Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Describe the constituents of bile and their functions.
Understand the solubility of the bile acids and bile salts and how it affects their reabsorption in the small bowel.
Describe enterohepatic circulation and its role in bile acid synthesis and the secretion of bile.
Understand the process involved in the excretion of bile pigments and its relationship with jaundice.
Explain the function of the gallbladder.
Describe the regulation of bile secretion from the liver and its expulsion from the gallbladder.
Explain the abnormalities that may lead to the formation of gallstones.
Bile is responsible for the principal digestive functions of the liver. Bile in the small intestine is necessary for the digestion and absorption of lipids. The problem of the insolubility of fats in water is solved by the constituents of bile. The bile salts and other organic components of bile are responsible in part for emulsifying fat so that it can be digested by pancreatic lipase. The bile acids also take part in solubilizing the digestion products into micelles. Micellar formation is essential for the optimal absorption of fat digestion products. Bile also serves as the vehicle for the elimination of a variety of substances from the body. These include endogenous products such as cholesterol and bile pigments, as well as some drugs and heavy metals.
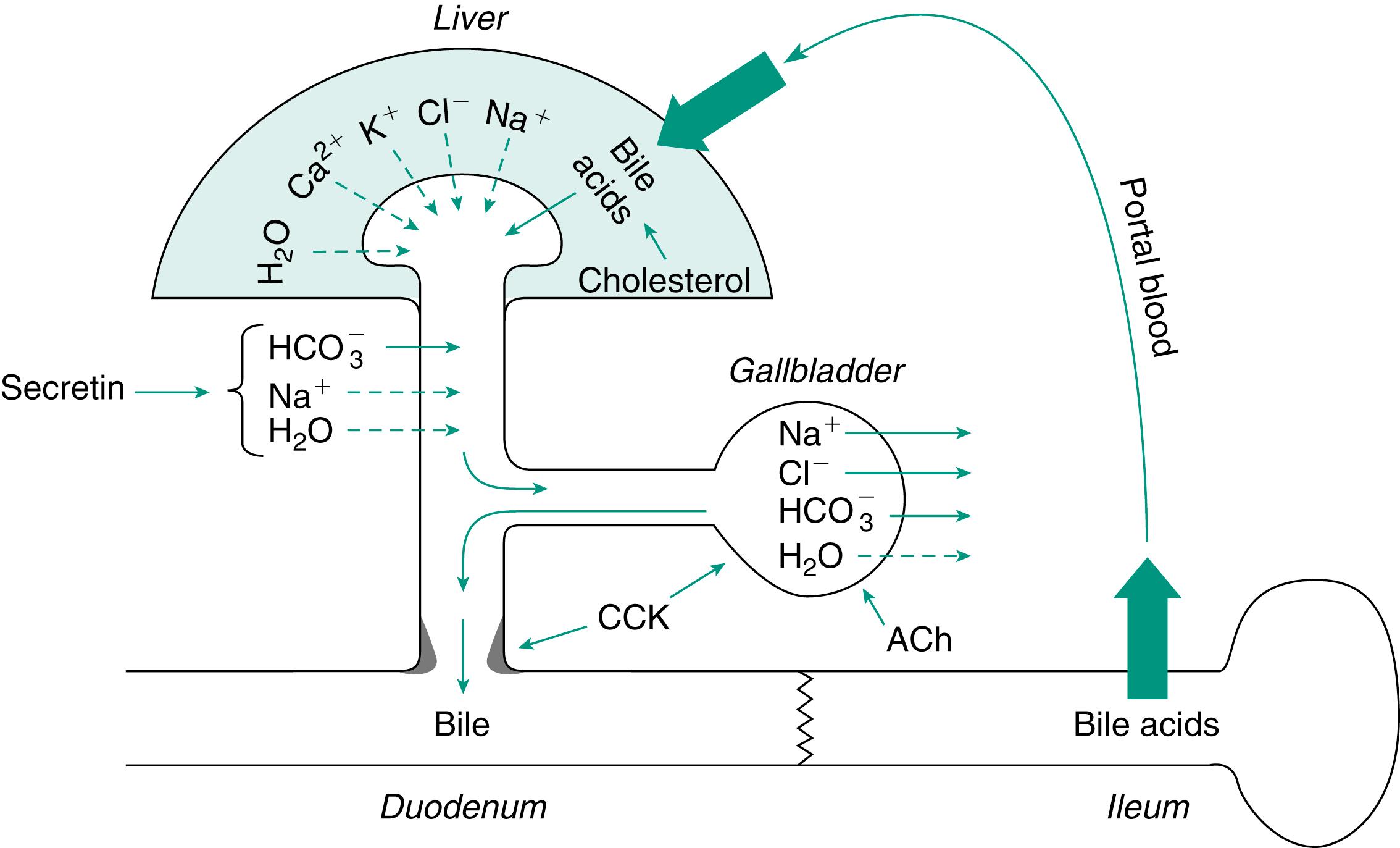
Fig. 10.1 is a schematic illustration of the biliary system and the circulation of bile acids between the intestine and liver. Bile is continuously produced by the hepatocytes. The principal organic constituents of bile are the bile acids , which are synthesized by the hepatocytes. The secretion of bile acids carries water and electrolytes into the bile by osmotic filtration. Additional water and electrolytes, primarily sodium bicarbonate (NaHCO 3 ), are added by cells lining the ducts. This latter component is stimulated by secretin and is essentially identical to the aqueous component of pancreatic