Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter discusses benign and malignant renal lesions ( Box 63-1 ) with a separate note on cystic lesions based on the Bosniak classification.
Simple renal cyst
Oncocytoma
Angiomyolipoma
Leiomyoma
Mesoblastic nephroma
Adenoma
Renal parenchymal tumors, including renal cell carcinoma subtypes
Urothelial carcinoma
Secondary renal tumors
Lymphoma and leukemia
Metastatic lesions
Pediatric malignant tumors
Wilms' tumor
Nephroblastomatosis
Clear cell sarcoma
Rhabdoid tumor
The incidental detection of cystic renal lesions has dramatically increased with the widespread use of ultrasonography and cross-sectional imaging. As many as 27% of patients older than 50 years have a benign renal cyst on computed tomography (CT) examination. Using strict criteria and proper technique, a simple renal cyst can be diagnosed using ultrasonography, CT, and magnetic resonance imaging (MRI).
The sonographic criteria for a simple cyst are well established and include (1) anechoic contents, (2) sharp smooth walls, (3) posterior acoustic enhancement, and (4) lack of internal blood flow.
Findings characteristic of a simple renal cyst on CT are (1) water density (−20 to 20 Hounsfield units [HU]) on precontrast images, (2) smooth thin borders with a sharp interface with the adjacent renal parenchyma, and (3) does not enhance with contrast.
A lesion can be categorized as a simple renal cyst on MRI, if the lesion (1) has a signal intensity that follows simple fluid, (2) has smooth thin borders with a sharp interface with the adjacent renal parenchyma, and (3) does not enhance on postcontrast images.
A renal cyst that meets each of the criteria for any given imaging modality can be confidently diagnosed as a benign simple cyst and requires no additional evaluation ( Figure 63-1 ).
Angiomyolipoma is a benign renal tumor composed of varying amounts of blood vessels, fat, and smooth muscle. The majority of angiomyolipomas are sporadic (80%) and are typically identified in adults, with a strong female predilection as compared to men with a ratio as high as 4 : 1. A smaller percentage of approximately 20% are associated with tuberous sclerosis complex, and it can occur in as many as 55% 75% of patients with it.
Angiomyolipomas are often an incidental finding on imaging. In a minority of cases, symptoms such as flank pain, nausea, vomiting, and fever are produced by mass effect and intratumoral or perirenal hemorrhage. The risk for bleeding has been reported to be proportional to the size of the lesion once it is larger than 4 cm in diameter.
Isolated angiomyolipomas are usually solitary, whereas angiomyolipomas associated with tuberous sclerosis are often multiple, larger, and bilateral. The majority of these tumors are intraparenchymal, but 25% are exophytic.
Angiomyolipomas belong to the PEComa family. PEComas are considered a family of perivascular epithelioid cell (PEC) tumors (PEComa) and are essentially mesenchymal neoplasms. Abnormalities of the blood vessels associated with angiomyolipomas explain the propensity for these lesions to hemorrhage.
On gross examination, angiomyolipomas are composed predominantly of fat and have a homogeneous yellow appearance. Tumors with more varied proportions of fat, smooth muscle, and blood vessels have a heterogeneous gross pathologic appearance. Angiomyolipomas are often well circumscribed but lack a true capsule.
The imaging appearance of angiomyolipomas varies significantly because of its variable composition and can cause a diverse radiographic appearance of these lesions. However, when a large fat component is present, the imaging characteristics are usually pathognomonic and vice versa.
Intravenous urography and plain radiography are generally not sensitive techniques for identifying these tumors.
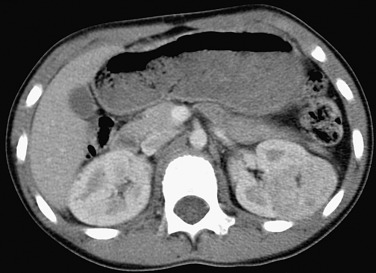
The value of CT is in identifying any fat content present in these lesions because the presence of intratumoral fat is nearly pathognomonic of angiomyolipoma ( Figure 63-2 ). Lesions with a component demonstrating an attenuation value of less than 20 Hounsfield units (HU) are typical of angiomyolipoma on unenhanced CT. Typically, the lesion is a well-defined, cortical, heterogeneous mass with a fat component. The presence of hemorrhage may cause the lesion to appear poorly defined. Soft tissue attenuation of the lesion may be due to hemorrhage, smooth muscle, or fibrosis.
