth retardation, hyperlipidemia, endothelial cell damage with consumption coagulopathy, and microangiopathic hemolytic anemia. Inducible HO-1 is particularly important for cytoprotection in vascular endothelium and renal tubular epithelium. HO-1-deficient mice show early atherosclerosis, particularly when they are also hypercholesterolemic.
In addition to these cellular effects, the products of the HO reaction, CO and bilirubin, have more distant effects. CO is a signaling molecule with vasodilatory effects and effects on intestinal motility and sphincters. Moreover, a possible negative correlation between circulating bilirubin levels and coronary heart disease has been reported. Whether this protective effect of bilirubin also holds for patients with Gilbert syndrome is unclear.
Binding of heme to HO requires the propionic acid substituents in the C-6 and C-7 positions and a metal, such as iron, tin, or zinc. Oxygen binds to ferrous heme and undergoes reductive activation. Noniron metalloprotoporphyrins, such as tin and zinc protoporphyrins, bind HO with even greater affinity but do not activate oxygen and are therefore not degraded by HO. These metalloporphyrins are dead-end inhibitors of heme degradation. Tin and zinc protoporphyrins also disrupt the integrity of HO-2 but not that of HO-1. The loss of integrity of HO-2, the more abundant form of HO, may partly account for the suppression of bilirubin formation by tin protoporphyrin.
The immediate product of HO-mediated ring opening is the green pigment biliverdin, which is the major bile pigment in many amphibian, avian, and fish species. In most mammals, biliverdin is converted to the orange pigment bilirubin. Being less polar, bilirubin crosses placental membranes more readily than does biliverdin, although some placentate animals, such as nutria and rabbits, excrete biliverdin as the main bile pigment. Conversely, bilirubin formation has been found in early vertebrates, such as teleost and elasmobranch fish, that precede the evolution of the placenta.
Reduction of biliverdin to bilirubin is catalyzed by biliverdin reductase (see Fig. 58-1 ), a family of cytosolic enzymes that use the reduced form of nicotinamide adenine dinucleotide at pH 6.7 and the reduced form of nicotinamide adenine dinucleotide phosphate at pH 8.5 as cofactors. Guinea pig liver biliverdin reductase is a 70-kDa protein. Several interconverting forms of biliverdin reductase found in rat liver and spleen are produced by tissue-specific posttranslational modification of a single gene product. Recently, biliverdin reductase was shown to induce activating transcription factor 2, which controls the HMOX1 transcription, thereby forming a regulatory loop in which HO-1 and biliverdin reductase expression are interdependent.
Both biliverdin and bilirubin are strong antioxidants, which may be particularly important in the newborn period, when the levels of other natural antioxidants are low. Biliverdin appears to attenuate graft rejection in both cardiac and small intestine transplant models. Bilirubin is also a strong antioxidant, and has been reported to have cytoprotective activity, although at higher concentrations it is neurotoxic. The evolutionary development and conservation of the energetically expensive mechanisms of bilirubin production and elimination suggest a physiologic benefit for bilirubin. In a large cohort of insurance applicants, the relative mortality rate was higher in individuals who had serum bilirubin levels lower than those in the middle 50% of the population. An inverse relationship between serum bilirubin levels and risk of coronary artery disease supports this hypothesis. Analysis of data from 16,865 individuals in the Third National Health and Nutrition Examination Survey in the United States showed a significant inverse relationship between serum bilirubin concentrations and history of colorectal cancer. The odds ratios for colorectal cancer per 1 mg/dL increase in serum bilirubin levels for men and women were 0.295 (95% confidence interval 0.291 to 0.299) and 0.186 (95% confidence interval 0.183 to 0.189) respectively. This observation is consistent with another large study showing an inverse relationship between serum bilirubin levels and cancer mortality in a Belgian population. In a 4.5-year study of a nationally representative cohort of 4303 participants aged 60 years or older in the United States, a higher mortality rate was observed in participants with total bilirubin levels of 0.1 mg/dL to 0.4 mg/dL than in those with total bilirubin levels of 0.5 mg/dL to 0.7 mg/dL, the hazard ratio being 1.36. Overweight children developing nonalcoholic fatty liver disease were reported to have lower mean serum bilirubin levels than those who did not develop fatty liver. However, despite the impressive difference in odds ratios, such database analyses do not establish firmly a cause-and-effect relationship, because of the possible existence of known and unknown confounding variables. Notably, HO and biliverdin reductase, the two enzymes involved in bilirubin production, may also exert beneficial effects directly. For example, cell surface biliverdin reductase has been implicated in biliverdin-induced antiinflammatory effects via phosphatidylinositol 3-kinase and Akt signaling.
At the steady state, bilirubin production equals the synthesis and breakdown of hemoproteins. Normally, bilirubin is almost quantitatively excreted in bile; therefore one can measure bilirubin production by determining its biliary excretion in bile duct–cannulated experimental animals. Although bilirubin is excreted in bile predominantly as glucuronides, a small fraction is excreted as unconjugated bilirubin, which may undergo enterohepatic cycling. This may become important in patients with terminal ileum dysfunction, such as in Crohn disease, where unabsorbed bile acids may spill over into the cecum, solubilizing the unconjugated bilirubin formed by bacterial deconjugation of bilirubin glucuronides and thereby increasing bilirubin reabsorption.
One can measure bilirubin production by determining the turnover of radioisotopically labeled bilirubin. Plasma bilirubin clearance (the fraction of plasma from which bilirubin is irreversibly extracted) is calculated from the area under the radio-labeled bilirubin disappearance curve. Bilirubin removal is measured as the product of plasma bilirubin concentration and clearance. At steady-state levels of plasma bilirubin, the bilirubin removal rate equals the rate of its synthesis. This method does not take into account a small portion of bilirubin that is produced in the liver and excreted directly into bile without appearing in the circulation and therefore slightly underestimates bilirubin production.
Because HO-mediated oxidation of the α-carbon bridge of heme is the main source of endogenous CO, one can also quantify bilirubin formation by measuring CO production. The subject breathes into a closed rebreathing system. In the absence of anoxia, the body CO stores equilibrate rapidly with the CO in the rebreathed air. CO production is calculated from the CO concentration in the breathing chamber or from an increment in blood carboxyhemoglobin saturation. In addition, normal intestinal bacteria contribute a small fraction of the CO. Therefore in the absence of intestinal bacterial overgrowth, CO production exceeds plasma bilirubin turnover by 12% to 18%.
