Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lung cancer remains the leading cause of cancer death in the United States, with 236,000 new cases estimated annually and 130,180 estimated deaths (about 350 deaths per day). Benign and borderline malignant tumors of the lung are far outnumbered by their malignant counterparts and because of this less attention has been given to them. Nevertheless, because they often simulate malignant disease on imaging and are subject to biopsy, knowledge of their appearance under the microscope is an absolute requirement for the practicing pathologist. These neoplasms have little in common with each other but comprise an interesting array of lesions with diverse lineages and unknown etiologies in most cases. This chapter provides an overview of such tumors, with an emphasis on the differential diagnosis when they are encountered.
The clinical characteristics of benign tumors in the lower respiratory tract can be considered in an overview because they are generally not specific to any particular diagnosis. Most benign intrapulmonary lesions are associated with no symptoms or signs whatsoever and are found incidentally with screening radiographic studies. As discussed elsewhere in this book, hamartomas are often separable from other benign but truly neoplastic masses of the lung on imaging studies because of their common content of distinctive calcifications, fat densities, or both ( Fig. 20.1 ). Otherwise, the radiologist is not typically able to distinguish between specific histologic entities in this context. Endotracheal and endobronchial tumors are more often related to clinical symptoms such as wheezing, hemoptysis, obstructive pneumonia, and postobstructive pulmonary hyperinflation, depending on their anatomic location.
In the past, radiologists were comfortable simply observing a pulmonary mass over time, if it had reassuring morphologic characteristics. In fact, statistical paradigms have been constructed to aid in this process. However, because of the unfortunate pressure of litigation involving putative delays in diagnosis of malignant neoplasms at various sites —together with the modern availability of techniques such as video-assisted thoracoscopic surgery —many more benign lesions of the lungs are excised today than in previous years.
Bronchoscopic examination and endobronchial biopsy are productive diagnostically if the tumor in question protrudes significantly into the lumen of the airway. However, brushing cytology is only variably effective in sampling such growths, depending on whether the mucosa over them is intact and the level of intercellular cohesion in the tumor itself. Transthoracic or transbronchial aspirates or needle biopsies are usually necessary for adequate sampling of deeply seated masses in the parenchyma. In this setting, these procedures are most effective in the diagnosis of malignant neoplasms; if the pathologist sees only histologically banal tissue elements in the latter specimens, it is often impossible to discern whether they truly represent the lesion or are simply part of the adjacent lung. Moreover, in small biopsy specimens, the morphologic attributes of some cytologically low-grade malignancies may be virtually identical to those of benign tumors belonging to the same general cellular lineage.
The World Health Organization (WHO) has lumped squamous, glandular, and squamoglandular papillomas into a single entity of bronchial papilloma. These lesions are characterized by their endobronchial growth location, exophytic growth architecture, and variable presence of squamous and glandular components. Squamous papilloma of the tracheobronchial tree (SPTT) is the most commonly encountered variety.