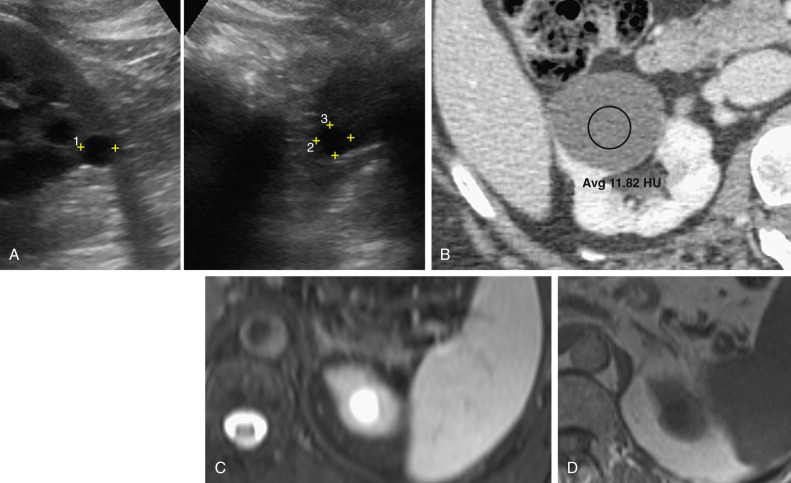
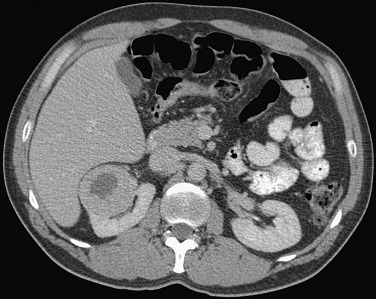
Approximately 5% of angiomyolipomas have insufficient fat to be recognized on CT and cannot be distinguished from renal cell carcinoma (RCC). Angiomyolipomas without visible fat appear homogeneous and demonstrate higher attenuation than adjacent normal renal parenchyma ( Figure 63-3 ). These lesions also can demonstrate contrast enhancement because of the relatively larger smooth muscle and vascular components.
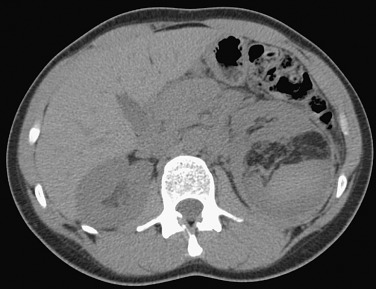
Calcification is rarely seen but may be present after hemorrhage. In the presence of significant calcification, the diagnosis of angiomyolipoma should be reconsidered.
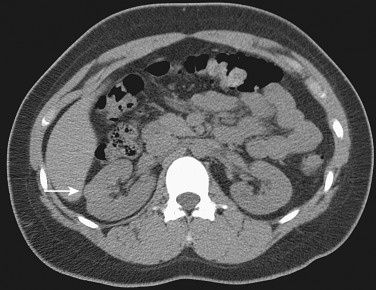
CT is useful to evaluate for retroperitoneal hemorrhage that may be associated with angiomyolipomas ( Figure 63-4 ). Blood products may obscure the fat content of the lesion, and in these cases carcinoma cannot be excluded.
MRI can be used to depict intratumoral fat. Variable areas of high signal are seen on T1- and T2-weighted images owing to the fat content. However, high signal intensity on T1-weighted images also can be seen with high protein content or hemorrhage. In these cases, fat suppression techniques are useful. Israel and coworkers showed that angiomyolipomas also can be diagnosed using opposed-phase chemical shift MRI in which an India ink artifact at the mass/kidney interface or within a renal mass is suggestive of the tumor ( Figure 63-5 ). Clear cell RCC also may demonstrate signal loss on opposed-phase imaging; therefore, this is not a specific finding for angiomyolipoma.