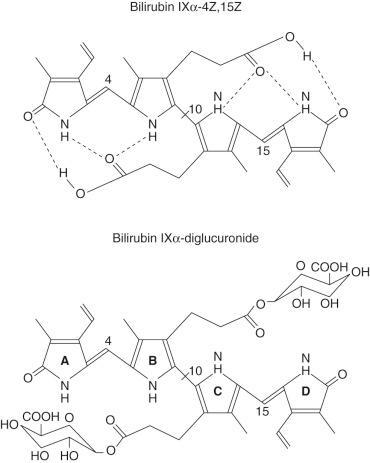
The systemic name of bilirubin IXα is 1′,8′-dioxo-1,3,6,7-tetramethyl-2,8-divinylbiladiene- a,c -dipropionic acid. The planar chemical structure of bilirubin, was determined by Fischer and Plieninger, and confirmed by X-ray diffraction analysis. The carbon bridges between pyrrolenone rings A and B (C-4) and rings C and D (C-15) are in trans or Z configuration. The oxygen attached to the outer pyrrolenone ring is in a lactam rather than a lactim configuration. Carbon nuclear magnetic resonance spectra and potentiometric and spectrophotometric titrations in aqueous solutions indicate that the p K of the two carboxyl groups is 4.4 and that of the two lactam groups is 13.0.
Crystallized bilirubin IXα- Z , with two protonated carboxyl groups, is virtually insoluble in water but is readily soluble in polar solvents, provided the intramolecular hydrogen bonds can be disrupted. Bilirubin and polar ligands, such as sulfonamides, share a binding site in the polar region of albumin with other polar substances, such as sulfonamides. Therefore, despite its insolubility in water at physiologic pH, bilirubin should be considered a relatively polar substance, the mechanism of toxicity of which may differ from that of truly lipophilic toxins, such as dichlorodiphenyltrichloroethane (also known as DDT ).
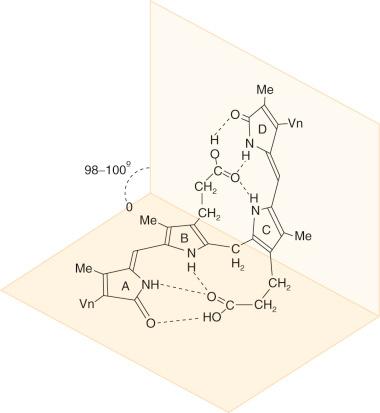
Insolubility of bilirubin IXα in water despite the presence of two propionic acid side chains, four amino groups, and two lactam oxygens is explained by internal stabilization of the molecule by hydrogen bonding between the carboxyl and the two external pyrrolenone rings ( Fig. 58-2 ). X-ray diffraction studies of crystalline bilirubin confirmed hydrogen bonding between each propionic acid side chain and the pyrrolic and lactam sites in the opposite half of the molecule. These hydrogen bonds constrain the molecule into a ridge-tile conformation (see Fig. 58-2 ) and make it insoluble in water by engaging the polar groups of the bilirubin. The integrity of the hydrogen-bonded structure requires the interpyrrolic bridges at positions 4 and 15 of bilirubin to be in trans or Z configuration. Addition of methanol, ethanol, or 6 mol/L urea interferes with the hydrogen-bonded structure and makes bilirubin more labile, water soluble, and rapidly reactive with diazo reagents. In the liver, conjugation of the propionic acid carboxyls of bilirubin with glucuronic acid moieties disrupts the hydrogen bonds, resulting in the formation of water-soluble conjugates that are readily excreted in bile. Resonance Raman spectroscopic studies of bilirubin-sphingomyelin complexes suggest that the intramolecular hydrogen bonds are disrupted in such complexes, and the propionic acid carboxyls form ion pairs with the quaternary ammonium ion of the choline moiety of sphingomyelin.
Bilirubin IXα has a main absorption band at 450 nm to 474 nm in most organic solvents with an extinction coefficient of 48.0 mmol/L to 63.4 mmol/L at its absorption maximum for a 1-cm path length. Circular dichroism spectroscopy shows that biliverdin preferentially adopts a minus-helicity conformation when bound to human serum albumin, whereas bilirubin IXα prefers a plus-helicity conformation. Therefore reduction of human serum albumin–bound biliverdin to bilirubin results in a conformational inversion from minus to plus helicity. Such sign inversion also occurs on addition of halothane, chloroform, or other volatile anesthetics to the albumin-bilirubin complex, suggesting that the volatile anesthetics alter the internal topography of receptor sites, influencing the stereoselectivity of ligand binding.
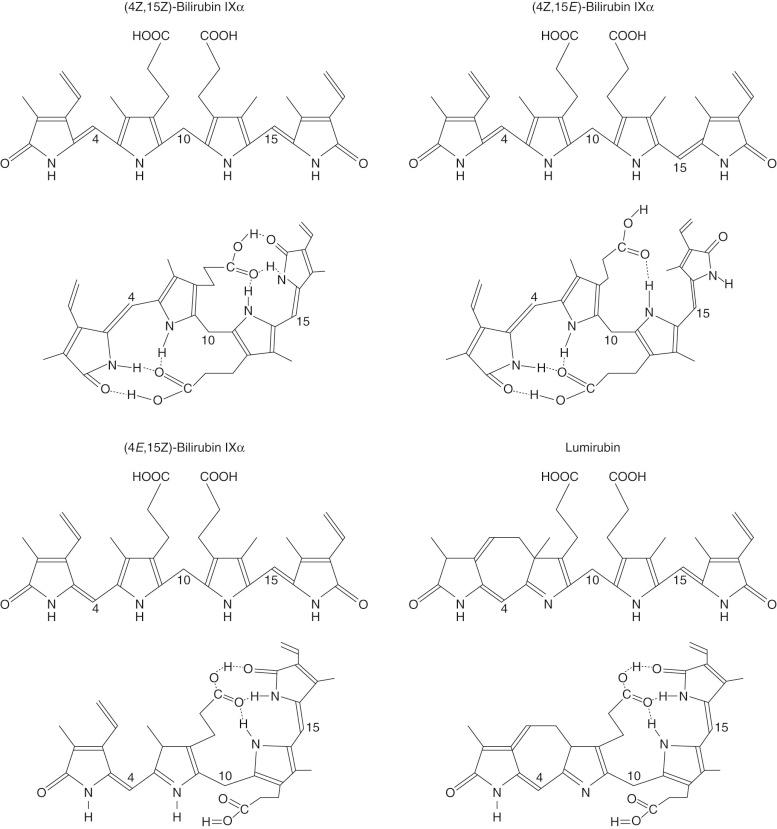
Normally, bilirubin IXα remains in a ZZ form, in which both of the interpyrrolic bridges at positions 5 and 15 are in the Z ( trans ) configuration. Exposure of circulating bilirubin to light changes the configuration of one or both of the interpyrrolic bridges from Z ( trans ) to E ( cis ) ( Fig. 58-3 ). Blue light is most efficient in mediating the conformational changes. As hydrogen bonding requires the Z configuration, (4 Z ,15 E )-bilirubin IXα and (4 E ,15 Z )-bilirubin IXα lack hydrogen bonds in one half of the molecule, whereas ( E,E )-bilirubin IXα lacks hydrogen bonds in both halves. Of these conformational isomers, (4 Z ,15 E )-bilirubin IXα is more abundant. Following absorption of two photons, the vinyl substituent at position C-3 of (4 E ,15 Z )-bilirubin IXα is cyclized with the methyl substituent on the internal pyrrole ring, forming the structural isomer ( E )-cyclobilirubin, or lumirubin. Although cyclization of bilirubin occurs at a slower rate than formation of configurational isomers, because of the relative stability of cyclobilirubin, this form may be quantitatively more important in phototherapy of neonatal jaundice. The conformational isomers are more polar than is ( Z,Z )-bilirubin IXα and can be excreted in bile without conjugation.