secretion. The secretion of bile increases pressure in the hepatic ducts and causes the gallbladder to fill. Within the gallbladder, bile is stored and concentrated by the absorption of water and electrolytes. When a meal is eaten, the gallbladder is stimulated to contract by CCK and vagal stimulation. Within the lumen of the intestine bile participates in the emulsification, hydrolysis, and absorption of lipids. Most bile acids are absorbed either passively throughout the intestine or actively in the ileum. Bile acids lost in the feces are replaced by synthesis in the hepatocytes. The absorbed bile acids are returned to the liver via the portal circulation, where they are extracted actively from the blood. Together with newly synthesized bile acids, the returning bile acids are secreted into the bile canaliculi. Canalicular bile is secreted by ductule cells in response to the osmotic effects of anion transport. In humans, almost all bile formation is driven by bile acids and is therefore referred to as bile acid dependent . The portion of bile stimulated by secretin and contributed by the ducts is termed bile acid independent or ductular secretion .
Bile is a complex mixture of organic and inorganic components. Taken separately, some of the components are insoluble and would precipitate out of an aqueous medium. Normally, however, bile is a homogeneous and stable solution whose stability depends on the physical behavior and interactions of its various components.
Bile acids, the major organic constituents of bile, account for approximately 50% of the solid components. Chemically, they are carboxylic acids with a cyclopentanoperhydrophenanthrene nucleus and a branched side chain of three to nine carbon atoms that ends in a carboxyl group ( Fig. 10.2 ). They are related structurally to cholesterol, from which they are synthesized by the liver. Indeed, the synthesis of bile acids is a major pathway for the elimination of cholesterol from the body. Conversion of cholesterol to bile acids occurs via two main synthetic pathways.