SPTT most often arises in adults—typically middle-aged—with a slight male predominance. Occasionally, even though only one papillomatous lesion of the lower respiratory tract may be present, it may coexist with papillary squamous proliferations of the larynx or oropharynx.
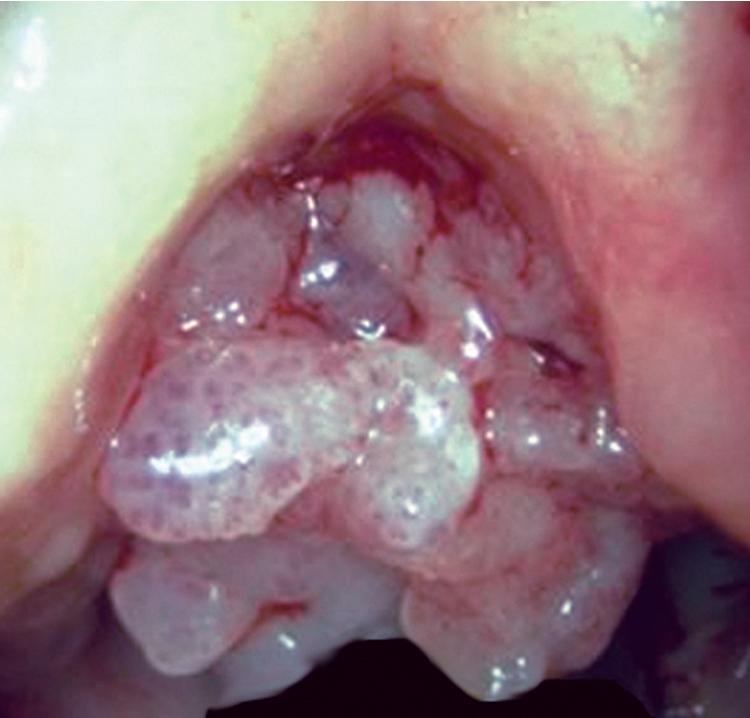
Grossly, SPTTs form a pedunculated tan-white polypoid excrescence in the mucosa of the airway, with variable luminal compromise. The surface may be smooth or slightly verrucoid.
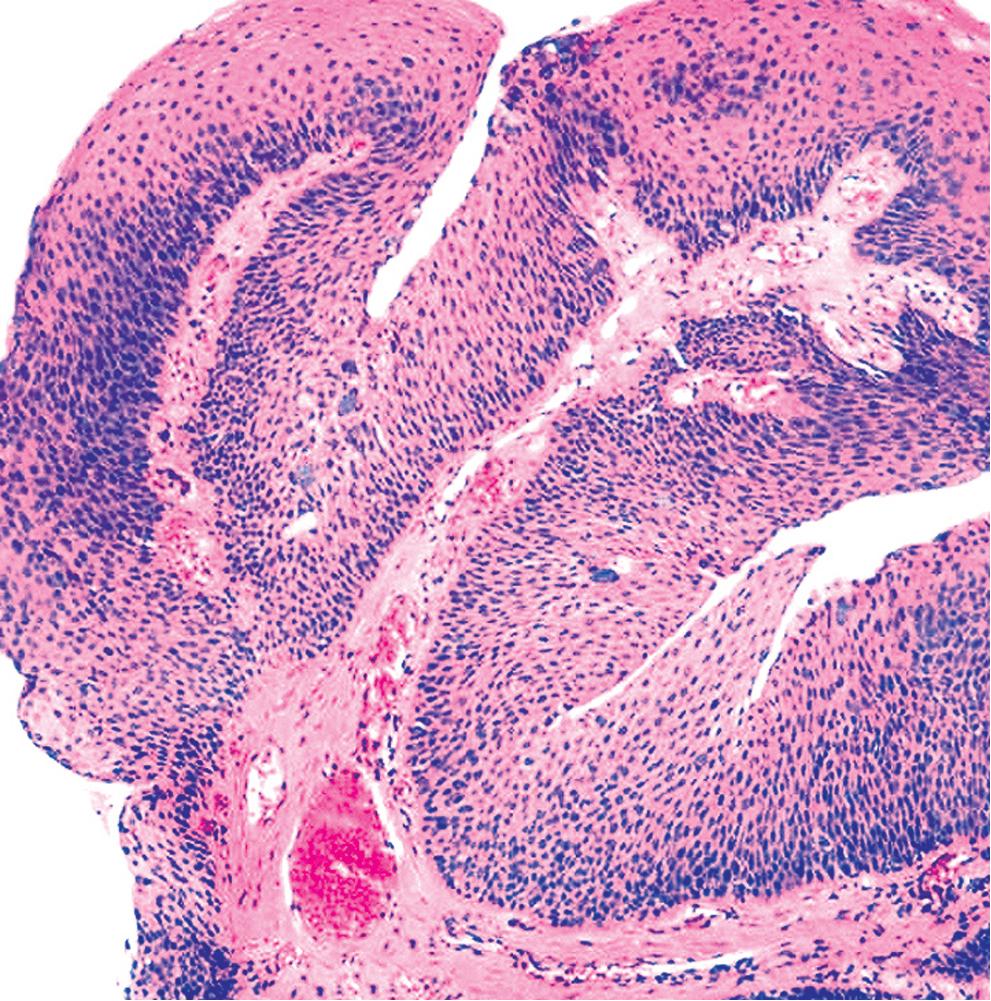
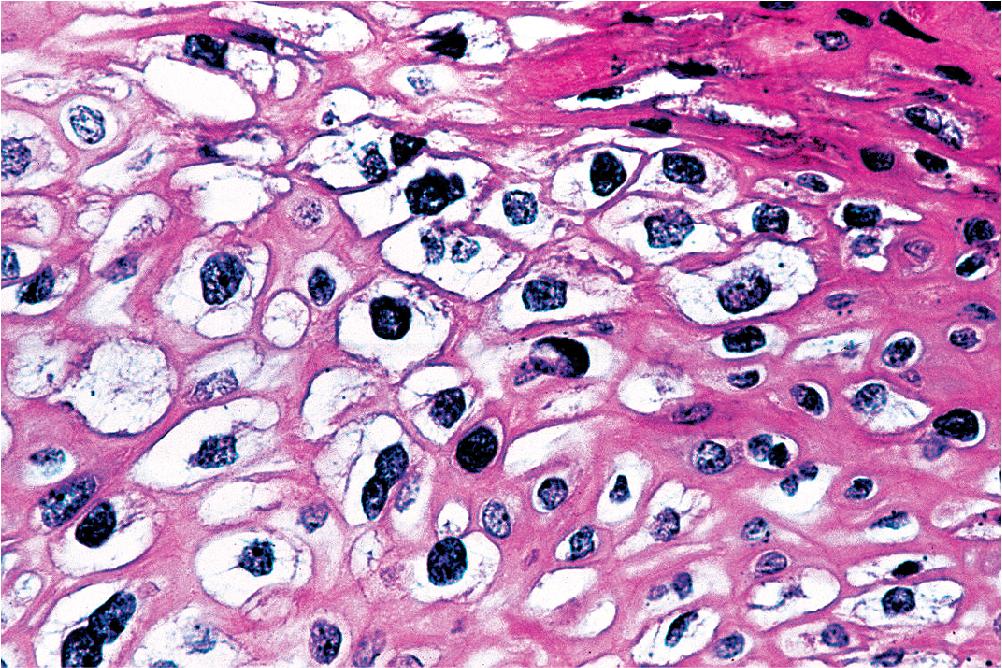
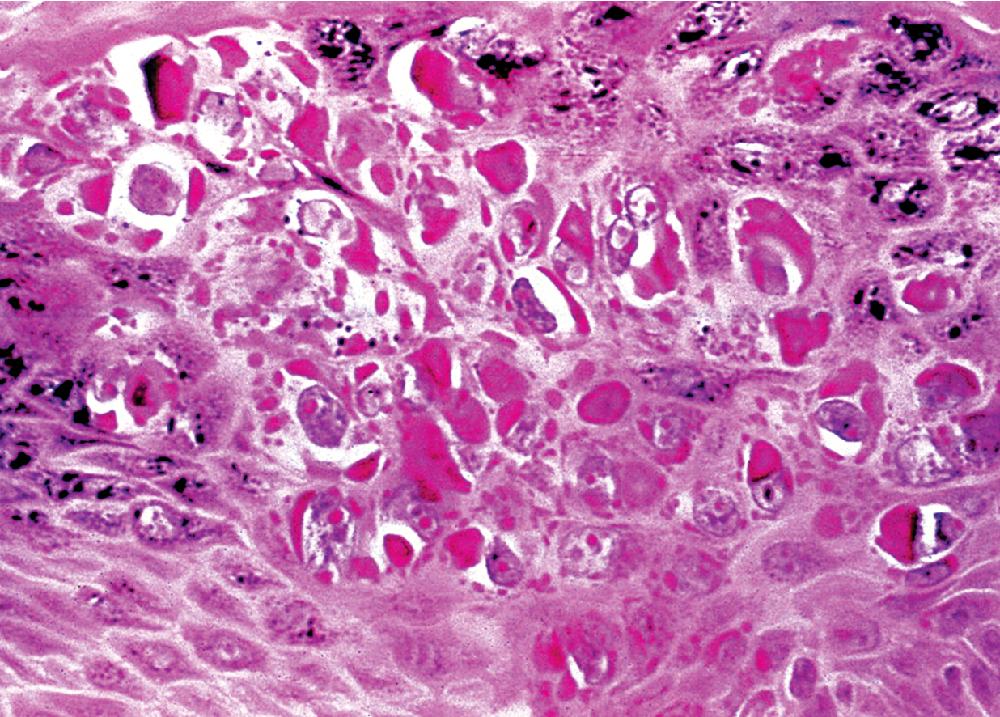
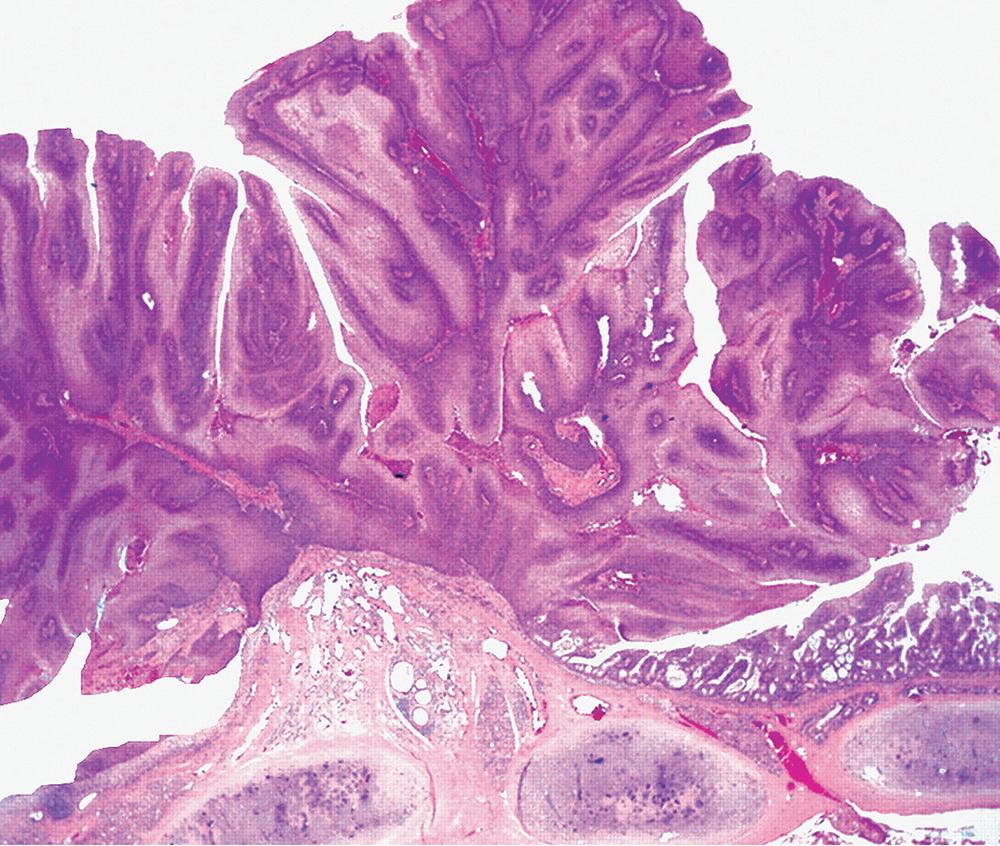
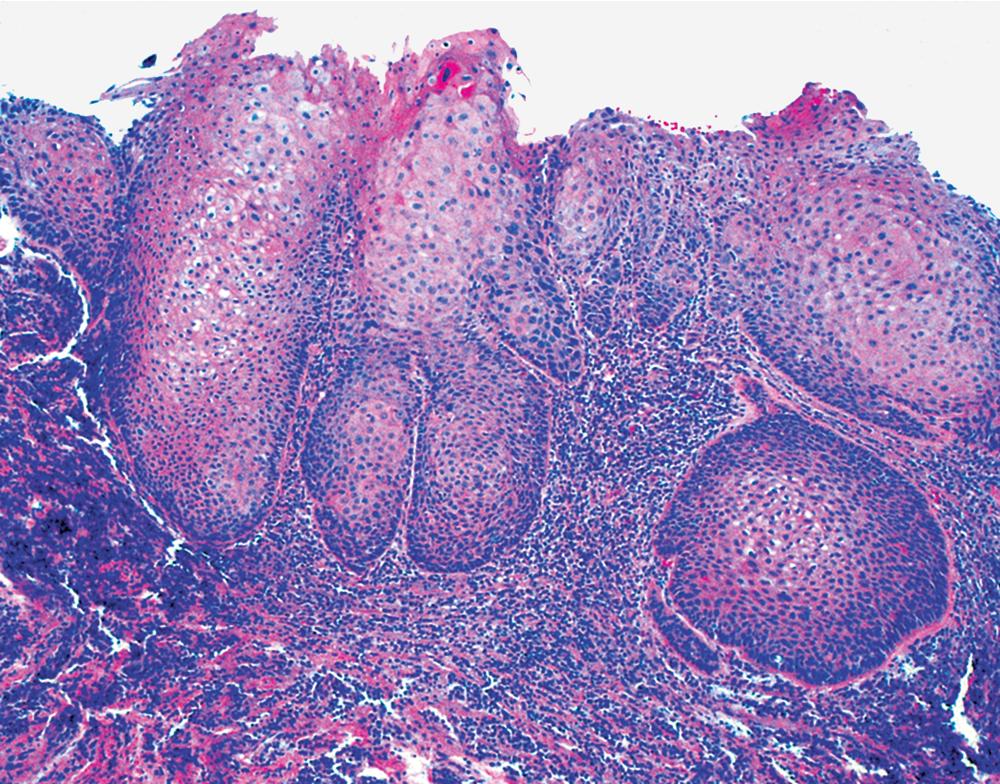
Microscopically, SPTTs are similar to viral papillomas elsewhere in the body, particularly in the genitoperineal region. They are constituted by arborizing fronds with fibrovascular cores, mantled by relatively bland squamous epithelial cells ( Fig. 20.2 ). These often exhibit nuclear hyperchromasia and crenation, but the nuclear chromatin may be homogenized and glassy. The cytoplasm is commonly unremarkable; alternatively, it may show perinuclear clearing, eosinophilic globular inclusions, or hypergranulation with clumping of keratohyaline granules ( Figs. 20.3 and 20.4 ). Nuclear atypia has been observed in squamous SPTT, and squamous carcinoma may develop in this setting. ,
Another even rarer variant of SPTT is the columnar papilloma, composed of columnar epithelial cells rather than squamous elements. Putatively, it has no potential for malignant change.
Interestingly, the biologic associations between squamous SPTT and human papillomavirus (HPV) types are comparable to those in the genital tract. Specifically, HPV types 7 and 11 are most commonly seen in uncomplicated SPTT; in contrast, HPV types 16 and 18 are associated with dysplastic nuclear features and a higher risk of carcinomatous transformation. , The presence of these viral agents can be evaluated using in situ hybridization ( Fig. 20.5 ), the hybrid capture method, or polymerase chain reaction.
In light of the usual behavior of SPTT, conservative therapeutic approaches, such as endoscopic removal, cryotherapy, or fulguration, are typically applied. Lesions in which malignancy develops must be treated in a manner appropriate for ordinary lung cancers ( Fig. 20.6 ).
Glandular papilloma are comprised of papillary fibrovascular cores lined by stratified or pseudostratified glandular epithelium lacking cilia. The epithelium is stratified or pseudostratified and cells are either cuboidal or columnar. A variable number of cells with mucin vacuoles may be present.
Squamoglandular papilloma have various components of being both squamous proliferations and glandular proliferations.
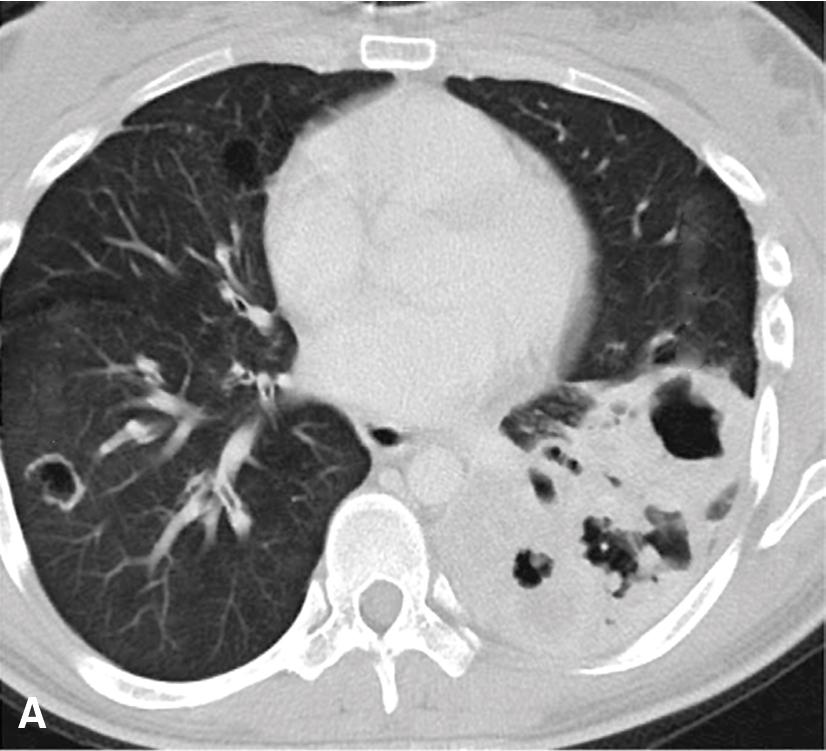
Respiratory tract papillomatosis (RTP) is the multifocal form of viral papillomagenesis, as described earlier. Its onset is early in life because the mode of transmission is natal inhalation of virally infected genital tract secretions during vaginal delivery. Children with RTP typically have oropharyngeal or laryngeal disease initially; it may remain localized or spread to involve the lower airways as well, as seen in 5% of cases. In the latter instance, growth into the lung parenchyma may supervene, with the eventual appearance of cavitary lesions that can simulate carcinomas radiographically. , Obstruction of the tracheal or bronchial lumina is associated with recurrent pneumonia, hemoptysis, and asthma-like symptoms. Even though RTP is often said to be recurring, the entire respiratory tract is at risk for infection by HPV in this disorder. Thus it is more apropos to consider separate lesions as metachronously or synchronously independent of one another pathogenetically.