Although pure bilirubin does not fluoresce, when dissolved in detergents, albumin, or alkaline methanol it exhibits intense fluorescence at 510 nm to 530 nm, which has been used for quantification of blood bilirubin concentrations and the unsaturated bilirubin-binding capacity of albumin.
In the presence of light and oxygen, bilirubin undergoes a self-sensitized reaction involving singlet oxygen, resulting in the formation of colorless fragments, chiefly maleimides and propentdyopent adducts. A small amount of biliverdin is also formed.
Bilirubin IXα is asymmetric because of the difference in the side chains of the two halves of the molecule. On exposure to light, two bilirubin IXα molecules undergo free-radical disproportionation, forming two symmetric nonphysiologic isomers termed bilirubin IIIα and bilirubin XIIIα . The reaction is enhanced in the presence of acid and oxygen, and is inhibited by ascorbate.
The cerebral toxicity of bilirubin in neonatal jaundice has been known for at least 5 centuries. Degeneration of brain tissues associated with yellow pigmentation was reported in 1949. The association of kernicterus, or bilirubin-induced encephalopathy with severe unconjugated hyperbilirubinemia, was established subsequently. The study of mutant rats (Gunn strain) that lack hepatic bilirubin glucuronidating activity has contributed greatly to the current knowledge of bilirubin toxicity. Bilirubin neurotoxicity is caused by the non–protein-bound fraction of unconjugated bilirubin that can diffuse across cell membranes. Moderately increased intracellular bilirubin levels affect astrocytes and neurons, causing mitochondrial damage, which impairs energy metabolism and may induce apoptosis. Cell membrane perturbation can also inhibit the transport of neurotransmitters. Protective mechanisms against bilirubin encephalopathy include active export of bilirubin from cells to plasma by ATP-consuming pumps in the brain capillary endothelium and the choroid plexus epithelium. Binding to cytosolic proteins lowers the intracellular free bilirubin concentration, thereby reducing the toxicity of bilirubin. Because bilirubin cytotoxicity is modified by multiple pathophysiologic factors, the incidence and extent of bilirubin encephalopathy cannot be predicted simply on the basis of plasma bilirubin and albumin concentrations.
Bilirubin deposition in specific areas of the brain accompanied by structural damage is termed kernicterus . The Gunn rat is the only spontaneous mutant animal model in which bilirubin-induced brain damage has been observed. Normally, albumin binding inhibits bilirubin deposition in the brain. Displacement of bilirubin from albumin binding sites by drugs such as salicylates or sulfonamides increases bilirubin accumulation in the brain and may precipitate kernicterus. Gunn rats that are rendered genetically analbuminemic by crossbreeding with Nagase analbuminemic rats have serum bilirubin levels that are only 25% of those of other Gunn rats, whereas their cerebral bilirubin content is 1.2-fold to 2.7-fold higher. Such hybrid rats die within 3 weeks of birth. Therefore for clinical purposes, it is important to calculate the molar ratio between plasma albumin and bilirubin. However, the plasma free bilirubin level does not correlate well with brain bilirubin concentration, and it is not certain whether unbound bilirubin is the only toxic species of the pigment.
Various degrees of hearing deficiency due to abnormalities of the cochlear nuclei occur commonly as a complication of neonatal hyperbilirubinemia. Brainstem auditory evoked potential studies in Gunn rats indicate functional abnormalities of the central auditory pathways at and rostral to the cochlear nuclei beginning at 17 days of age. Similar changes are found in human neonates with severe hyperbilirubinemia.
Sulfonamides displace bilirubin from albumin binding, thereby promoting the net transfer of bilirubin into neural tissues. Administration of sulfonamides results in reversible abnormalities of brainstem auditory evoked potentials in Gunn rats. Under these conditions, focal bilirubin staining occurs in Purkinje cells of the cerebellum, hippocampus, and basal ganglia. Similar changes occur in human infants with fully developed kernicterus. A large number of Purkinje cells are affected in the cerebellum of Gunn rats at the age of 7 days; most of these cells degenerate and disappear between day 12 and day 30, resulting in cerebellar hypoplasia. The remaining Purkinje cells recover and persist into adult life. However, synapse formation among these Purkinje cells or with other neural cells may remain abnormal. Cerebellar mitochondria are enlarged and distorted in Gunn rats. Increased activities of the lysosomal enzymes arylsulfatase and cathepsin occur in the cerebellum of Gunn rats by the eighth day of life. Cerebellar cyclic GMP concentrations decrease progressively from day 15 to day 30, but cyclic AMP levels remain normal.
Except in patients with severe inherited deficiency of bilirubin glucuronidation (discussed later in this chapter), the occurrence of kernicterus is usually limited to the neonatal period and the first few months of life. Bilirubin encephalopathy may present with a broad spectrum of neurologic features. In the severest cases, overt kernicterus presents between the third and sixth days of life. The normal Moro reflex is lost, the muscles become hypotonic, the cry is high-pitched, athetoid movements appear, and reflex opisthotonos occurs in response to a startling stimulus. This may progress to lethargy, atonia, and death. Occasionally, in some children with Crigler-Najjar syndrome type 1, bilirubin encephalopathy may present late with cerebellar symptoms as the presenting feature. Those who survive acute kernicterus may develop chronic hearing abnormalities, athetoid movements, paralysis of upward gaze, and mental retardation, in various combinations. The cochlear nucleus is commonly affected by hyperbilirubinemia. Cells of the auditory system that receive synaptic input from end-bulbs or calyces appear to be early targets. In Gunn rat pups, these morphologic changes correlate with abnormalities of brainstem auditory evoked potentials. The sensitivity of auditory evoked potential testing can be increased by the recording of binaural difference waves obtained by subtraction of the sum of two monaural brainstem auditory evoked potentials from a binaural brainstem auditory evoked potential.