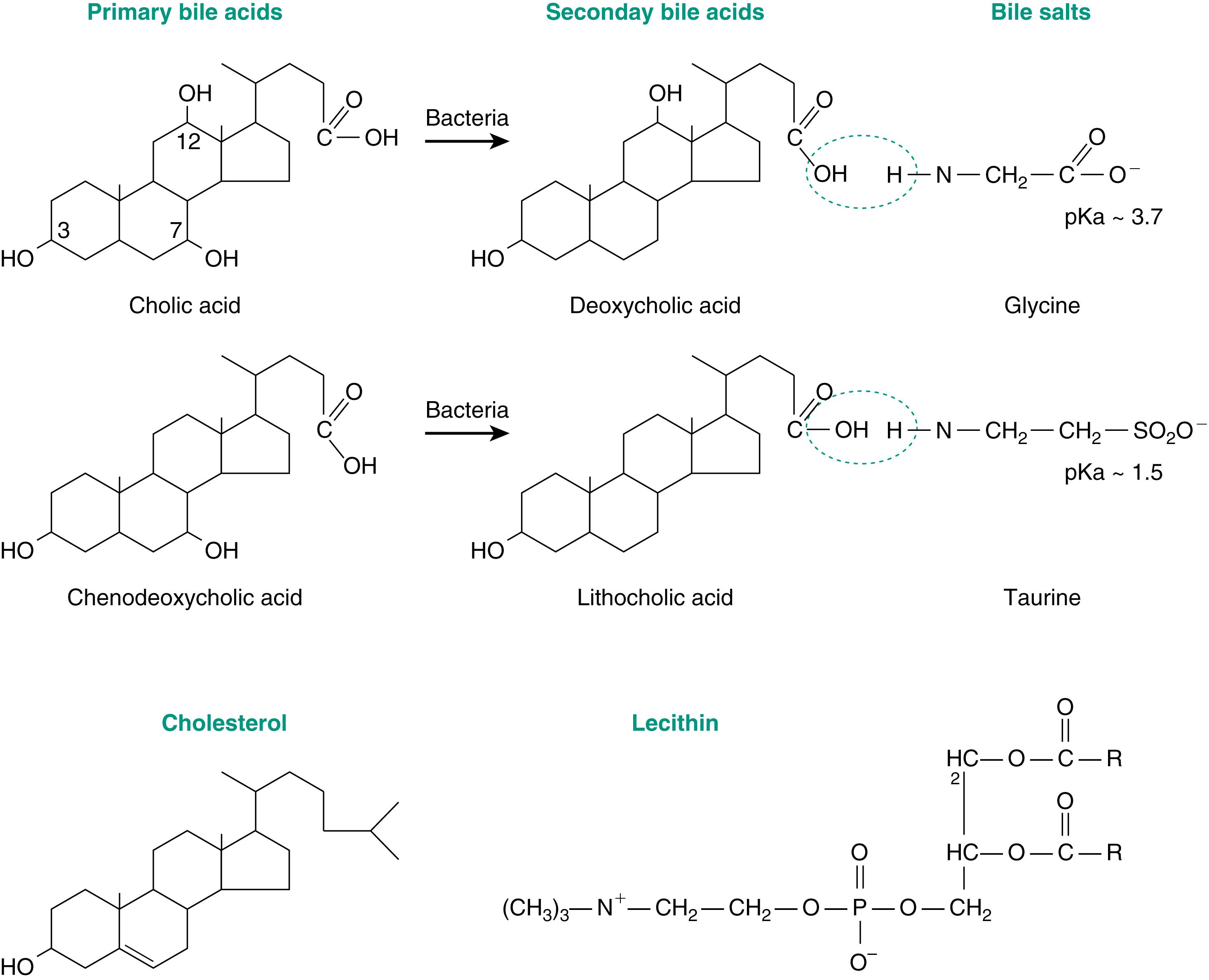
The major pathway begins with the rate-limiting step of 7α-hydroxylation of cholesterol by the hepatic enzyme 7α-hydroxylase. A secondary pathway begins with the conversion of cholesterol to 27-hydroxycholesterol, a reaction that takes place in many tissues. Four bile acids are present in bile, along with trace amounts of others that are modifications of the four. The liver synthesizes two bile acids, cholic acid and chenodeoxycholic acid . These are the primary bile acids (see Fig. 10.2 ). Within the lumen of the gut, a fraction of each acid is dehydroxylated by bacteria to form deoxycholic acid and lithocholic acid . These are called secondary bile acids. All four are returned to the liver in the portal blood and are secreted into the bile. Their relative amounts in bile are approximately four cholic to two chenodeoxycholic acid to one deoxycholic to only small amounts of lithocholic acid.
The solubility of bile acids depends on the number of hydroxyl groups present and the state of the terminal carboxyl group. Cholic acid, with three hydroxyl groups, is the most soluble, whereas lithocholic, a monohydroxy acid, is least soluble. The dissociation constant (pK) of the bile acids is near the pH of the duodenal contents, so there are relatively equal amounts of protonated (insoluble) forms and ionic (soluble) forms. The liver, however, conjugates the bile acids to the amino acids glycine or taurine with a pKa of 3.7 and 1.5, respectively. Thus at the pH of duodenal contents, bile acids are largely ionized and water soluble. Conjugated bile acids exist as salts of various cations, primarily Na + , and are referred to as bile salts (see Fig. 10.2 ).