The gross and histologic features of RTP are largely the same as those of solitary respiratory tract papilloma (SRTP) ( Figs. 20.7 and 20.8 ). Exceptions include the multiplicity of lesions and the greater tendency for “inverting” or tissue-destructive growth of the papillomatous lesions in RTP ( Fig. 20.9 ). The HPV profiles of RTP and SSTP are comparable, with the notable proviso that HPV-11 is overwhelmingly the most common viral type seen in RTP. Mutations of the p53 gene have been linked to malignant transformation of individual lesions in both conditions. , , ,
It does not appear as though treatment with HPV-targeted antiviral medications (specifically, cidofovir) produced morphologic changes in persistent papillomatous lesions of the airway.
The incidence of previous HPV integration was assessed by Clavel et al., using the hybrid capture method in a series of bronchopulmonary carcinomas. They found evidence of oncogenic HPV integration in only 2.7% of those tumors. On the other hand, Yousem et al. demonstrated similar positivity by in situ hybridization in 30% of squamous carcinomas and 17% of large cell undifferentiated carcinomas of the lung; Syrjanen likewise observed histologic viral changes in adjacent metaplastic bronchial mucosa in 26 of 104 squamous carcinomas (25%). Based on these data, it must be acknowledged that HPV may play a greater role in the etiology of lung carcinomas (particularly of the squamous type) than previously thought.
The term bronchial adenoma has been plagued by many misconceptions and misapplications since its introduction by Liebow in 1952. However, two benign neoplastic entities could still properly be called bronchial adenomas—mucous gland adenoma (MGA) and mixed tumor (MT; pleomorphic adenoma; discussed later).
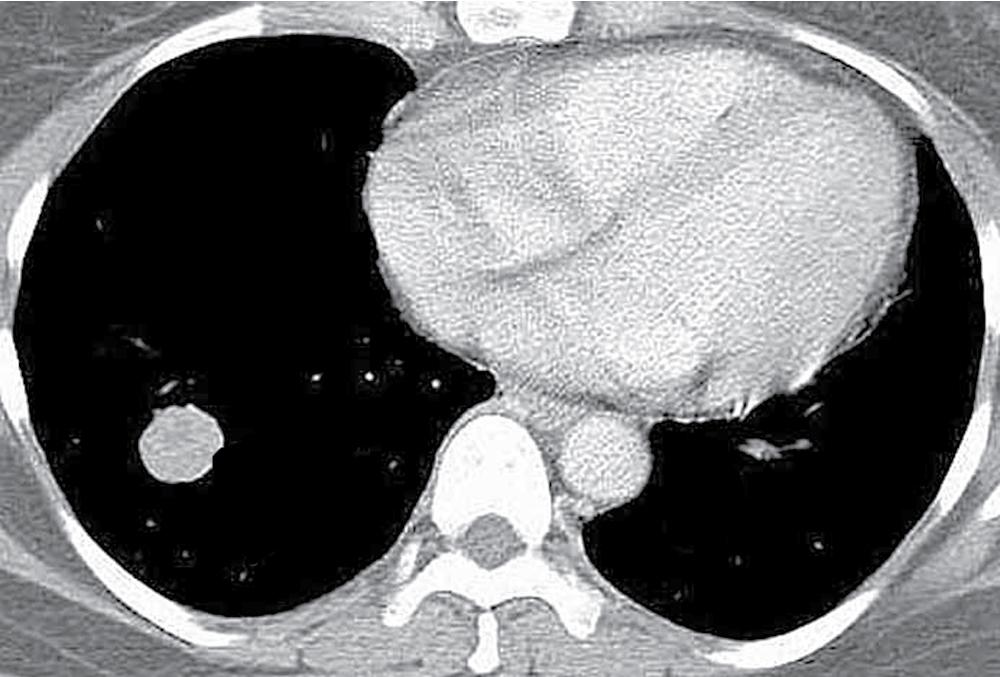
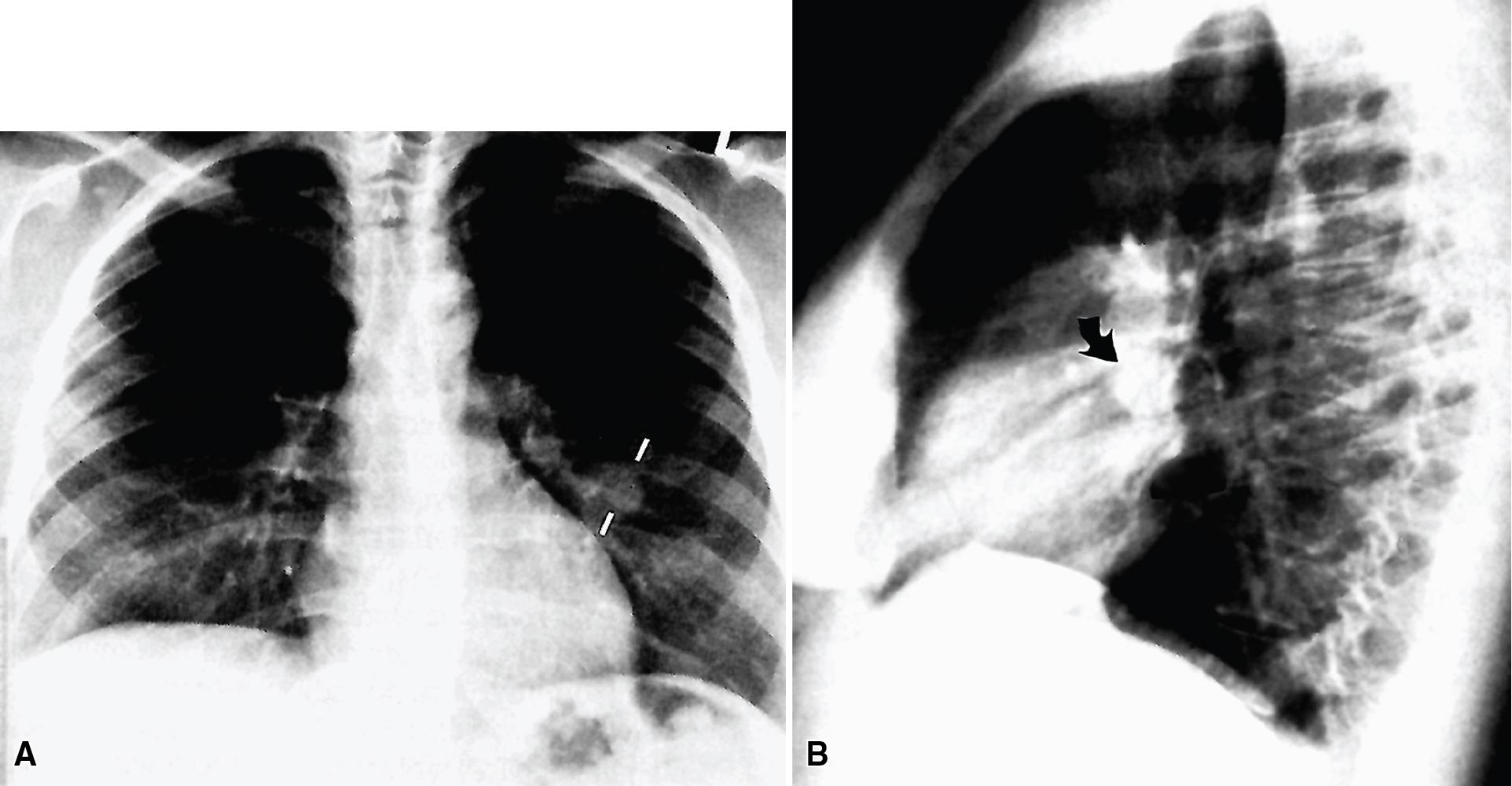
MGA is an extraordinarily rare lesion. England and Hochholzer noted that one series of more than 3000 pulmonary tumors included no examples of MGA, and only 1 MGA was represented in another report of 130 benign neoplasms of the lung seen at a large referral center. In the vast experience of the US Armed Forces Institute of Pathology, only 10 examples of MGA were found. Men and women were equally affected, and the patients ranged in age from 25 to 67 years. , There is no particular predilection for anatomic location; MGAs may arise in any of the major lobar or segmental bronchi. Radiographs either show changes of postobstructive pneumonia or hyperinflation, or demonstrate a discrete nodule or coin lesion that is centered on a bronchus ( Fig. 20.10 ). Kwon et al. have noted that MGA may produce the “air meniscus sign” on computed tomogram (CT) of the airways.
MGAs measure between 0.5 and 1 cm in maximal dimension. They often demonstrate encapsulation, with mucoid cut surfaces; internal fibrous septations may yield a loculated appearance as well. Occasional lesions may completely occlude the bronchial lumen ( Fig. 20.11 ).