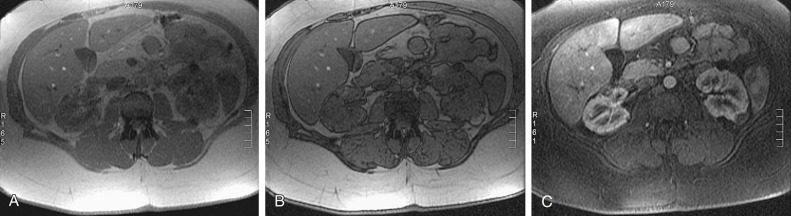
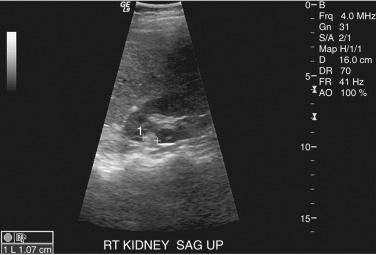
The typical ultrasound appearance of an angiomyolipoma is a well-defined hyperechoic mass without acoustic shadowing ( Figure 63-6 ). This echogenicity is not necessarily due to the fat component because some lesions that contain little or no fat also can be echogenic. Although RCC can appear echogenic, angiomyolipomas are more likely to show posterior shadowing. These differences, however, cannot distinguish between angiomyolipomas and carcinoma with the same degree of confidence as CT.
Nuclear medicine has a limited role in the assessment of angiomyolipoma. When surgical management is indicated, it can be used to assess differential renal function.
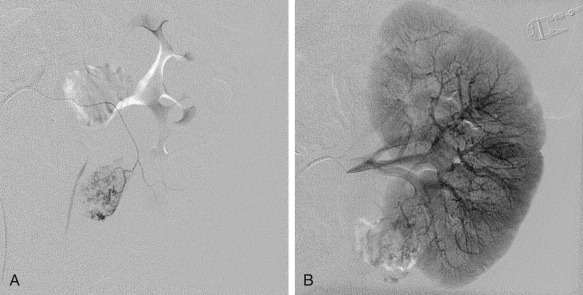
Angiography characteristically demonstrates clusters of saccular microaneurysms or macroaneurysms. Other findings on angiography include hypervascularity, venous pooling with a whorled appearance, and lack of arteriovenous shunting ( Figure 63-7 ).
Thin-section noncontrast CT is the preferred and most widely available method for detection of angiomyolipoma because demonstration of fat is pathognomonic. Although MRI can detect fat within an angiomyolipoma, it is not as sensitive as CT for detecting fat in small lesions. Opposed-phase chemical shift MRI has been investigated and found to be useful as a method for detecting angiomyolipomas ( Table 63-1 ).
Fat-containing lesion on CT and MRI
Well-circumscribed echogenic mass with posterior shadowing evident on ultrasonography
Retroperitoneal hemorrhage associated with a renal lesion (usually >4 cm)
| Modality | Accuracy | Limitations | Pitfalls |
|---|---|---|---|
| Radiography | Poor | Limited sensitivity unless tumor is large and is predominantly fat, which may be seen as radiolucency | Exophytic mass (25%) may not be seen as a space-occupying lesion in the renal sinus |
| CT | High | Minimal fat angiomyolipomas Hemorrhage may mask intratumoral fat |
Minimal fat angiomyolipomas Rarely, other benign and malignant lesions may contain fat |
| MRI | High | Failure to detect small lesions or small amount of intratumoral fat because of volume-averaging artifacts and limitations of spatial resolution | Same as for CT Clear cell renal cell carcinoma may demonstrate signal loss on opposed-phase imaging |
| Ultrasonography | Cannot assess for actual presence of fat because hyperechogenicity is not necessarily caused by fat | Echogenic renal cell carcinomas |
Rare lesions that can contain fat include perirenal fat entrapment or fat necrosis, which can occur in RCC. Other rare entities include myolipoma, liposarcoma, lipoma, oncocytoma, and Wilms' tumor. In the absence of intratumoral fat, the differential diagnosis includes RCC, leiomyosarcoma, and malignant epithelioid angiomyolipoma.