Several features are unique to the bile acids and account for their behavior in solution. Three-dimensionally, the hydroxyl and carboxyl groups are located on one side of the molecule. The bulk of the molecule is composed of the nucleus and several methyl groups ( Fig. 10.3 ). This structure renders bile acids amphipathic to the extent that the hydroxyl groups, the peptide bond of the side chain, and either the carbonyl or sulfonyl group of glycine or taurine are hydrophilic, and the cholesterol nucleus and methyl groupings are hydrophobic. In solution, the behavior of bile acids depends on their concentration. At low concentrations, little interaction occurs among bile acid molecules. As the concentration is increased, a point is reached at which aggregation of the molecules takes place. These aggregates are called micelles , and the point of formation is called the critical micellar concentration . Hydrophobic regions of the micelles interact with one another, and the hydrophilic regions interact with the water molecules (see Fig. 10.3 ).
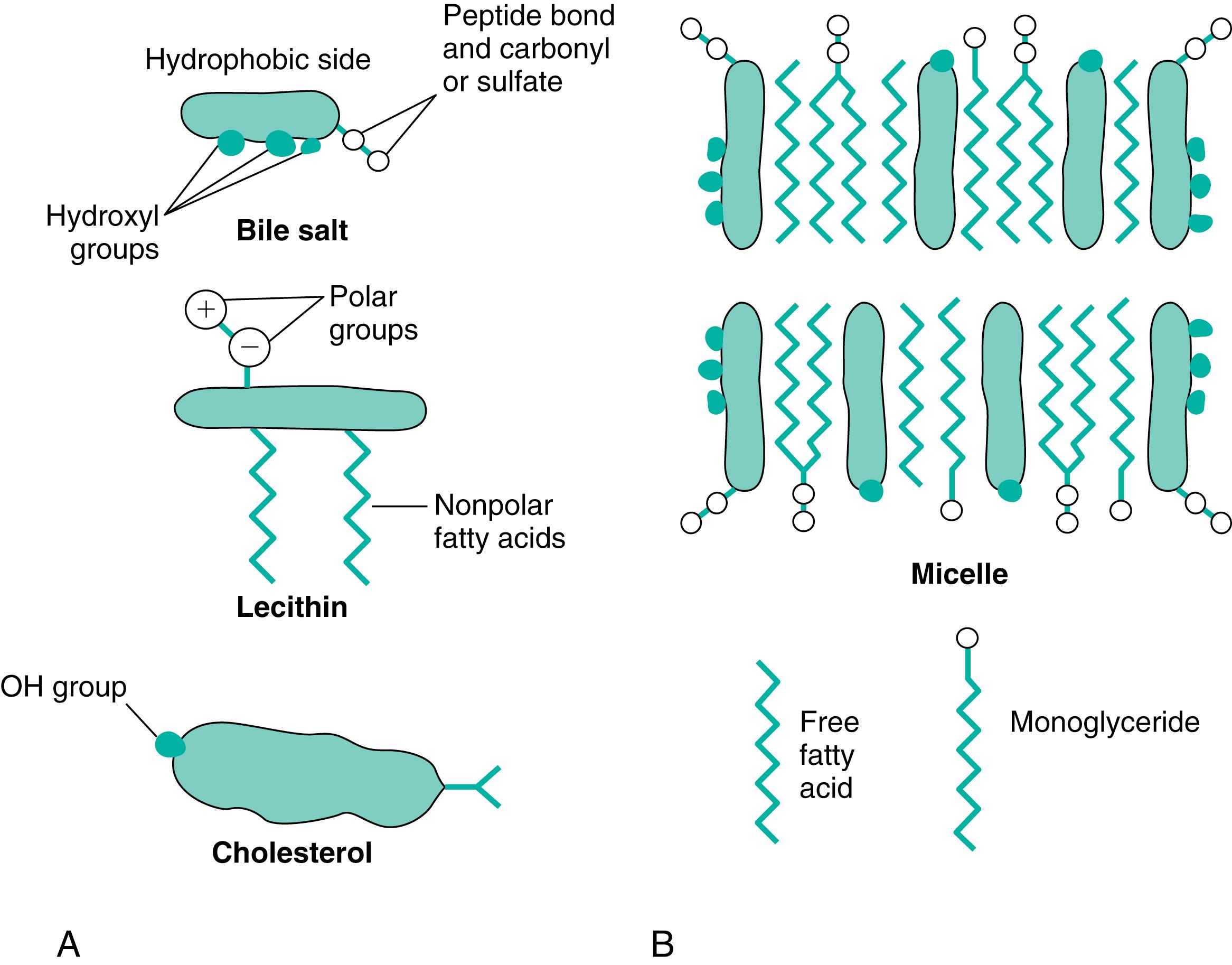
The second most abundant group of organic compounds in bile consists of the phospholipids , and the major ones are the lecithins (see Fig. 10.3 ). Phospholipids also are amphipathic, insofar as the phosphatidylcholine grouping is hydrophilic, whereas the fatty acid chains are hydrophobic. Although amphipathic, the phospholipids are not soluble in water but form liquid crystals that swell in solution. In the presence of bile salts, however, the liquid crystals are broken up and solubilized as a component of the micelles. Bile salts possess a large capacity to solubilize phospholipids; 2 moles (mol) of lecithin are solubilized by 1 mol of bile salts. The combination of bile salts and phospholipids also is better able to solubilize other lipids—mainly cholesterol and the products of fat digestion—than is a simple solution of bile salts.