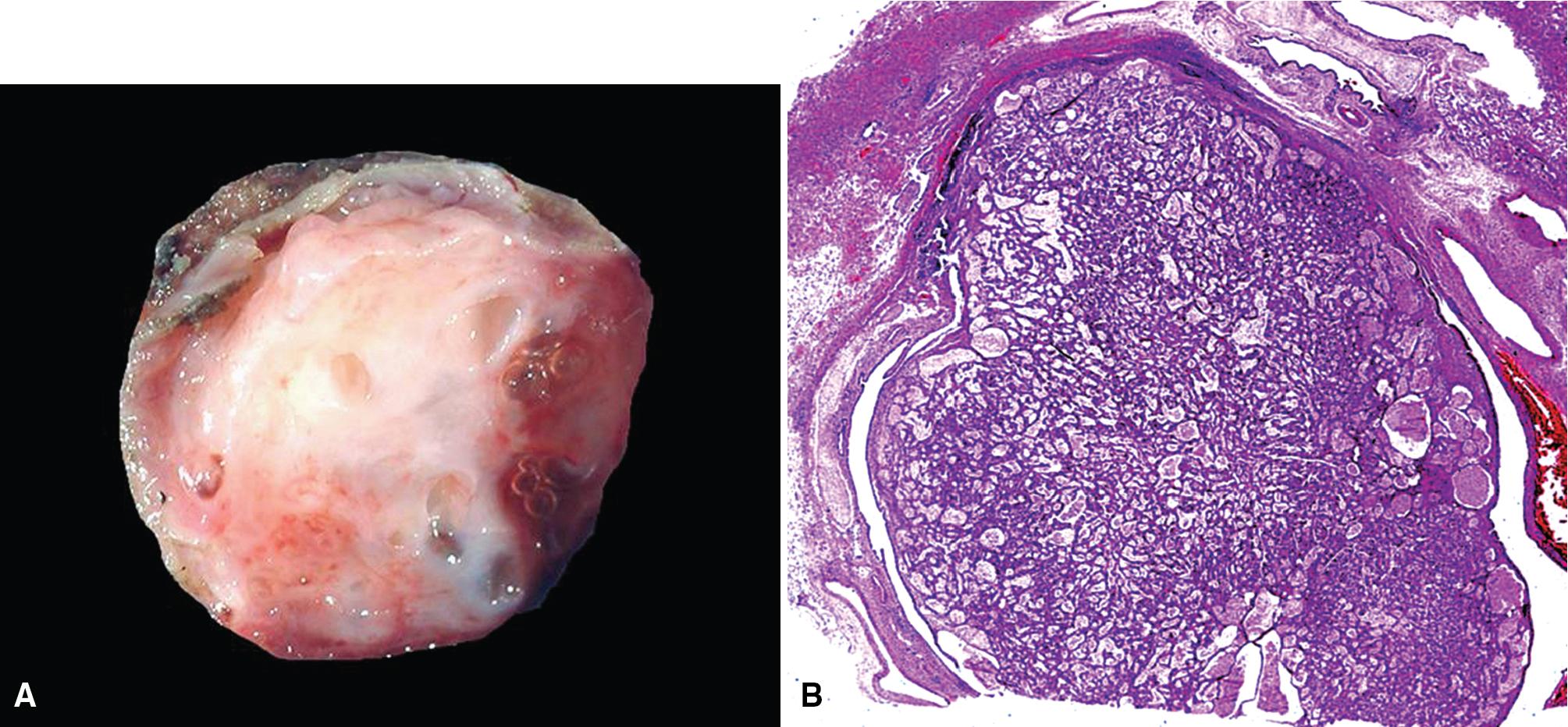
As succinctly stated by England and Hochholzer, “cystic change [is] the cardinal feature of MGA of the bronchus.” Microcystic arrays of cuboidal or columnar tumor cells may permeate the bronchial wall to the level of the cartilaginous plates; however, growth beyond that point is absent. Cystic contents are either overtly mucinous or more serous. Secondary formation of cholesterol clefts and dystrophic calcifications may also be seen, and squamous metaplasia in the most luminal aspect of the lesion may occur. Nuclei are generally bland, with small nucleoli, and cytoplasm may be amphophilic, oxyphilic, clear, or foamy. Mitotic figures are scarce.
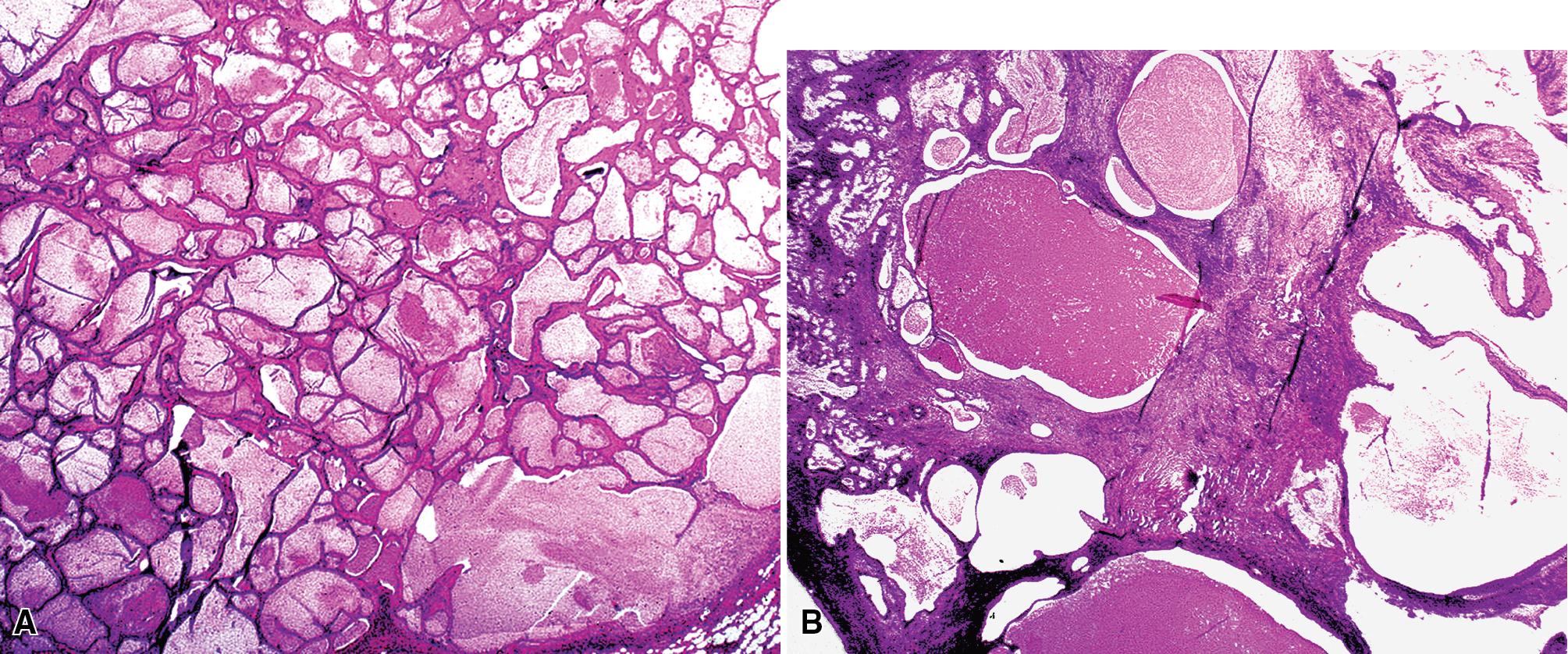
The internal substructure of MGA has been divided into two morphologic patterns: glandular-tubulocystic ( Fig. 20.12 ) and papillocystic ( Fig. 20.13 ). Within each of these categories, there may be a spectrum of appearances, ranging from a monotonous tubular pattern ( Fig. 20.14 ) to a complex arborizing aggregation of papillary structures. Stromal sclerosis may be present.
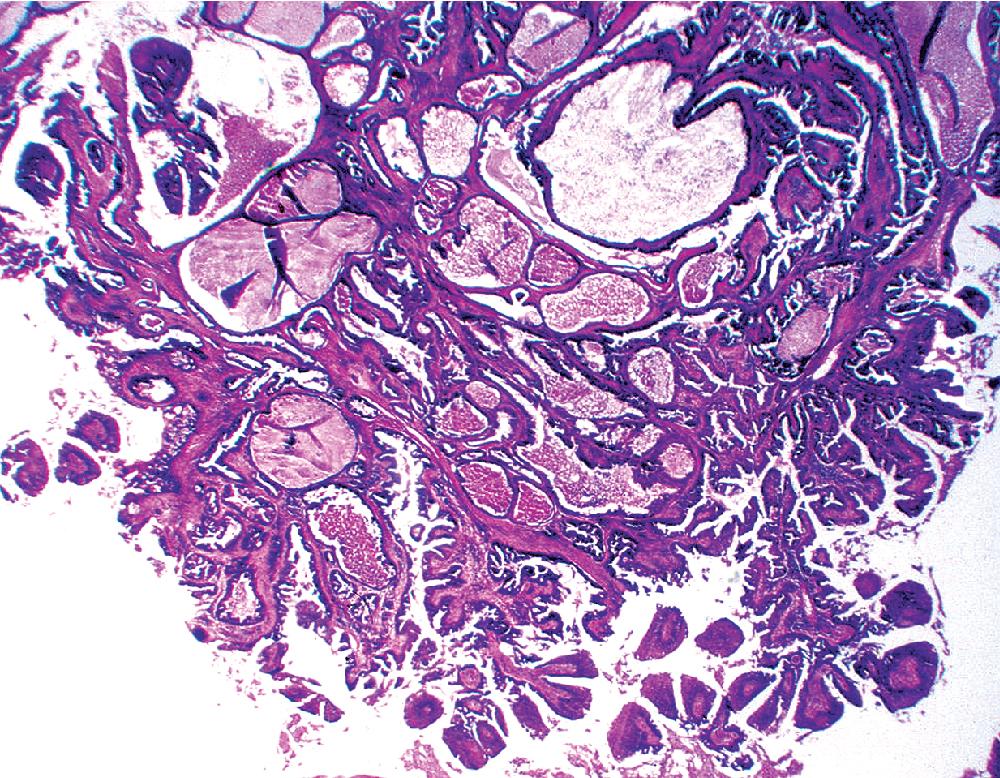

Immunohistologic features of MGA are comparable to those of nonneoplastic bronchial glands. The constituent cells are consistently labeled for cytokeratin, epithelial membrane antigen (EMA), and blood group isoantigens, with less uniform reactivity for carcinoembryonic antigen. Stromal cells show myoepithelial features, with concurrent staining for keratin, actin, and S-100 protein. Squamous-type (“high-molecular-weight”) keratins may be expressed in MGAs. This could be a diagnostic trap vis-à-vis the alternative interpretation of mucoepidermoid carcinoma (discussed later).
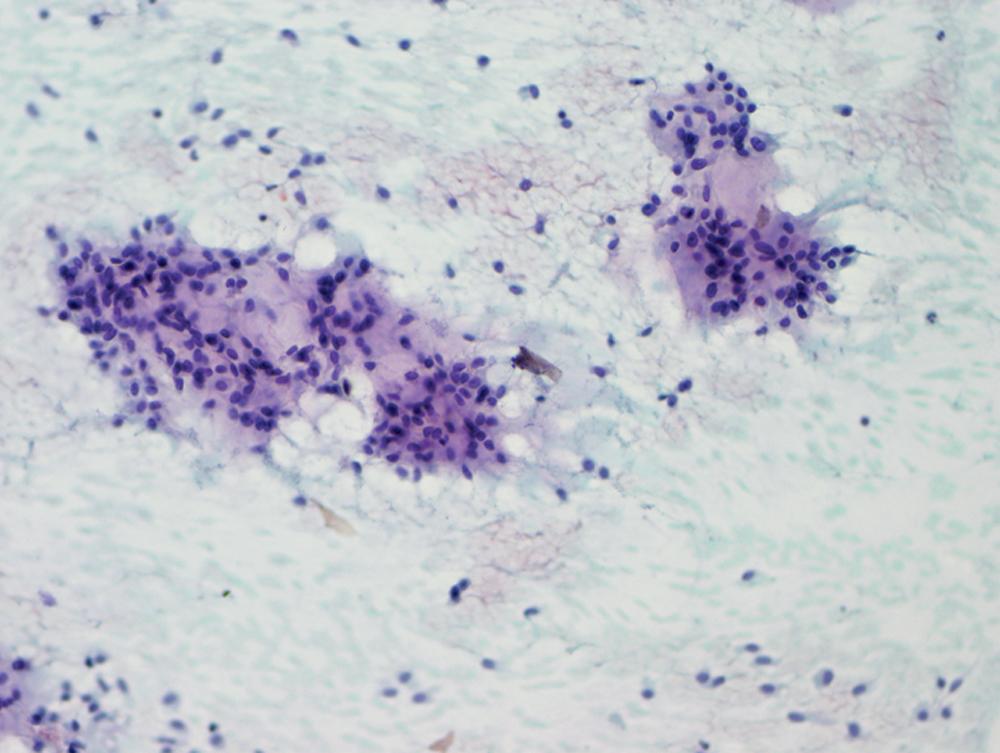
The differential diagnosis includes predominantly cystic mucoepidermoid carcinoma, MT, sclerosing hemangioma (pneumocytoma), and primary or metastatic mucinous (“colloid”) adenocarcinoma. Of these possibilities, the first two are the most problematic, necessitating adequate biopsies for visualization of the tumor architecture. Cytologically, MGA shows bland nests and sheets of monotonous epithelioid cells ( Fig. 20.15 ). Diagnostic separation from mucoepidermoid carcinoma or MT usually is not possible using fine-needle aspiration (FNA) biopsy or bronchial brushing specimens.