When lesions are small, asymptomatic, and discovered incidentally, no treatment is necessary. Larger lesions if considered at risk for hemorrhage can be prophylactically embolized. In severe acute presentation, total nephrectomy may be required if conservative measures fail.
Most commonly this is an incidental diagnosis in asymptomatic patients.
Lesions greater than 4 cm have increased risk for hemorrhage.
These tumors are often multiple and bilateral in patients with tuberous sclerosis.
Oncocytomas arise from the distal tubule or collecting ducts of the kidney. An oncocyte is a large transformed epithelial cell with a finely granular eosinophilic cytoplasm. These cells increase in number with age and are seen in many organs.
Oncocytomas comprise 3% to 7% of all renal neoplasms. Patients typically present in their sixth to seventh decades. The tumors are more common in males than females in a ratio of 2 : 1.
As many as 75% of oncocytomas are asymptomatic and incidentally diagnosed, but patients may uncommonly present with a flank mass, pain, or hematuria.
Most oncocytomas are solitary. Approximately 3% are bilateral and 5% are multicentric within the same kidney. They average 7 cm in diameter and are most often symptomatic when larger than 5 cm.
These tumors are well encapsulated and classically have a central scar. On gross inspection, they are tan-brown similar to the renal cortex and well defined. Necrosis, hemorrhage, and calcification are rare.
Plain radiographic findings are nonspecific and include a soft tissue mass distorting the renal silhouette with displacement of the fat planes. Calcification is an uncommon feature.
The classic findings are a spokewheel arrangement of vessels, homogeneous dense tumor blush during the capillary phase, and sharp demarcation from the kidney. Bizarre neoplastic vessels are absent, in contrast to RCC.
An oncocytoma typically appears as a well-defined solid renal mass with smooth margins. A central stellate scar is seen in one third of cases, but this is a nonspecific finding that cannot be differentiated from central necrosis in RCC.
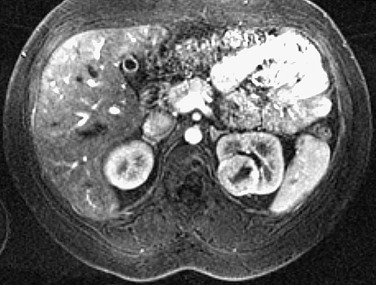
On noncontrast CT, oncocytoma is isodense or hyperdense relative to normal renal parenchyma. On the nephrographic phase of contrast-enhanced CT, this tumor is most commonly hypodense to renal parenchyma ( Figure 63-8 ).
Typically, oncocytoma demonstrates low signal on T1-weighted images and high signal on T2-weighted images. The central scar, if present, shows as a low T1 and a low T2 signal, in contrast to necrosis in RCC, which usually has a low T1 and a high T2 signal.
After administration of a contrast agent, oncocytoma typically demonstrates homogeneous enhancement. The central scar generally does not enhance ( Figure 63-9 ).
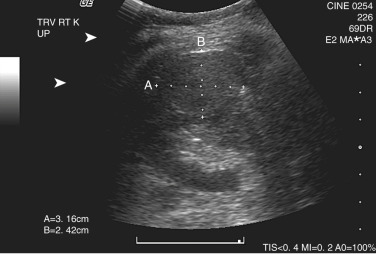
On ultrasonography, an oncocytoma shows as a well-defined hypoechoic to isoechoic mass ( Figure 63-10 ). If visualized, the central scar may appear echogenic. Doppler imaging may show central radiating vessels in a spokewheel distribution.
Nuclear medicine is not routinely used in the evaluation of renal tumors.
PET/CT is not routinely performed for evaluation of oncocytoma. It may show equal to slightly higher uptake of fluorodeoxyglucose (FDG) than normal renal parenchyma.
Contrast-enhanced CT is the examination of choice for evaluating solid renal masses. Evaluation for vascular involvement, lymphadenopathy, and extent of the lesion is possible ( Table 63-2 ).