Bilirubin staining of the hippocampus, basal ganglia, and nuclei of the cerebellum and brainstem occurs in infants dying in the acute phase of bilirubin encephalopathy ; however, such staining is not found in children dying in the chronic stage of the disorder. Clinical manifestations precede histologic evidence of brain damage by approximately 72 hours. Focal necrosis of neurons and glial cells occurs later. Gliosis of the affected areas is seen in chronic cases. As these histologic lesions are not present from the onset of clinical kernicterus, they may not be the initiating pathophysiologic events in bilirubin-induced brain damage. Nonspecific signs of encephalopathy in the neonate may result from other causes, such as cerebral hemorrhage, and therefore kernicterus cannot always be diagnosed without pathologic documentation. Conversely, focal bilirubin staining of the brain may occur in other forms of brain injury. Thus, in the absence of neuronal degeneration, bilirubin staining alone does not establish the diagnosis of classic kernicterus.
Peak serum bilirubin levels of up to 10 mg/dL or 12 mg/dL are usually considered safe. The prognostic significance of a moderate degree of hyperbilirubinemia is not entirely clear. Serum bilirubin levels that are not high enough to cause kernicterus have been reported to result in an increased incidence of neurologic abnormalities or decreased intellectual performance later in life.
The equilibration of hydrophilic water-soluble substances and proteins between the blood and the brain is restricted by a functional blood-brain barrier. Tight junctions between capillary endothelial cells and foot processes of astroglial cells represent the structural component of this barrier. Specific transport mechanisms for translocation of ions, water, and nutrients from plasma to brain may provide the functional counterpart of the blood-brain barrier. Conventionally, immaturity of the blood-brain barrier in neonates has been implicated in the high incidence of kernicterus in this age group. However, it has been difficult to confirm a more rapid passage of labeled markers or lipophilic substances into the immature brain. Therefore there is no firm evidence to support the concept of an immature blood-brain barrier in the neonate.
The efficiency of cerebral bilirubin clearance may be inversely related to the cerebral toxicity of bilirubin. Experimentally, the blood-brain barrier can be unilaterally and reversibly opened without causing brain damage by infusion of hypertonic urea or arabinose. The hyperosmolarity-associated shrinkage of the capillary endothelial cells results in temporary opening of the tight junctions. When the blood-brain barrier is opened in newborn rats by this technique, intravenously administered albumin-bound bilirubin rapidly enters the brain. Following the reversal of the blood-brain barrier, bilirubin is rapidly cleared from the brain. The clearance of bilirubin from brain parallels its clearance from plasma, suggesting that bilirubin is cleared by diffusion or active transport back into the general circulation. Slower bilirubin clearance from the injured edematous brain may make the damaged brain more vulnerable to bilirubin toxicity.
Endothelial cells of cerebral microvessels and the choroid plexus together form the blood-brain barrier and cerebrospinal fluid–blood barrier. These endothelial cells strongly express the ATP-binding cassette (ABC) superfamily of transporting proteins. Notably, multidrug resistance protein 1 (MDR1; a P-glycoprotein) and the multidrug resistance–associated proteins (MRPs) MRP1, MRP4, MRP5, and, to a lesser extent, MRP6 are expressed in these tissues. The MRPs located in the microvascular endothelial cells and the basolateral membranes of the choroid plexus epithelial cells act as export pumps for drugs from the brain and the cerebrospinal fluid to the blood. MRP1 is preferentially expressed in the astroglial component of the blood-brain barrier. Recent evidence indicates that bilirubin is a substrate for MRP1 (ABCC1). According to these concepts the blood-brain barrier is not merely a passive anatomic structure but is an active tissue that can pump bilirubin and other metabolites and drugs out of the brain, thus reducing their intracellular concentrations. Substrate competition at the level of these pumps may be another way by which drug exposure can modulate bilirubin brain toxicity.
In cell culture systems, bilirubin shows a very broad range of toxicity. It is not clear which of the toxic effects observed in monotype cell cultures are relevant in bilirubin encephalopathy, and results observed with cultured cells do not always mirror findings in vivo. Although bilirubin is an antioxidant, bilirubin toxicity has been found to be associated with oxidative stress. This is partly explained by proteomic analysis of cerebella of Ugt1a1 -knockout jaundiced mice, which showed reduced levels of several proteins that are involved in the cellular defense against reactive oxygen species. These included protein deglycase DJ-1, superoxide dismutase, and peroxiredoxins 2 and 6. Reduction in the levels of these proteins occurred despite increased messenger RNA (mRNA) levels of nuclear factor erythroid-derived 2–like 2, which regulates the expression of the antioxidant genes. The mechanism of reduced expression of these proteins appears to be posttranscriptional because the corresponding mRNA levels were not altered. Thus a variety of protective mechanisms fail to protect the cells in the presence of excessive amounts of bilirubin, ultimately leading to neurodegeneration. Inhibition of cell division and enhancement of cellular apoptosis by bilirubin have been linked to the induction of a tumor suppressor protein, phosphatase and tensin homolog. In a cell-free system, bilirubin irreversibly inhibits calcium-activated, phospholipid-dependent protein kinase C activity and cAMP-dependent protein kinase activity. Because protein phosphorylation is the final common pathway of neuronal signal transmission, inhibition of protein kinase C by bilirubin may play a role in bilirubin encephalopathy in the newborn.
Physiologic mechanisms of protection against bilirubin-induced injury and cellular mechanisms of bilirubin toxicity are summarized in Table 58-1 .