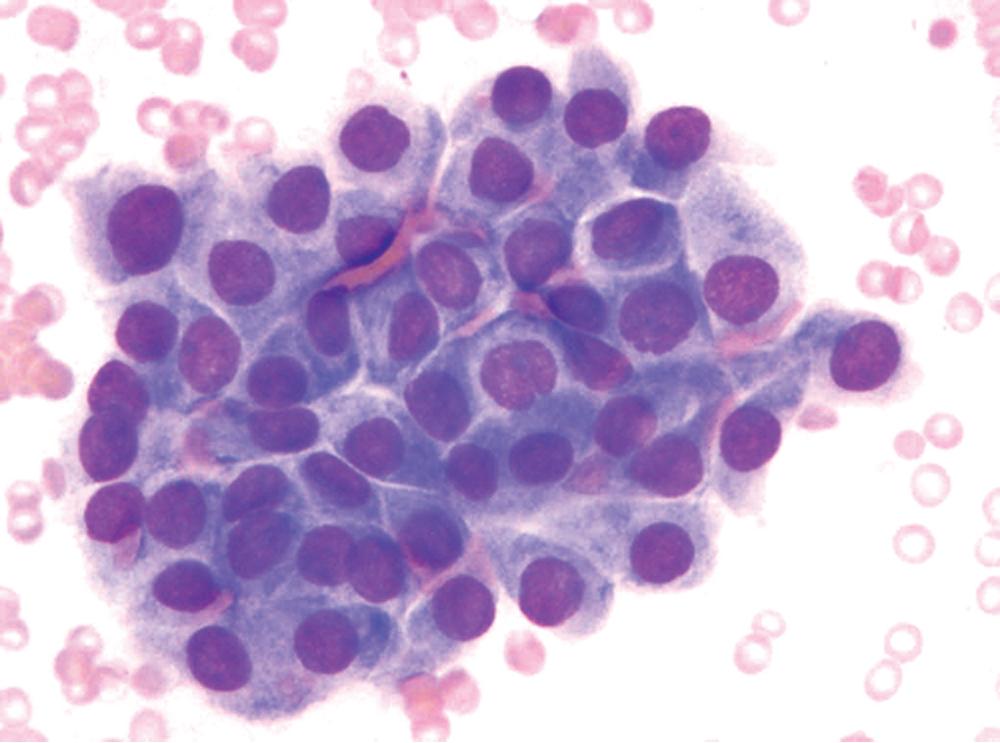
An admixture of squamous elements throughout the mass, infiltrative growth through the bronchial wall, or both would tend to argue for a diagnosis of mucoepidermoid carcinoma. Although MGA does not manifest chondromyxoid stroma or immunoreactivity for glial fibrillary acidic protein, as seen in some MTs, the possibility that MGA is related nosologically to monomorphic adenoma—a variant of MT—cannot be dismissed. In any event, it represents a distinctive clinicopathologic entity that is believed to deserve its own diagnostic designation. A recent report identified a GNAS gene (R201C) mutation in a case of MGA, suggesting a role in MGA tumorigenesis, as has been postulated for mucinous epithelial neoplasms of other organs.
Treatment of MGA can be conservative, with sleeve resection of the bronchus and reconstruction when clinically feasible. ,
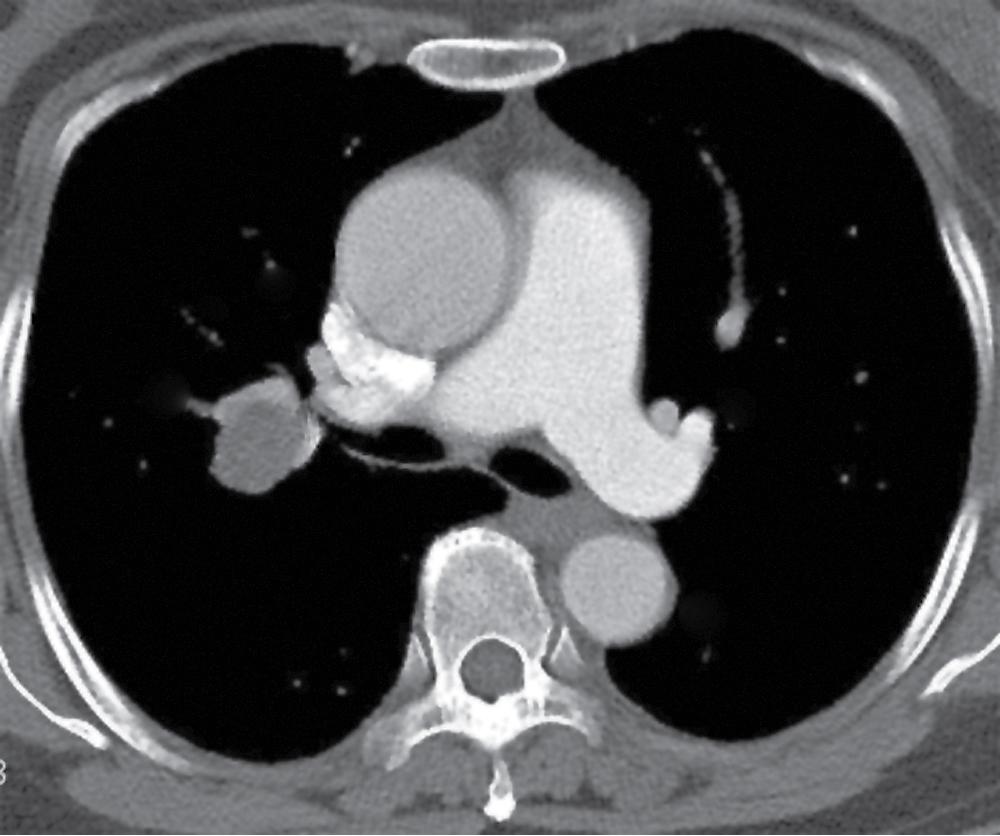
Pleomorphic adenoma, or MT, occurs predominantly in adults, with a slight preference for women. , The age range is 8 to 75 years. The lesion may present as either a central or a peripheral mass ( Fig. 20.16 ).
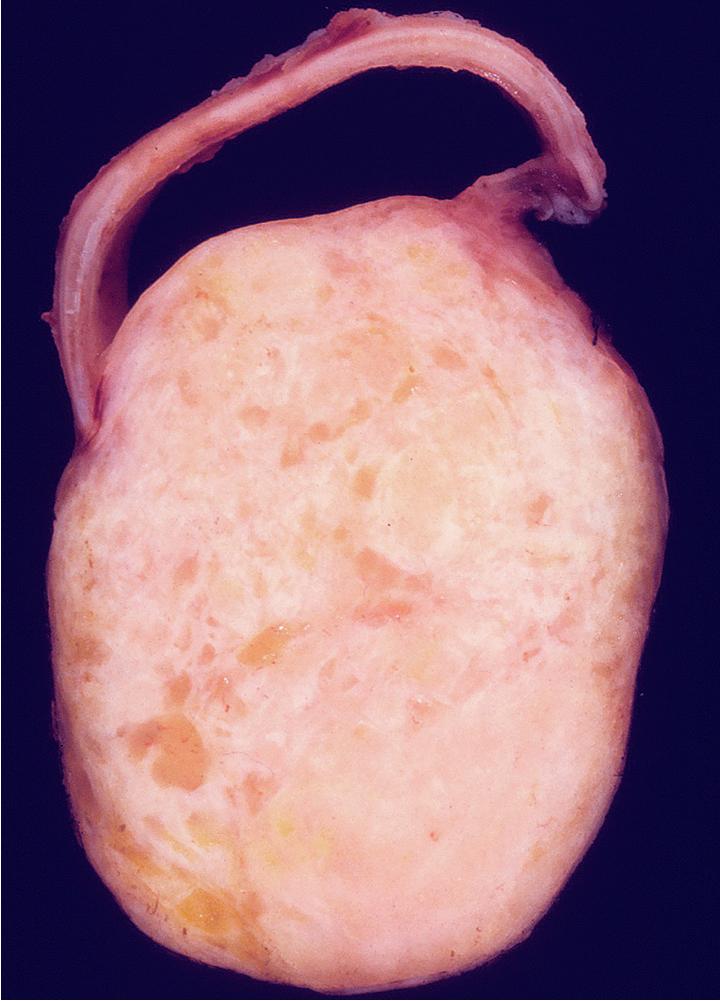
Grossly, central lesions of the main bronchus are commonly polypoid, whereas those in the periphery present as well-circumscribed tumors usually attached to bronchi ( Fig. 20.17 ). The size of the neoplasms varies from 1 to 16 cm in greatest diameter. The cut surfaces of MTs may be chondroid and firm, or may have a soft consistency, with only focal induration.