Central scar on CT and MRI
Spokewheel pattern of vessels on angiography and Doppler imaging
| Modality | Accuracy | Limitations | Pitfalls |
|---|---|---|---|
| Radiography | Poor sensitivity | Cannot distinguish from other space-occupying renal masses | |
| CT | Sensitive but not specific | Cannot distinguish from renal cell carcinoma | Central necrosis of renal cell carcinoma can mimic central scar of oncocytoma. |
| MRI | Sensitive but not specific | Cannot distinguish from renal cell carcinoma | Central scar is not specific for oncocytoma. |
| Ultrasonography | Poor sensitivity | Small isoechoic lesions may be missed. Larger lesions cannot be distinguished from other renal masses. | |
| Nuclear medicine | Poor sensitivity | ||
| PET/CT | Poor sensitivity |
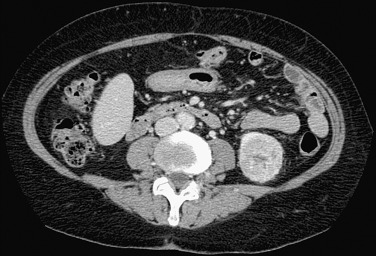
The main differential diagnostic consideration is RCC. Although central scar on CT/MRI and central radiating vessels on angiography and Doppler imaging are suggestive of oncocytoma, RCC cannot be excluded and histopathologic diagnosis is necessary ( Figure 63-11 ).
If radiographic findings suggest the possibility of oncocytoma, percutaneous biopsy may be considered. It is controversial whether oncocytoma can be reliably differentiated from oncocytic RCC on biopsy. Consequently, many clinicians choose pathologic sampling with partial or total nephrectomy. Many oncocytic RCCs are of low metastatic potential and include granular cell carcinoma, chromophobe RCC, and the eosinophilic variant of papillary RCC. Notably, oncocytoma cannot always be reliably distinguished from oncocytic RCC on pathologic examination.
Statistically, RCC is more common than oncocytoma.
Because of overlap in imaging and pathologic findings between oncocytoma and RCC, preoperative diagnosis remains difficult.
Whether oncocytoma can be reliably diagnosed on biopsy is controversial.
Mesoblastic nephroma is usually present at birth. It also has been called congenital Wilms' tumor or fetal mesenchymal hamartoma.
This mesenchymal renal tumor is typically benign but may demonstrate aggressive features such as local invasion or recurrence. There is a male predominance with a peak age at presentation of 3 months. Mesoblastic nephroma is the most common benign fetal renal neoplasm.
The most common manifestation is a large, palpable abdominal mass in a neonate. Less common signs and symptoms include hematuria, hypertension, vomiting, and hypercalcemia. Prenatal hydrops and polyhydramnios also may be seen.
On gross examination this tumor has a homogeneous rubbery appearance. The cut surface has a whorled appearance similar to that of uterine leiomyoma. Hemorrhage and necrosis are uncommon. Histologically, there are sheets of fibromatous cells. There is an aggressive variant that is highly cellular with immature mesenchymal cells and a high number of active mitotic figures. This variant has a poorer prognosis and is usually seen in infants and children older than 3 months of age.
A large mass may be seen involving the entire kidney. Calcification is uncommon, and radiography is not a reliable modality for evaluating for the presence of renal tumors.
A solid, homogeneous mass arising from the kidney that may replace all or part of the involved kidney. There can be areas of necrosis, although this is uncommon. The tumor does not enhance after contrast agent administration. However, entrapment of nephrons within the mass may cause excretion of contrast agent within the mass. No calcification is evident.
There is low signal intensity on T1-weighted imaging, and the tumor is typically nonenhancing.
A well-circumscribed, homogeneous and hyperechoic mass is evident. Concentric hypoechoic and hyperechoic rings are a helpful imaging feature.
Prenatal or pediatric ultrasonography is usually the first imaging study performed when the abdominal mass is palpated. Ultrasonography is easily and widely available; it is inexpensive and involves no ionizing radiation. CT has an important role in evaluating recurrent or metastatic disease.
This homogeneous solid renal mass affects neonates.
The cut surface resembles that of uterine leiomyoma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here