| Site of Action | Physiologic Mechanism | Pathophysiology | Clinical Consequences |
|---|---|---|---|
| Plasma | Albumin-binding keeps UCB in solution and inhibits its entry into tissues (other than hepatocytes). | When the albumin-to-bilirubin ratio decreases, free UCB may enter tissues. | Risk of bilirubin toxicity increases when albumin levels are low. |
| Drugs that displace bilirubin from albumin can precipitate kernicterus. | |||
| Drugs displacing bilirubin from albumin can increase the free UCB fraction. | |||
| Liver sinusoid, hepatocytes | At the hepatocyte cell surface, UCB dissociates from albumin, enters hepatocytes by facilitated diffusion, is detoxified by glucuronic acid conjugation, and is excreted into bile. | Delayed development of UGT1A1 during the newborn period or inherited deficiency of UGT1A1 activity can result in marked elevation of plasma UCB levels. | Very high UCB levels can cause kernicterus in neonates and even in adults with inherited UGT1A1 deficiency (Crigler-Najjar syndrome). |
| BBB consisting of (1) endothelial cells (their tight junctions and adherens junctions), (2) astrocyte foot processes), (3) perivascular microglia, and (4) pericytes | BBB protects the astrocytes, oligodendrocytes, and neurons from direct interaction with UCB. | BBB may be less efficient in neonates because of lower expression of Pgp and MRP1. | Immaturity of BBB has been implicated in the increased risk of kernicterus in the neonatal period. |
| Pgp of endothelial cells and MRP1 on astrocytes and other brain cells export UCB. | Under oxidative stress caused by UCB, astrocytes release glutathione disulfide via MRP1, resulting in reduced levels of the intracellular antioxidant glutathione. | UCB interaction with developing neurons can impair neurogenesis and cause neuritic atrophy and cell death by necrosis or apoptosis. | |
| Astrocytes and microglia | Normally, intracellular Ca 2+ modulates glutamate exocytosis. | UCB-mediated ER stress increases intracellular Ca 2+ levels, causing exocytosis of glutamate and opening of mitochondrial permeability transition megapores. | Excess extracellular glutamate causes excitotoxicity, further increasing intracellular Ca 2+ levels, thereby activating enzymes that cause cell death. |
| The levels of the inflammatory cytokines TNF-α and IL-1α, and their corresponding receptors are low. | |||
| UCB increases the secretion of the inflammatory cytokines TNF-α and IL-1α and up-regulates their receptors. | Activation of the intracellular inflammatory cascade causes apoptosis and necrosis of astrocytes and neurons. | ||
| Oligodendrocytes | Oligodendrocytes support brain neurons by providing myelination of axons. | Increased extracellular glutamate levels after UCB exposure can cause excitotoxicity of oligodendrocytes. | Loss or dysfunction of oligodendrocytes can cause neural cell demyelination. |
Renal medullary deposition of unconjugated bilirubin results in medullary necrosis and formation of bilirubin crystals on the renal papillae in Gunn rats and in hyperbilirubinemic infants. In adult Gunn rats, abnormality of the ascending loop of Henle leads to an impairment of urinary concentration, which is ameliorated by reduction of serum bilirubin levels. The urinary concentration defect has not been found in mature neonates with hyperbilirubinemia or in patients with Crigler-Najjar syndrome type 1 who survive to adult age.
Bilirubin is transported in the circulation bound to plasma proteins, predominantly albumin. In the hepatic sinusoids the albumin-bilirubin complex dissociates and bilirubin is internalized by facilitated diffusion. Bilirubin is stored in the hepatocytes bound to cytosolic proteins. Bilirubin uridine diphosphoglucuronate glucuronosyltransferase (UGT) family 1 member A1 (UGT1A1), located in the endoplasmic reticulum, mediates the conjugation of bilirubin with glucuronic acid. Conjugated bilirubin exits from the endoplasmic reticulum and is eventually transported across the bile canalicular membrane into the bile by an energy-consuming process that is rate limiting in bilirubin throughput. A fraction of the bilirubin glucuronides is transported into the plasma and is subsequently internalized by other hepatocytes located downstream (toward the central vein) of the sinusoidal blood flow This multistep process is summarized in Fig. 58-4 . Many of these steps are shared by other organic anions and cholephilic metabolites. These processes are briefly discussed in the following sections.
Bilirubin is carried in the circulation bound to plasma albumin, which keeps it in solution at physiologic pH. Binding of bilirubin to albumin prevents its precipitation and deposition in tissues, thereby facilitating the transport of bilirubin from its site of production to its organ of elimination—the liver. Albumin binding prevents bilirubin from entering the brain. The reserve bilirubin binding capacity of albumin serves as a buffer by accommodating for sudden increases of bilirubin load (e.g., during acute hemolysis). The fenestrated endothelium of the hepatic sinusoids permits the albumin-bilirubin complex to enter the space of Disse, where bilirubin has direct contact with the sinusoidal and basolateral plasma membrane domains of the hepatocyte (see Fig. 58-4 ). Albumin enables bilirubin to traverse the unstirred water layer, a thin layer of water that surrounds the hepatocytes. Bilirubin, but not albumin, passes into the hepatocyte, indicating that bilirubin dissociates from albumin close to or at the hepatocyte surface. It is unclear whether or not an albumin receptor at the hepatocyte surface facilitates this dissociation.
There is a primary and a secondary binding site on albumin for bilirubin. Affinity labeling studies indicate that the primary binding site of bilirubin is located at residues 124 to 297 and, to a lesser extent, at residues 446 to 547. Lysine 240 in human albumin and lysine 238 in bovine serum albumin appear to be critical in bilirubin binding.
Albumin inhibits the neurotoxic effects of unconjugated bilirubin following intravenous injection in puppies. The role of albumin in preventing bilirubin toxicity is clearly shown in analbuminemic-Gunn hybrid rats, which die of bilirubin encephalopathy shortly after birth. Normally, bilirubin binding to albumin is reversible and can be affected by alternative ligands. Ligands that bind to the bilirubin-binding site of albumin, such as sulfonamides, antiinflammatory drugs, and cholangiographic contrast media, displace bilirubin competitively from albumin, increasing the non–protein-bound fraction of bilirubin. Prophylactic use of sulfonamides in newborns enhances bilirubin encephalopathy, probably by enhancing the dissociation of bilirubin from albumin, thereby increasing the net uptake of bilirubin into neural tissues. On the other hand, binding at non–bilirubin-binding sites may cause configurational changes that can enhance (cooperative binding) or diminish (anticooperative) bilirubin binding.
Because of the influence of many metabolites and drugs on albumin binding of bilirubin and its transfer from plasma to the central nervous system, measurement of total plasma bilirubin concentration does not accurately estimate the risk of brain damage from unconjugated bilirubin. Unbound bilirubin in serum can be quantified by gel chromatography, peroxidase treatment, electrophoresis on cellulose acetate, and fluorometry of serum with or without detergent treatment. Unbound bilirubin concentration may be roughly estimated as the product of serum bilirubin concentration, the concentration of reserve bilirubin-binding sites on albumin, and the association constant for bilirubin.
During prolonged conjugated hyperbilirubinemia, bilirubin becomes covalently bound to albumin. This fraction of bile pigments is not cleared by the liver or kidneys and persists for a long time in the circulation, reflecting the long half-life of serum albumin.
In view of this discussion it is interesting to note that analbuminemia is compatible with life and that elimination of amphipathic compounds such as bilirubin and bromosulfophthalein (BSP) is disturbed to a relatively minor degree in analbuminemic rats. For example, elimination of a tracer dose of bilirubin was entirely normal, although the biliary recovery after a loading dose was decreased. Other plasma proteins, such as high-density lipoproteins, can assume some of the assigned roles of albumin.
For efficient liver uptake, bilirubin needs to be delivered to hepatocytes via the sinusoidal blood. In the presence of portosystemic collaterals that develop in patients with portal hypertension, or surgically produced portosystemic shunts, significant fractions of bilirubin generated in the spleen are diverted to the systemic circulation, bypassing the liver. In these circumstances, first-pass clearance of bilirubin by the liver does not occur, resulting in mild hyperbilirubinemia. Similarly, an open ductus venosus in the newborn may intensify and prolong the physiologic jaundice in premature infants. Hepatic uptake of amphiphilic organic anions is carrier mediated. A brief discussion of these mechanisms follows.