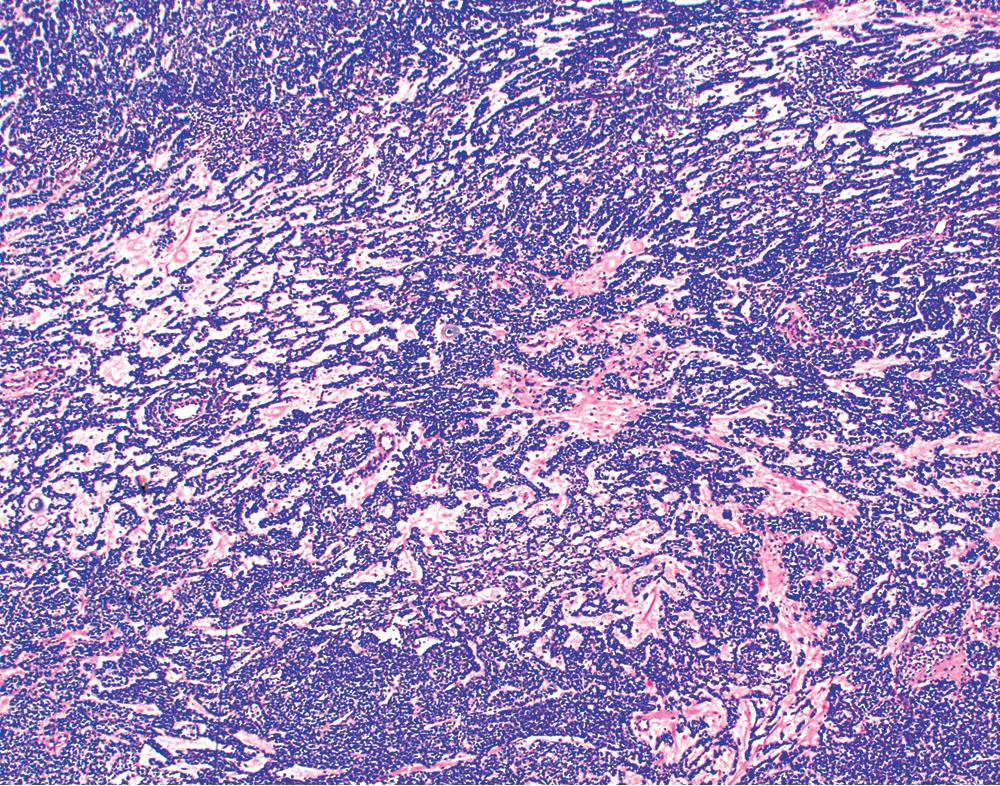
By definition, pleomorphic adenomas show at least a biphasic microscopic image; they are characteristically composed of epithelial tubules and nests that are embedded in a chondromyxoid stroma ( Figs. 20.18 and 20.19 ). Interestingly, intrapulmonary MTs rarely show the amount of mature cartilaginous stroma present in MTs of the salivary glands. In some lesions, the predominant growth pattern may be solid myoepithelial proliferation that approximates that of cellular MTs in the head and neck. Those neoplasms comprise compact epithelioid cells with round or oval nuclei, which generally lack nuclear atypia, necrosis, hemorrhage, and mitotic activity ( Fig. 20.20 ). Notably, there have been no well-documented cases of carcinoma arising from preexisting MTs of the lung, as may rarely occur in salivary glandular sites. Other variants of MT include a myoepitheliomatous subtype that may manifest either a spindle cell composition ( Fig. 20.21 ) or a plasmacytoid constituency; a form with extensive squamous metaplasia ( Fig. 20.22 ); a subtype in which sizable zones of the lesion demonstrate an adenoid cystic carcinoma-like cribriform architecture; and a chondroid-rich form that simulates pulmonary chondroma or chondromatous hamartoma.
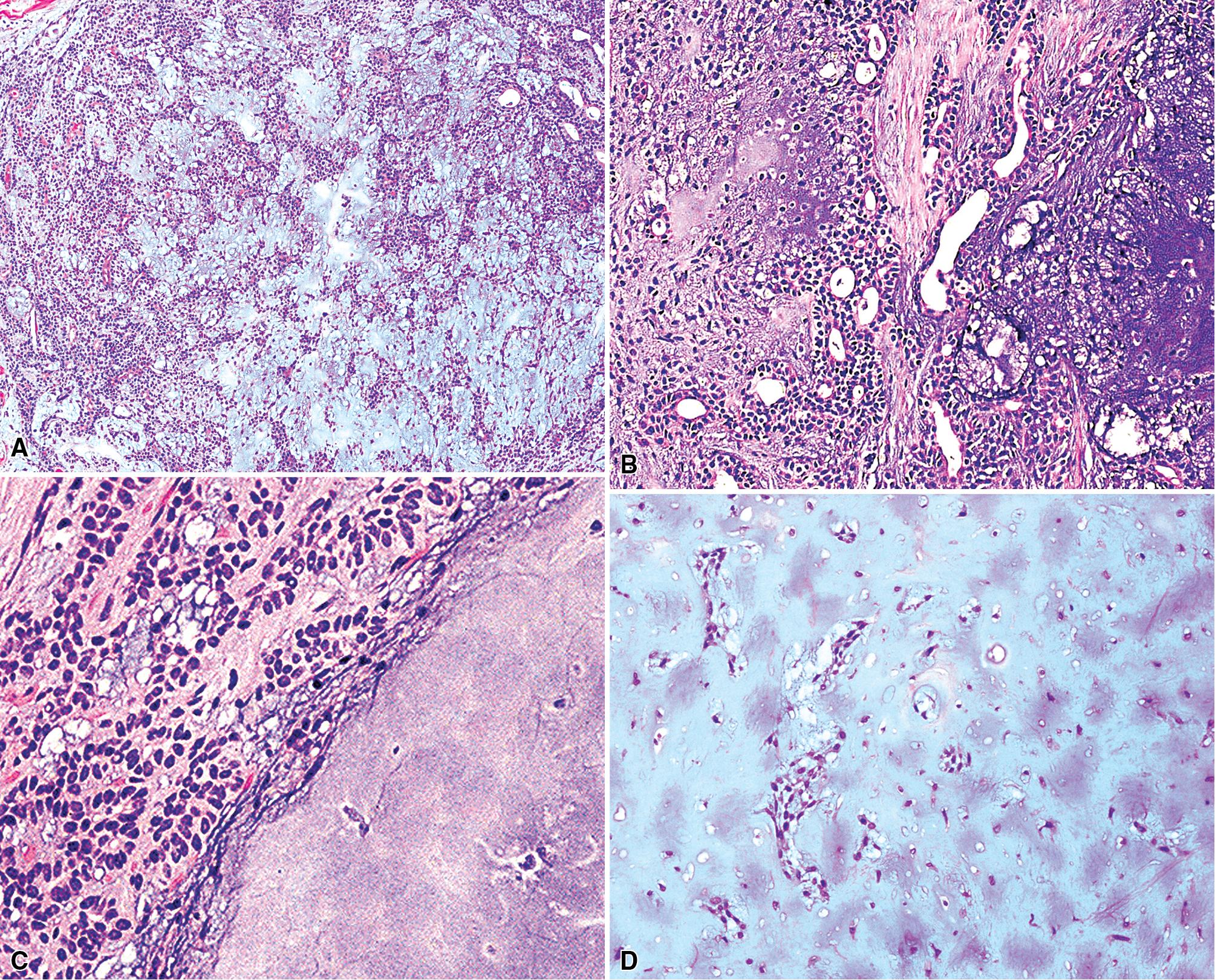
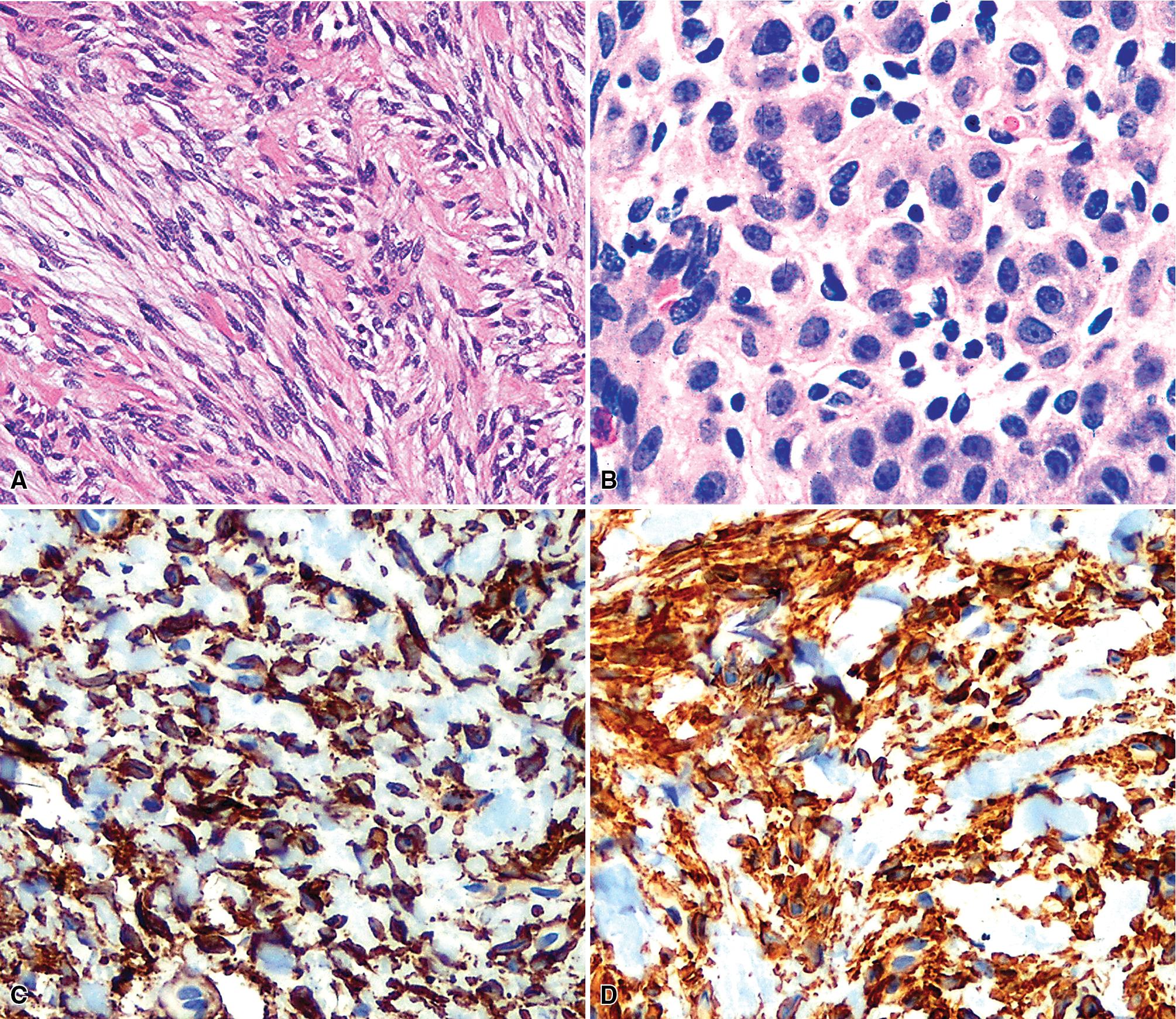
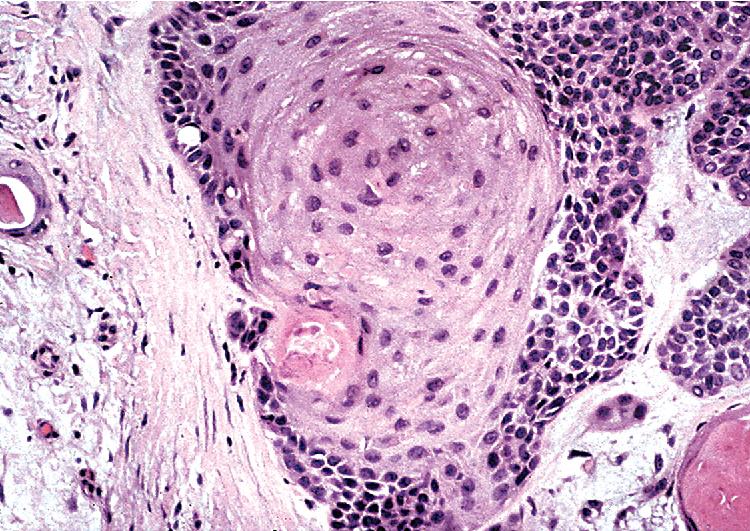
The differential diagnosis depends on whether one is dealing with a biopsy specimen or a complete resection of the tumor. In the former instance, MTs can be confused with other salivary glandlike tumors, such as adenoid cystic carcinoma, as well as with hamartoma/chondroma, squamous cell carcinoma (when squamous metaplasia is dominant), and biphasic malignancies, such as sarcomatoid carcinoma. In resection specimens, the diagnosis is typically straightforward. However, if MTs demonstrate an overwhelmingly prominent solid pattern with spindle cell (myoepitheliomatous) differentiation, sarcomas may also be considered. In this setting, concurrent immunoreactivity for keratin, vimentin, S-100 protein, actin or caldesmon, p63 protein, and glial fibrillary acidic protein , provides the necessary evidence for a conclusive diagnosis of MT.
