Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Children with primary disorders of the bile ducts present early in life with classic signs of prolonged conjugated jaundice, pale stools, and dark urine. They represent an important group within the so-called neonatal cholestasis syndrome. Disorders of the bile ducts can be due to developmental anomalies, an inflammatory process, or genetic causes. If corrective surgical treatment is available, it should be instituted early to minimize the progression of chronic liver disease.
Biliary atresia (BA) is the most common surgically correctable liver disorder in infancy, affecting sporadically between one in 8000 (Far East, Oceania) to 16,000 (Europe, North America) live-born infants. , It is characterized by complete obstruction of the bile flow due to progressive ascending destruction and obliteration of part of or the entire extrahepatic biliary tree. The intrahepatic bile ducts become affected as well. Studies of bile duct remnants removed at surgery, and from serial sectioning and reconstruction of surgical and necropsy liver specimens, indicate that BA arises from a sclerosing inflammatory process affecting previously formed bile ducts. Comparative anatomic studies have suggested that, at least in some cases, BA may be caused by failure of the intrauterine remodeling process at the hepatic hilum, with persistence of fetal bile ducts poorly supported by mesenchyme. As bile flow dramatically increases postnatally, bile leakage from these abnormal ducts may trigger an intense inflammatory reaction, with consequent obliteration of the biliary tree. The extrahepatic ducts are primarily affected, whereas the intrahepatic bile ducts remain patent in early infancy but eventually also become inflamed and obliterated and eventually disappear. Biliary cirrhosis with complications such as portal hypertension may develop at any time from 2 months of age; few unoperated children survive beyond 18 months of age.
Two forms of BA are described: (1) a more common (around 85% to 90% of cases) perinatal or postnatal sporadic form (“acquired”), possibly virus-related, and (2) a less common (around 10% to 15% of cases) fetal or embryonic form (“congenital”), with a high frequency of associated malformations. BA splenic malformation (BASM) syndrome is characterized by polysplenia or asplenia, various laterality defects such as abdominal or complete situs inversus, mediopositioned liver and intestinal malrotation, cardiac laterality defects, and positional abnormalities of the major abdominal blood vessels. , Intriguingly, BASM syndrome is less commonly seen in the Far East and Asia, where BA is twice as common as in the rest of the world. An increased incidence of