The basolateral hepatocyte membrane contains various transporter proteins that function as carriers for the uptake of a multitude of endogenous and exogenous substances. Internalization of organic anions from plasma into the hepatocytes occur by a non–energy-consuming process termed facilitated diffusion . Organic anion–transporting polypeptides (OATPs), encoded by a family of solute carrier anion transporter genes carry bile acids, bilirubin, interleukins, and hormones such as thyroid and steroid hormones into the hepatocyte across the basolateral membrane. OATPs also transport numerous drugs, such as tyrosine kinase inhibitors and statins. The uptake carriers in human hepatocytes are listed in Table 58-2 . Na + -taurocholate–cotransporting polypeptide (NTCP), encoded by SLC10A1 in humans and Slc10a1 in rodents, has a narrow range of substrate specificity and is a specialized carrier for the Na + -dependent hepatic uptake of bile salts. Although non–carrier-mediated transmembrane diffusion was proposed as the mechanism of bilirubin influx, because this process is hepatocyte preferred, specific carriers facilitating bilirubin diffusion have been sought. OATP1B1 (encoded by SLCO1B1 ) was reported to transport unconjugated bilirubin but other studies did not confirm this conclusion. Thus the mechanism by which unconjugated bilirubin is transported across the sinusoidal surface membrane of the hepatocytes remains to be conclusively identified. On the other hand, OATP1B1 and OATP1B3 (encoded by SLCO1B3 ) have been shown to transport bilirubin glucuronide into hepatocytes, which is physiologically relevant in reuptake of bilirubin glucuronides that are pumped out into the hepatic sinusoids by the ATP-utilizing pump MRP3 (ABCC3).
| OATP-A | OATP-C | |||||
|---|---|---|---|---|---|---|
| SLC10A1 (NTCP) | SLC21A3 (OATP1, OATP) | SLC21A9 (OATP-B) | SLC21A6 (LST-1 OATP2) | SLC21A8 (OATP8) | SLC21A2 (PGT) | |
| Bilirubin | + | − | ||||
| Bromosulfophthalein | ++ | + | − | + | +/− | |
| Taurocholate | + | + | + | +/− | ||
| Estrone 3-sulfate | − | + | − | + | + | |
| Estradiol | − | ++ | + | + | + | |
| 17β-Glucuronide | − | + | − | − | + | |
| Dehydroepiandrosterone sulfate | − | − | − | − | + | |
| Ouabain | + | |||||
| Digoxin | − | +++ | − | − | − | |
| Pravastatin | − | − | − | + | + | |
| N -Methylquinine | − | + | − | + | − | ++ |
| Leukotriene C 4 | − | − | − | − | − | |
| Prostaglandin E 2 | ||||||
| Tissue distribution | H | B | H, B | H | H | W |
| References | ||||||
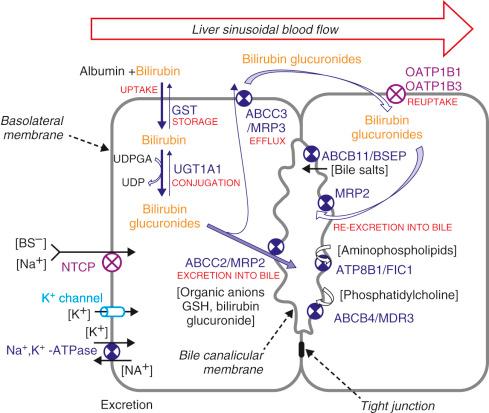
The expression of hepatic uptake carrier proteins is regulated at both the transcriptional level and the posttranscriptional level. Endotoxin, tumor necrosis factor α, interleukin-1β, and interleukin-6 reduce bile salt and organic anion uptake by down-regulating the expression of NTCP and OATP1B3. The significance of OATP1B3 in bilirubin metabolism is highlighted by the association of polymorphisms of the encoding gene with a statistically significant increase in serum total bilirubin levels in adults, as well as in neonates. OATP1B1 and OATP1B3 are now known to be involved in hepatocellular reuptake of bilirubin glucuronides that are transported into the plasma by hepatocytes. Simultaneous inactivating mutations of SLCO1B1 and SLCO1B3 , the genes that encode these two transporters, result in mild conjugated hyperbilirubinemia, which is seen in Rotor syndrome. Notably, the SLC family of genes is also involved in the transport of numerous organic anions, including hormones, cytokines, endogenous metabolites, and drugs, as highlighted by a high incidence of myopathy, induced by statins in individuals carrying SLCO1B1 variants. High intracellular bile salt concentrations, as found during cholestasis, decrease the expression of NTCP and OATP1B1. Bile salts bind to the farnesoid X receptor (FXR), a cytosolic nuclear hormone receptor. On binding bile salts, this receptor associates with retinoid X receptor (RXR), and the heterodimer then translocates to the nucleus. Here it binds to an FXR-RXR response element that is present in a number of genes, including the NR0B2 gene (also known as SHP ). Thus high bile salt concentration activates small heterodimer partner (SHP) expression and the up-regulated SHP interferes with RAR-RXR binding to the SLC10A1 gene, inhibiting transcription. Also OATP1B1 expression is down-regulated in cholestasis. OATP1B1 expression is under transcriptional control of hepatocyte nuclear factor 1α, which in turn is controlled by hepatocyte nuclear factor 4α. SHP inhibits hepatocyte nuclear factor 4α–mediated transactivation of the hepatocyte nuclear factor 1α promoter in cotransfection assays. In addition, the human hepatocyte nuclear factor 4α gene promoter is repressed by chenodeoxycholic acid through an SHP-independent mechanism. This explains why, both NTCP and OATP1B1are down-regulated in cholestatic liver disease. As discussed later in this chapter, MRP2 (ABCC2), the biliary export pump for conjugated bilirubin, is down-regulated during cholestasis, whereas MRP3 (ABCC3), a transporter with affinity for conjugated bilirubin, is up-regulated in the basolateral membrane. Via this transporter, conjugated bilirubin is pumped from the hepatocyte into blood. As OATP1B1is involved in reuptake of conjugated bilirubin, its down-regulation in cholestatic diseases may be expected to further increase the plasma accumulation of conjugated bilirubin. Subsequent clearance of bilirubin conjugates by the kidneys in cholestasis is facilitated by the up-regulation of renal MRP2 expression.
Separate transport proteins for bilirubin and bile salt internalization by hepatocytes explains the discrepancy between the extent of elevation of plasma bile acid and bilirubin levels in some clinical situations. For example, early in the course of primary biliary cirrhosis, bile acid levels are elevated but bilirubin levels may still be normal. Reduced hepatic bilirubin uptake has been observed in some cases of Gilbert syndrome (discussed later).