MTs of the lung behave in an indolent fashion. There are only anecdotal reports of metastasizing lesions of this type, in analogy to rare examples in the salivary glands. No particular pathologic features of such neoplasms can be used to predict this unusual adverse behavior. Complete but conservative excision is the treatment of choice. ,
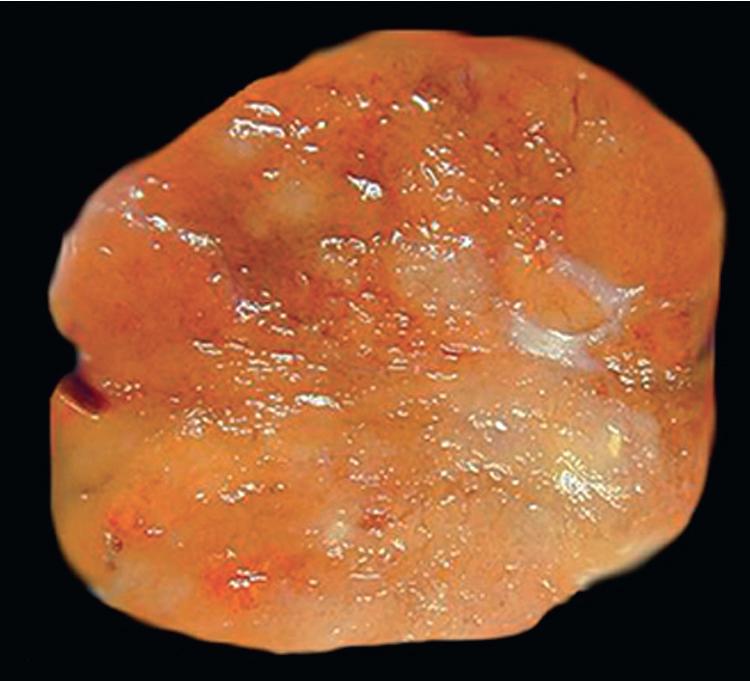
There are only a few reported cases of pulmonary oncocytoma. These tumors exhibit morphologic similarities to comparable tumors in the salivary glands, showing a brownish gross appearance ( Fig. 20.23 ). They are composed of nests of uniformly large polygonal cells with prominently eosinophilic granular cytoplasm and bland nuclei ( Figs. 20.24 and 20.25 ). In view of the existence of other, more common pulmonary tumors that can show oncocytic changes, it is important to properly exclude those other possibilities by adjunctive studies. Neuroendocrine tumors showing oncocytic features (oncocytic carcinoids; grade I neuroendocrine carcinomas) are far more common than oncocytomas and are recognizable by their immunoreactivity for chromogranin-A, synaptophysin, and CD56. , Metastatic tumors from the salivary glands and kidneys also must be considered, particularly in rare cases where pulmonary oncocytomas appear to be synchronously multifocal. Clinical information is important in this context because the electron microscopic and immunophenotypic properties of primary and metastatic oncocytic neoplasms may be very similar (see Chapter 17 ).
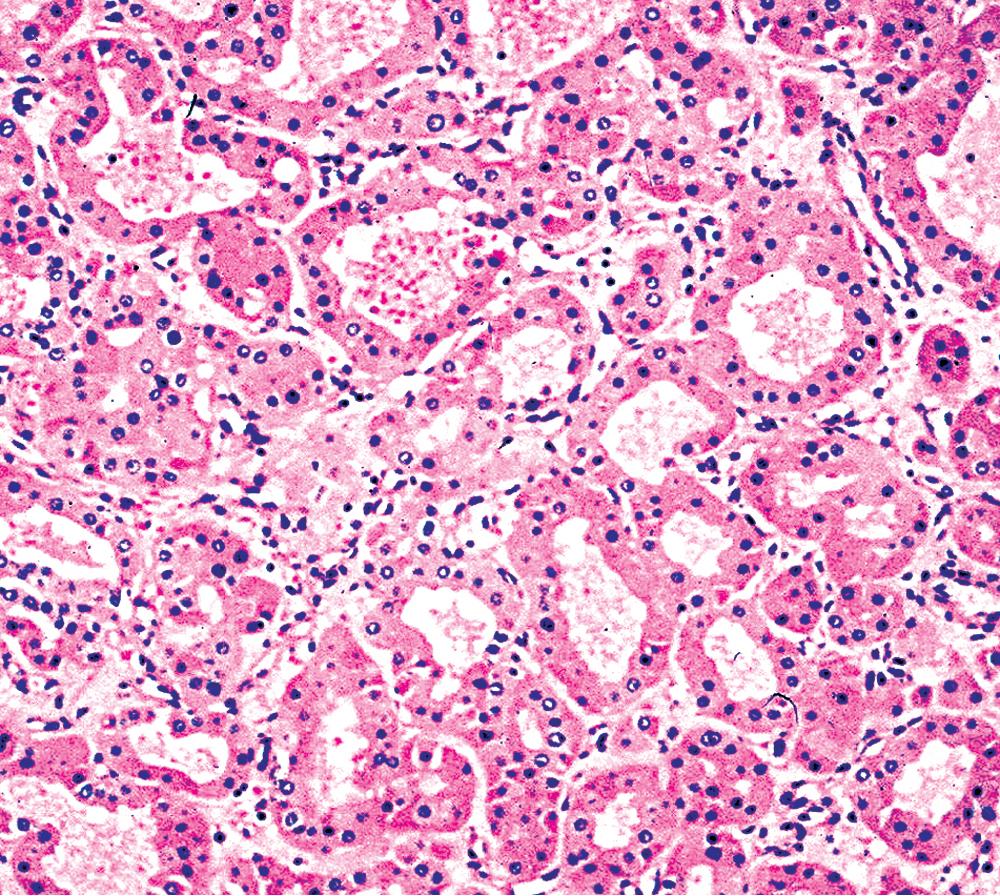
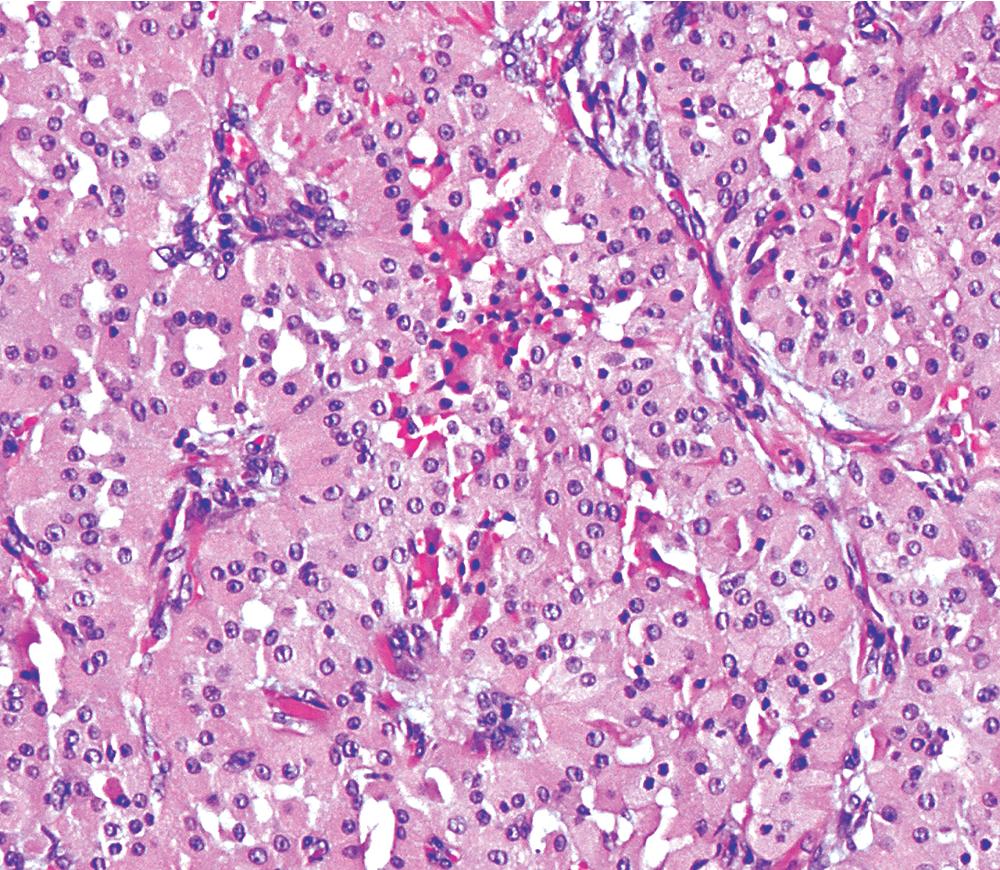
In general, oncocytomas in the lung and other anatomic sites are epithelial, demonstrating immunoreactivity for keratins and EMA; vimentin is inconsistently present, but markers of muscular, neural, or neuroendocrine lineages are absent. , Immunolabeling with antibodies to mitochondrial proteins is common, corresponding to the ultrastructural hallmark of these neoplasms.
FNA biopsy of oncocytic neoplasms yields a monomorphic population of large epithelioid cells with round to oval nuclei, dispersed chromatin, small nucleoli, and amphophilic to acidophilic cytoplasm. They are variably cohesive and typically show little nuclear pleomorphism.
Because of problems with the previous definition of pulmonary oncocytoma, as noted earlier, meaningful comments on its behavior are difficult. However, we have seen cases that obviously invaded the lung parenchyma and had atypical morphologic features, such as nuclear pleomorphism and atypical mitoses ( Fig. 20.26 ). Accordingly, such lesions are defensibly labeled as “malignant” oncocytomas.