maternal diabetes mellitus and female gender has been observed in the BASM syndrome. Children with this syndrome appear to have an increased frequency of infections, possibly leading to their poorer long-term prognosis compared with classic BA, including after liver transplantation, although no formal defect in their humoral immunity has been identified. It has also been suggested that the precarious blood supply to the biliary tree may be further jeopardized by their vascular abnormalities. A study suggests that among syndromic infants with BA, there may be a subgroup—around 6% of all BA children—who may have other, nonlaterality defects, including cardiovascular and genitourinary anomalies.
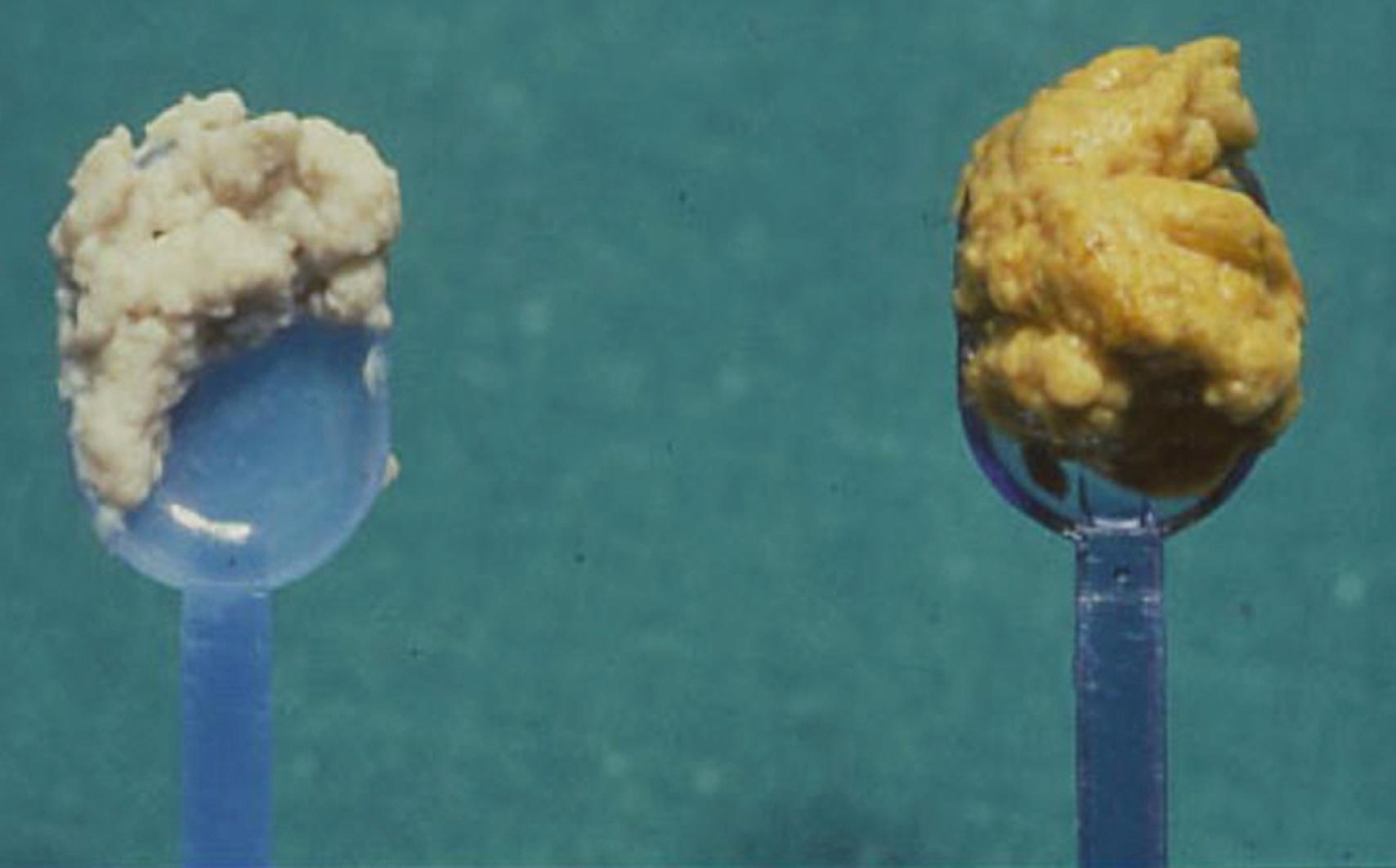
Clinical features of BA are jaundice, pale stools, and dark urine presenting at or soon after birth. Because physiologic jaundice, characterized by unconjugated hyperbilirubinemia, is common in neonates and most infants with BA have no major symptoms in the first few weeks of life, diagnosis is often delayed. This is a particular problem for infants with the perinatal or “acquired” form of BA, whose stools may have some pigment in the first few weeks of life before bile flow is completely obstructed ( Fig. 69.1 ). , Delayed diagnosis and surgical treatment carry a severe prognosis. Age at surgical correction is inversely correlated with the medium-term (up to 20 years) survival with native liver. Therefore, it is of paramount importance for health care professionals attending young infants to check the color of the urine and stools of all jaundiced babies, irrespective of their general health or age, and refer those with dark urine and pale stools promptly to specialized hepatology centers. No satisfactory screening test is available for BA, although promising results using universal neonatal stool color cards have been reported.
Physical examination and laboratory tests give little clue to the diagnosis of BA. Most of the affected infants have a mild degree of hepatomegaly and splenomegaly. Ascites or cutaneous signs of chronic liver disease are rarely detected in the early stages of the disease, when correct diagnosis is most important for effective surgical intervention. Biochemical findings are nonspecific, with levels of transaminases, γ-glutamyltranspeptidase (GGT), and alkaline phosphatase similar to those found in other forms of neonatal cholestasis. Coagulopathy, if present, is responsive to intravenous vitamin K. An ultrasound scan revealing an absent or abnormal gallbladder with an irregular wall or, in older infants, the “triangular cord” sign is suggestive of BA. However, a normal gallbladder or absence of the triangular cord sign does not exclude BA.