Within the hepatocyte bilirubin is kept in solution predominantly by binding to cytosolic proteins. Bilirubin and many other organic anions, drugs, and hormones were found to bind predominantly to a fraction of hepatocyte cytosolic proteins isolated by gel permeation chromatography. These proteins were termed Y protein , or ligandin . Later, ligandin was found to be a family of proteins, identical to the α class of glutathione S -transferases. Studies of bilirubin transport in isolated perfused rat liver showed that hepatic ligandin concentration did not affect the influx rate of bilirubin but increased the net uptake by reducing the efflux rate.
Biliary excretion of bilirubin requires its conversion to polar derivatives by enzyme-catalyzed conjugation of the propionic acid carboxyls with sugar moieties, particularly glucuronic acid, which disrupts the internal hydrogen bonds ( Fig. 58-5 ). Glucuronidation of one or both propionic acid carboxyls results in the formation of bilirubin monoglucuronide or bilirubin diglucuronide, respectively, both of which are efficiently excreted in bile. In normal bile from humans and most other mammals, bilirubin diglucuronide is the predominant conjugate.
Glucuronidation of bilirubin is catalyzed by a specific isoform (UGT1A1) of a family of enzymes termed uridine diphosphoglucuronate glucuronosyltransferase (UGT; EC 2.4.1.17). UGTs are concentrated in the endoplasmic reticulum and nuclear envelope of a variety of cells. These enzymes catalyze the transfer of the glucuronosyl moiety of UDP-glucuronate to a wide spectrum of aglycons, forming ether-, ester-, thiol-, and N -glucuronides. Substrates of UGT include hormones (e.g., steroid hormones, thyroid hormones, catecholamines), endogenous metabolites (e.g., bile salts, bilirubin), numerous drugs and their intermediate metabolites, toxins (e.g., carcinogens), and laboratory xenobiotics. Glucuronidation renders the aglycone substrates more polar and usually less biologically active. Thus UGTs constitute one of the most important detoxification mechanisms of the body.
UGTs are integral membrane proteins that require specific membrane lipids for function. In vitro, UGT activity in endoplasmic reticulum–derived microsomal vesicles is partially latent and the full catalytic activity is expressed only on membrane perturbation by physical, chemical, or enzymatic treatment. Two models have been proposed to explain such latency. The amino acid sequence of these enzymes suggests that the catalytic sites are compartmented inside the endoplasmic reticulum lumen, so a putative transporter is required to transfer the hydrophilic sugar donor substrate, UDP-glucuronic acid, across the unperturbed endoplasmic reticulum lipid membrane. Alternatively, the enzymes could be latent because of their being constrained by the lipid membranes. UDP- N -acetylglucosamine activates hepatic microsomal UGT activity at low concentrations and has been postulated to be a physiologic activator of the transferase. The action of UDP- N -acetylglucosamine is compatible with both the compartmental model and the constraint model of UGT latency, which may not be mutually exclusive.
The UGT system consists of a family of structurally related enzymes that accept UDP-glucuronic acid as the sugar donor substrate but are heterogeneous regarding their aglycone receptor substrates. The isoforms differ from each other in ontogenic development and their response to enzyme-inducing agents. Several laboratories have isolated multiple UGT isoforms from solubilized liver microsomes. Cloning of complementary DNAs and genomic DNA has provided a large amount of information on the structure and evolutionary divergence of UGTs. This topic has been reviewed. On the basis of the degree of structural homology among the various UGT complementary DNAs, UGTs may be separated into two major families. One family (UGT1) contains the bilirubin-conjugating form in human and rat liver, and a number of hormones, endogenous metabolites, drugs and toxins. The second family (UGT2) includes a number of UGT isoforms that mediate the conjugation of steroids and many other endogenous and exogenous substrates.
Members of the UGT1A family, including UGT1A1, which accepts bilirubin as a substrate, UGT1A6 and UGT1A7, which accept phenolic substrates, and several other isoforms, which are expressed from a gene locus, termed UGT1A. This locus, shown schematically in Fig. 58-6 , is located on human chromosome 2 at region 2q37. The 3′ domain of this gene contains four consecutive exons (exons 2 through 5) that are shared by all UGT isoforms expressed from the UGT1A locus and encode their identical carboxy-terminal domains. This common region is evolutionarily conserved and is responsible for UDP-glucuronic acid binding. Upstream of these exons are a series of 12 exons (exons 1A1 through 1A12). At least seven UGT1A isoforms are formed by the splicing of one of these unique region exons, encoding the aglycone-selective amino-terminal domain with the four common region exons. Each variable region exon is preceded by a separate promoter sequence. Depending on promoter selection during transcription, transcripts of various lengths are produced. During processing of the transcript to mRNA, only the unique region exon that is located at the 5′ end of the transcript is spliced to exon 2 (the first of the four common region exons), the whole intervening sequence being spliced out. The genes for the various UGT1A isoforms are named according to the unique exon used in that mRNA. For example, exon 1A1 encodes the amino-terminal domain of UGT1A1 and therefore this gene is termed UGT1A1. UGT1A1 is the only isoform that significantly contributes to the conjugation of bilirubin.
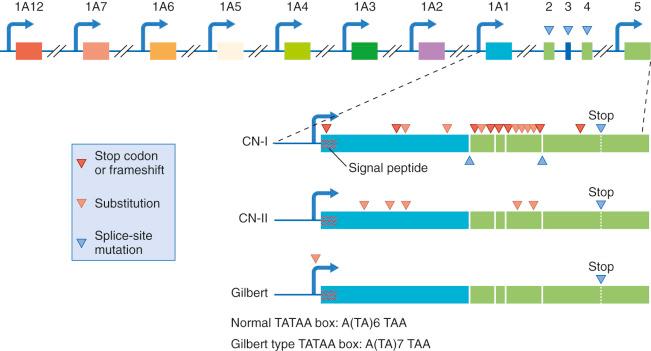
The presence of a separate regulatory cis element upstream of each unique exon permits different UGT1A isoforms to be independently regulated, explaining their different organ distribution and expression during ontogeny, enzyme induction, or carcinogenesis. Enzyme activity toward 4-nitrophenol and other simple phenolic substrates develops in late fetal life in rats, whereas activity toward bilirubin develops after birth. UGT1A6, a 3-methylcholanthrene-inducible isoform, is permanently overexpressed in carcinogen-induced preneoplastic nodules in rat liver. Triiodothyronine treatment results in a threefold increase in rat liver phenol-UGT activity, whereas bilirubin glucuronidation is reduced by 80%.
Excretion across the canalicular membrane is rate limiting in overall disposition of many cholephilic compounds and represents the most important concentrative step. For example, for organic anions such as dibromosulfophthalein, a liver-to-bile concentration ratio of 1 : 1000 can be reached. These concentration gradients are too large to be accounted for by the membrane potential difference across the canalicular membrane. For most classes of compounds, active, energy-consuming transport systems have been demonstrated.