A third organic component, cholesterol , is present in small amounts and contributes approximately 4% to the total solids of bile. Although present in small amounts, bile cholesterol is important because it may be excreted and therefore helps regulate body stores of cholesterol. Cholesterol appears mainly in the nonesterified form and is insoluble in water. In the presence of bile salts and phospholipids, however, it is solubilized as part of the micelle. Because it is a weakly polar substance, cholesterol is found in the interior of the micelle, where the hydrophobic portions of the bile salts, phospholipids, monoglycerides, and fatty acids interact (see Fig. 10.3 ). Within the liver, ducts, and gallbladder, bile is normally present as a micellar solution.
The fourth major group of organic compounds found in bile comprises the bile pigments . These constitute only 2% of the total solids, and bilirubin is the most important. Chemically, bile pigments are tetrapyrroles and are related to the porphyrins, from which they are derived. In their free form, bile pigments are insoluble in water. Normally, however, they are conjugated with glucuronic acid and are rendered soluble. Unlike the other organic compounds just mentioned, bile pigments do not take part in micellar formation. As their name implies, they are highly colored substances. Other than being responsible for the normal color of bile and feces, the pigment properties of these compounds are used to assess the level of function of the liver.
In addition to the organic compounds just discussed, many inorganic ions are found in bile. The predominant cation is Na + , accompanied by smaller amounts of potassium (K + ) and calcium (Ca 2+ ). The predominant inorganic anions are chloride (Cl − ) and
. Normally, the total number of inorganic cations exceeds the total number of inorganic anions. No anion deficit occurs, however, because the bile acids, which possess a net negative charge at the pH values found in bile, account for the difference. Bile is isosmotic even though the number of cations present is larger than expected. Because they are highly charged molecules, the bile acids attract a layer of cations that serve as counterions. These counterions are tightly associated with the micelles and thus exert little osmotic activity.