Histologic examination of the liver by an experienced histopathologist leads to the correct diagnosis of BA in up to 90% of cases. Typical histologic findings are edematous portal tracts with inflammatory changes, bile duct proliferation, and intraportal bile plugs ( Fig. 69.2A ), but in very young babies these features can be much less obvious. Biliary radionuclide scans are only useful if isotope is demonstrated in the gut, thereby excluding BA and avoiding laparotomy. If the liver biopsy is ambiguous, but the stools remain acholic, endoscopic retrograde cholangiopancreatography (ERCP) , is indicated to assess the patency of the biliary system. Currently, magnetic resonance cholangiopancreatography (MRCP) is a sensitive but nonspecific diagnostic tool for BA diagnostics. It is hoped that improvement in the magnetic resonance technique will offer a noninvasive way of assessing the biliary tree in suspected BA. , If the cholangiography is not informative, an explorative laparotomy with intraoperative cholangiography is required but should be undertaken by an experienced surgeon, because hypoplastic extrahepatic ducts caused by a severe intrahepatic cholestasis may be interpreted as atretic, leading to an unnecessary and possibly damaging operation. The diagnosis of BA should always be confirmed by histologic examination of the excised biliary remnants (see Fig. 69.2B ).
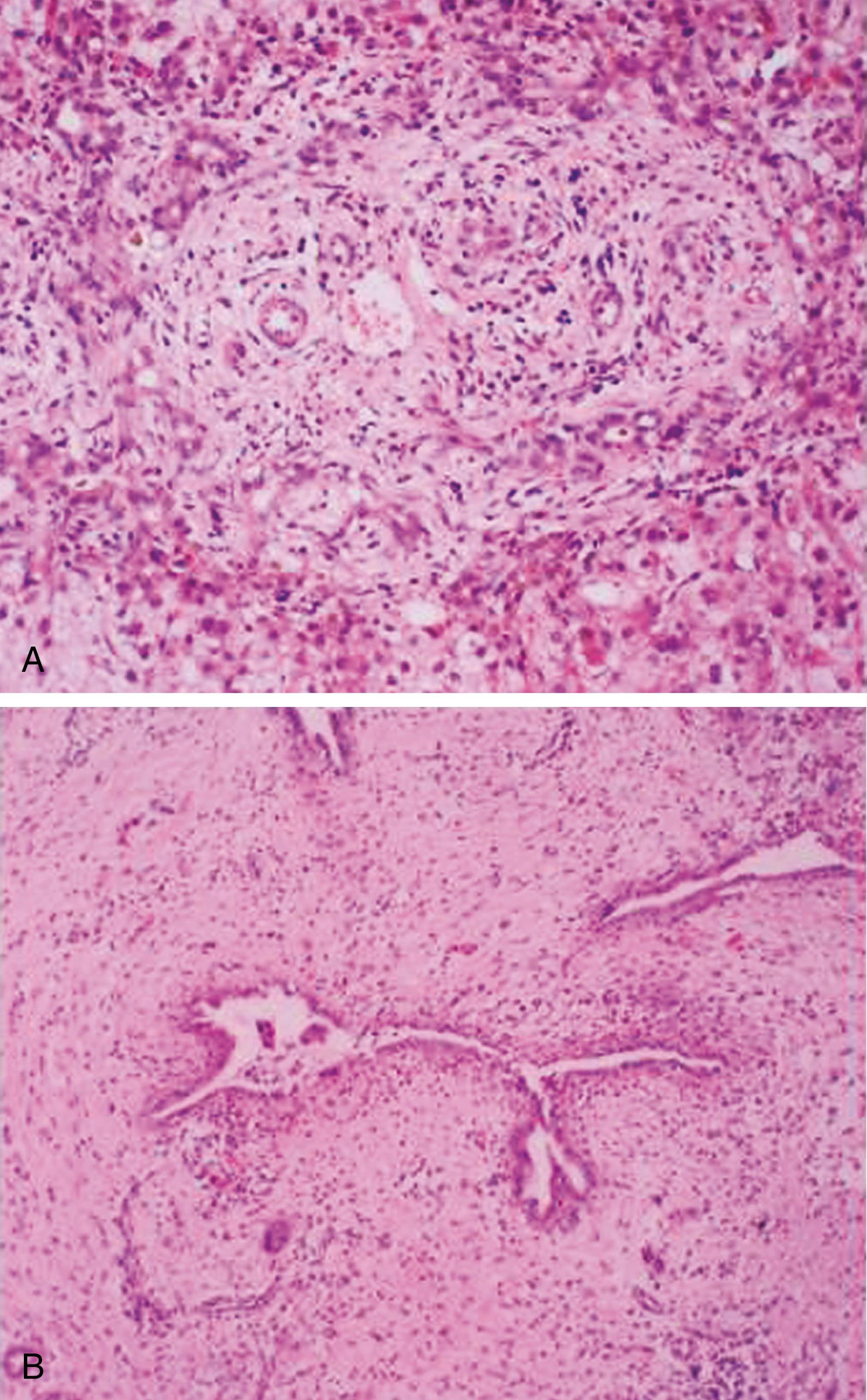
Studies of bile duct remnants removed at surgery and from serial sectioning and reconstruction of surgical and postmortem liver specimens indicate that BA is in most cases a sclerosing inflammatory process affecting previously formed bile ducts. , The cause of such inflammatory process remains unknown. It is conceivable that BA represents a common final phenotypic pathway of neonatal liver injury caused by diverse causes, including developmental, vascular, or infectious factors, which may act antenatally or within the first 3 months of life in a genetically predisposed individual. Although BA is not an inherited disorder, because identical twins are usually discordant for the disease and occurrence of BA within the same family is exceedingly rare, , it is possible that a genetic predisposition to an aberrant immune response against an exogenous agent and/or somatic mutations of genes regulating bile duct morphogenesis in fetal life are involved. Whatever the initiating event, as bile flow dramatically increases perinatally, bile leakage from the abnormal ducts is likely to trigger an intense inflammatory reaction, with consequent obliteration of the biliary tree. The detergent effect of the extravasated bile, however, cannot be the only explanation for the liver damage, because the disease can also progress in those patients in whom the Kasai portoenterostomy has achieved adequate bile flow. Proposed etiologic factors in BA include defective morphogenesis/genetic factors, vascular abnormalities, viral infection, exposure to toxins, and aberrant immune mechanisms.