ATP-dependent transporters, containing ABCs are important in the canalicular excretion of bilirubin glucuronides. MRP2 (ABCC2) is an organic anion efflux pump present in the canalicular surface of hepatocytes and apical surface of the renal and intestinal epithelia. for organic anions. In the liver, MRP2 functions as the efflux pump for many organic anions, including bilirubin monoglucuronide and bilirubin diglucuronide, most of which are products of phase II drug metabolism. MRP2 also contributes to bile formation by transporting glutathione, a major driving force for bile salt–independent bile flow. Genetic deficiency of MRP2 in humans leads to Dubin-Johnson syndrome, characterized by hyperbilirubinemia. The TR − rat is an animal model with conjugated bilirubinemia caused by a single nucleotide deletion in the ABCC2 gene. MRP1 and MRP3 are located in the basolateral membrane of hepatocytes. These export pumps are expressed at low levels in normal liver but are greatly induced during cholestasis and hyperbilirubinemia. MRP1 and MRP3 both transport glucuronides, including bilirubin glucuronides, from hepatocytes to blood, whereas MRP1 pumps glutathione S-conjugates. MRP3 normally transports a fraction of bilirubin glucuronides formed in the hepatocyte back to plasma. In conditions where MRP2 expression is down-regulated, such as in cholestasis and genetic MRP2 deficiency, up-regulation of MRP3 at the basolateral surface of the liver results in increased transport of bilirubin glucuronides to the blood.
Constitutive androstane receptor (CAR) is the dominant transcriptional regulator of MRP3. Phenobarbital is the prototypic substrate of CAR. MRP3 expression is enhanced in patients with Dubin-Johnson syndrome and in Eisai hyperbilirubinemic rats, suggesting that conjugated bilirubin may also be a CAR substrate. A dose- and time-dependent induction of Abcc2 expression was observed in isolated rat hepatocytes cultured in the presence of xenobiotics, including vincristine, tamoxifen, or the pregnane X receptor (PXR)-ligand rifampicin, indicating that Abcc2 gene transcription may respond to substrates of MRP2 itself. The promoter regions of the human ABCC2 and rat Abcc2 genes have been identified. Sequence analysis of the human ABCC2 promoter showed a number of putative consensus binding sites for both ubiquitous and liver-enriched transcription factors, including activating protein 1, specificity protein 1, hepatocyte nuclear factors 1 and 4, as well as CAR, PXR, and FXR. An unusual sequence was identified 440 base pairs upstream of the ABCC2 transcription initiation site that binds with high-affinity to PXR, CAR, and FXR as heterodimers with RXR. Human and rodent hepatocytes reacted with a robust induction of ABCC2 mRNA levels on exposure to PXR, CAR, and FXR agonists—rifampicin, dexamethasone, pregnenolone 16α-carbonitrile, and spironolactone (PXR); phenobarbital (CAR); and chenodeoxycholic acid (FXR). Ugt1A1 in rodents has also been reported to be a PXR target gene. Like ABCC2, UGT1A1 contains a multicomponent enhancer element in its promoter region with CAR, PXR, and aryl hydrocarbon receptor motifs. In addition, both glucuronidation and secretion are induced by PXR and CAR agonists. Thus PXR and CAR are major regulators of bilirubin uptake, glucuronidation, and secretion. The various secretion transporters and the substances that they handle are shown in Table 58-3 .
| MRP1 (ABCC1) | MRP2 (ABCC2, CMOAT) | MRP3 (ABCC3) | MRP6 (ABCC6) | |
|---|---|---|---|---|
| Glutathione | + | + | ||
| Glutathione disulfide | + | + | ||
| Leukotriene C 4 | + | + | ||
| Gluthatione S-conjugates | + | + | ||
| Bilirubin monoglucuronide | + | + | Probable | |
| Bilirubin diglucuronide | + | + | Probable | |
| Estradiol 17β-d-glucuronide | + | + | + | |
| Taurocholate | + | |||
| Glycocholate | + | |||
| 3α-Sulfotaurochenodeoxycholate | + | |||
| 6α-Glucuronosyl hyodeoxycholate | + | |||
| 3α-Sulfotaurolithocholate | + | + | ||
| Ochratoxin A | + | |||
| Methotrexate | + | + | + | |
| BQ-123 | + | |||
| Regulation | LPS↑ | LPS↓ ↑ | BDL | |
| PH↑ | BDL↓ dexamethasone↑ , | Eisai hyperbilirubinemic rats↑ | ||
| Gunn rats↑ | ||||
| Phenobarbital↑ | ||||
| Polarity | Basolateral | Canalicular | Basolateral | Basolateral |
| Canalicular | ||||
| Tissue distribution | H, E, B | H, I, K | H, C | H |
| References |
Transport of bile acids and other cholephilic organic anions into the bile canaliculus is important in bile formation and therefore excretion of conjugated bilirubin into the bile. Human MDR1 (ABCB1), multidrug resistance protein 3 (MDR3; ABCB4), ATPase class I type 8B member 1 (ATP8B1), and bile salt export pump (BSEP; ABCB11)—and their rat orthologues MDR1A and MDR1B, multidrug resistance protein 2 (MDR2), ATP8B1, and BSEP respectively—are P-glycoproteins that are constitutively expressed in the canalicular membrane of the hepatocyte. Human MDR3, rat MDR2, and human and rat BSEP are expressed in the liver only, whereas MDR1 and ATP8B1 are also expressed in various nonhepatic secretory epithelia. The canalicular BSEP is of paramount importance for bile formation and liver function. BSEP appears to be the principal driving force for the enterohepatic circulation of bile salts and the bile salt–dependent fraction of bile flow. Bile salts are the major, if not the only, substrates of BSEP. Inherited deficiency of ATP8B1 and BSEP leads to progressive familial intrahepatic cholestasis type I and type II respectively. These conditions both lead to severe life-threatening cholestatic liver disease, associated with both conjugated and unconjugated hyperbilirubinemia. Surprisingly, some mutations of ATP8B1 and BSEP also cause forms of benign intrahepatic cholestasis. MDR3 in humans (MDR2 in mice) is involved in the biliary secretion of phosphatidylcholine, the only phospholipid in bile. Mice with a knockout mutation of the Mdr2 gene produce bile in which phospholipids are absent. These mice and humans with a similar defect develop severe liver disease characterized by bile duct proliferation, portal fibrosis, and eventually cirrhosis. Heterozygosity for MDR3 deficiency is also in part responsible for intrahepatic cholestasis of pregnancy as well as for intrahepatic and gallbladder cholesterol stone formation. Approximately one third of adult patients presenting with unexplained cholestasis have mutations in the coding region of at least one ABCB4 allele. ABCB4 mutations also cause acute recurrent biliary pancreatitis, biliary cirrhosis, and fibrosing cholestatic liver disease in adults with or without biliary symptoms.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here