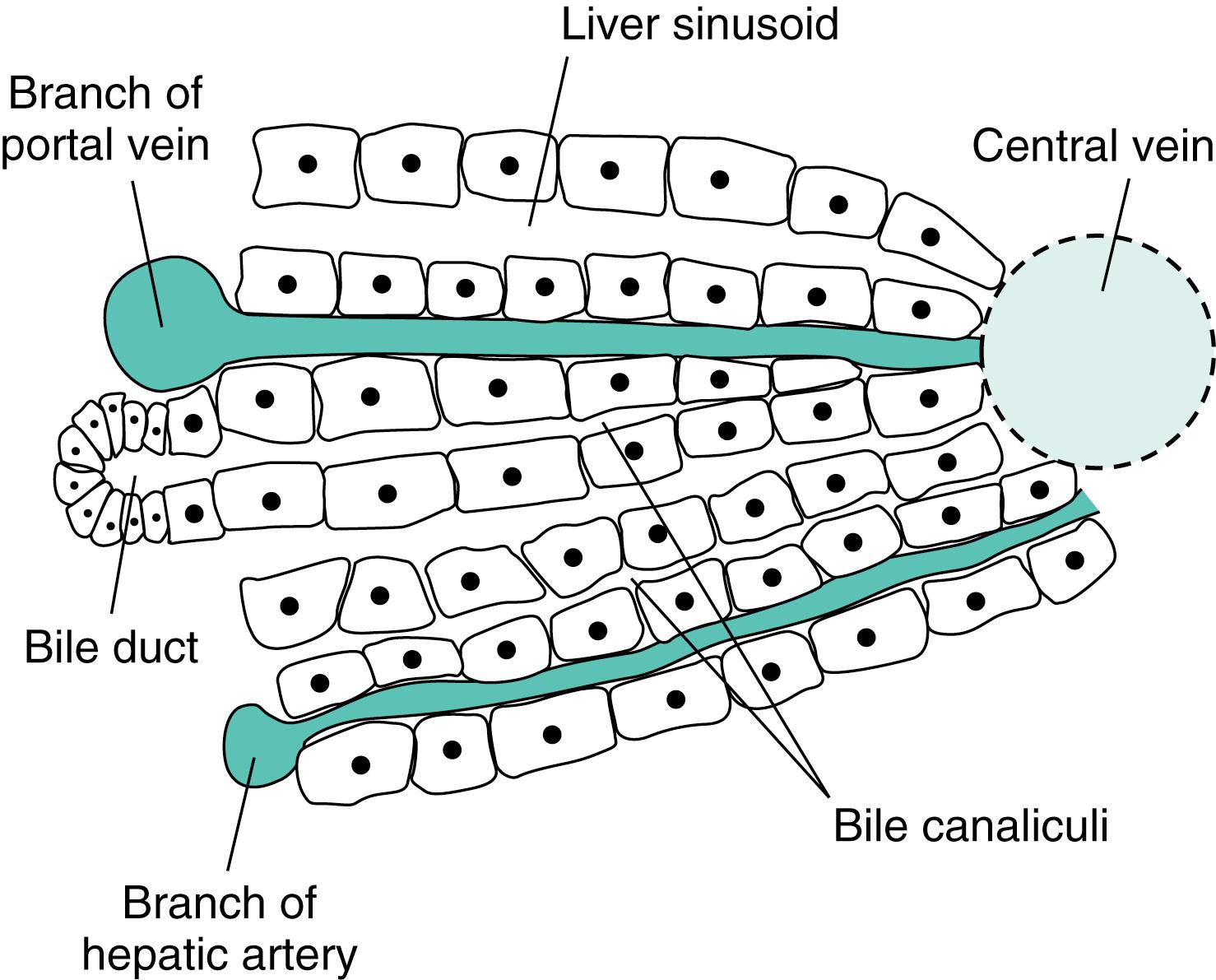
The functional organization of the liver is shown schematically in Fig. 10.4 . The liver is divided into lobules organized around a central vein that receives blood through separations surrounded by plates of hepatocytes. The separations are called sinusoids, and they in turn are supplied by blood from both the portal vein and the hepatic artery. The plates of hepatocytes are no more than two cells thick, so every hepatocyte is exposed to blood. Openings between the plates ensure that the blood is exposed to a large surface area. The hepatocytes remove substances from the blood and secrete them into the biliary canaliculi lying between the adjacent hepatocytes. The bile flows toward the periphery, countercurrent to the flow of the blood, and drains into bile ducts. This countercurrent relationship minimizes the concentration differences between substances in the blood and in the bile and contributes to the liver’s efficiency in extracting substances from the blood.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here