A separate clinical and etiologic subgroup, named BASM syndrome, is believed to be caused by defective morphogenesis of the biliary tree. , A recessive insertional mutation in the proximal region of mouse chromosome 4 or complete deletion of the inversion (INV) gene in a murine model leads to anomalous development of the hepatobiliary system. However, no consistent mutations in the human homologue INV gene were identified in patients with BA, including those with BASM syndrome, suggesting that the INV gene is unlikely to be involved in the fetal cases of BA. CFC1 , coding for the CRYPTIC protein, is another gene investigated in BA. Although the precise function of the CRYPTIC protein is unknown, it is believed to act as a cofactor in the nodal pathway that determines left–right axis development, disturbed in the BASM syndrome. A genetic mutation in exon 5 of the CFC1 gene, leading to the amino acid substitution Ala145Thr, was found in 5 of 10 infants with BASM syndrome. It is conceivable that CFC1 heterozygous mutations predispose to BASM, but then a second genetic or environmental factor is necessary to produce the disease phenotype.
Histologic features similar to the inherited group of disorders termed ductal plate malformation (DPM) syndrome, which include congenital hepatic fibrosis (CHF) and Caroli syndrome, have been reported in fetal-type BA, suggesting that abnormalities in hepatocyte growth factor signaling during a critical period for mesenchymal/epithelial differentiation or other defects in intracellular adhesion molecule systems might be involved in the pathogenesis of this form of the disease. Of note, however, among nine children with BA diagnosed in utero, only one had BASM syndrome and none had histologic appearances of “ductal plate malformation.”
Cui et al. have used genome-wide association studies to identify a potential susceptibility gene for BA at 2q37.3, encoding glypican 1 (GPC1) , one member of the proteoglycan family involved in intercellular signaling, including biliary development. They have used its analog GPC1 in a zebrafish model to demonstrate that GPC1 knockdown variant develops impaired bile production and intrahepatic biliary and gallbladder defects.
Tan et al. postulated that BA may derive from failure of the ductal plate structure remodeling between 11 and 13 weeks of gestation leading to the formation of an inadequate mesenchymal cuff around the developing hilar bile ducts, which could potentially be prone to rupture at the initiation of bile flow at 12 to 13 weeks of gestation.
Two studies report the presence of maternal microchimerism in children with BA. Hayashida et al. demonstrated three times more XX chromosome cells in males with BA compared with age-matched controls. Kobayashi et al. confirmed maternal microchimerism in male patients with BA and showed maternal microchimerism in females with BA by demonstrating presence of maternal human leukocyte antigens (HLAs). They suggested that maternal microchimerism could be a potential causative factor in BA, as maternal cells could elicit an immune response similar to graft-versus-host disease.
Intrahepatic and extrahepatic bile ducts receive their blood supply exclusively from the hepatic artery. An arteriopathy affecting branches of the hepatic artery has been reported in patients with BA. These observations have led to the proposal that a vasculopathy may be the cause of BA, although whether vascular problems are primary or secondary to bile duct damage remains to be clarified.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here