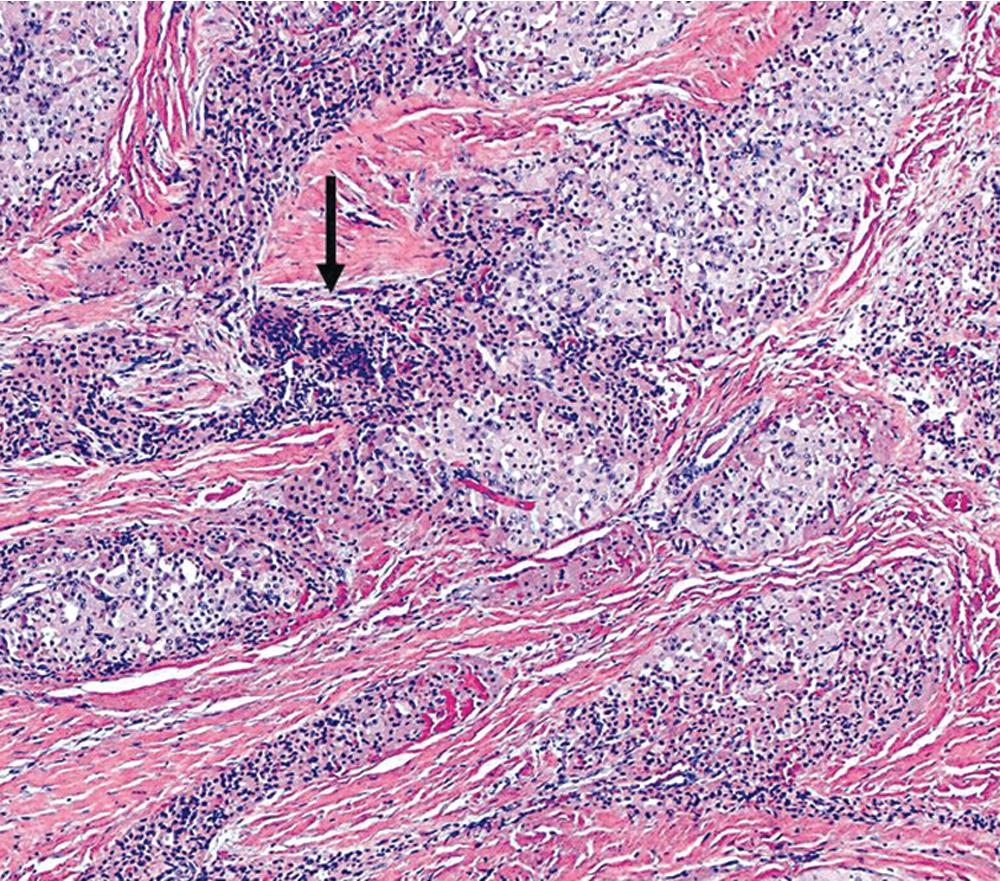
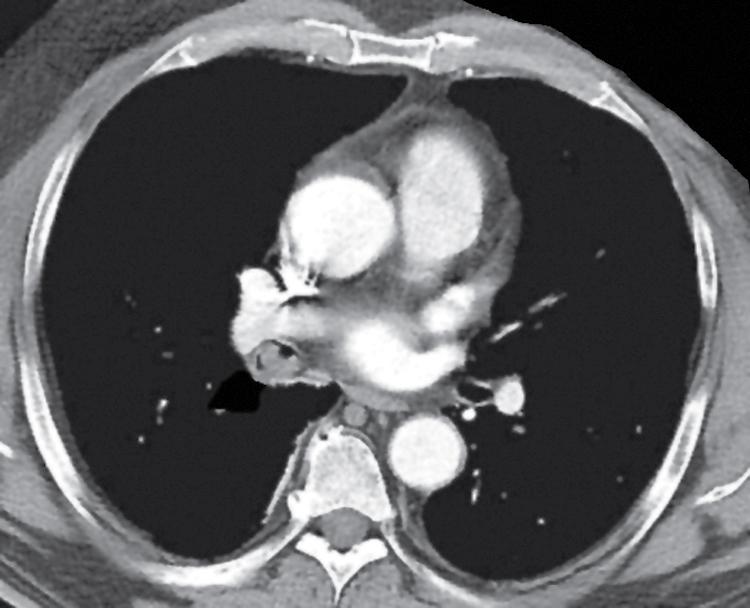
Primary neoplasms of the lung that demonstrate schwannian or perineurial differentiation are more often located in the walls of the major bronchi than in the peripheral lung. Chest radiographs show nodular or irregular masses associated with bronchi; secondary atelectasis is sometimes noted as well ( Fig. 20.27 ). Some patients with primary neurogenic pulmonary tumors have neurofibromatosis type 1 (NF1; von Recklinghausen disease), and that is true for both neurofibroma and neurilemmoma. Neurogenic sarcomas also arise in the lungs, but it is unclear how many have occurred in the context of NF1 in association with preexisting pulmonary neurofibromas. A recent review of 75 intrathoracic PNSTs by Boland and colleagues identified 21 in the lung and pleura.
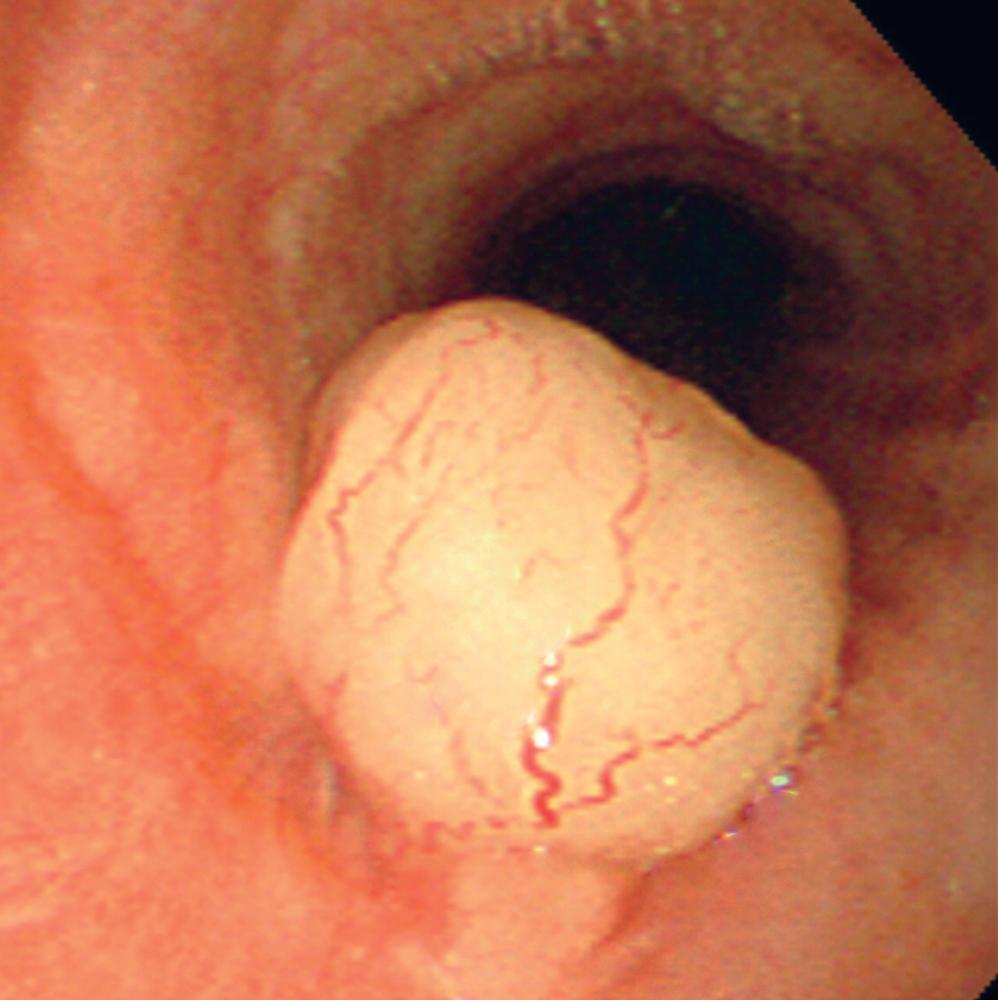
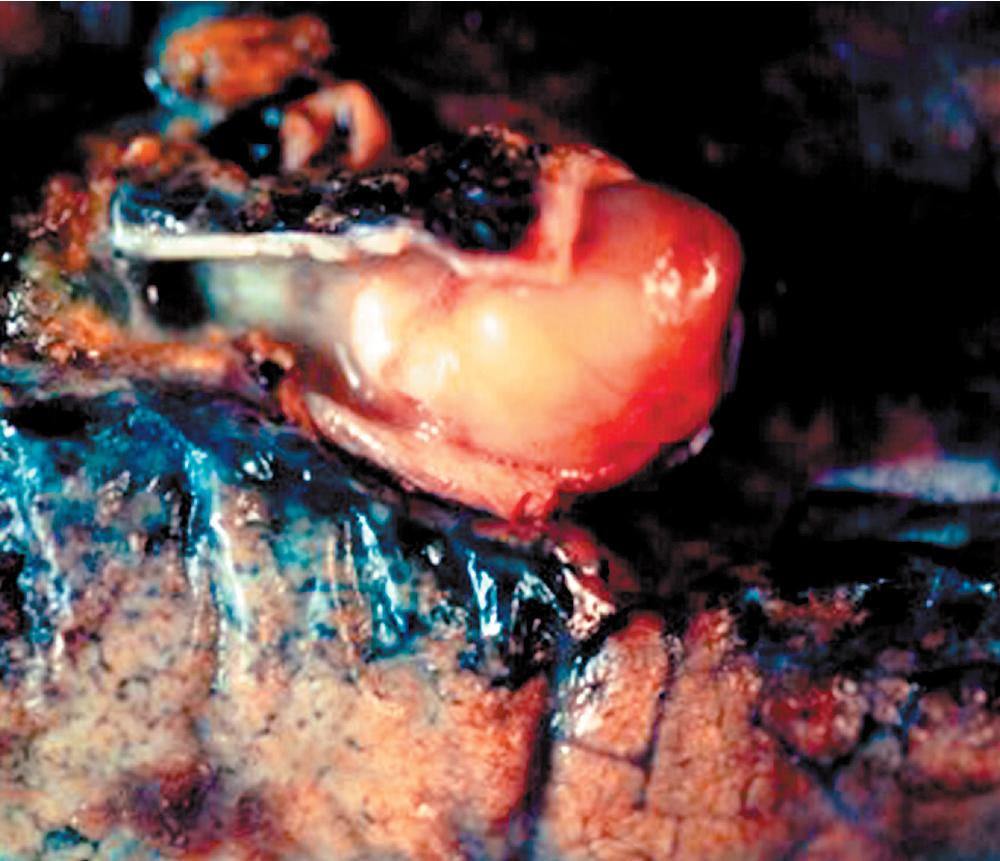
Grossly, peripheral nerve sheath tumors (PNSTs) of the bronchus are well-demarcated yellow-white masses centered on the bronchial wall and often protruding into the bronchial lumen ( Figs. 20.28 and 20.29 ). Gross foci of hemorrhage, necrosis, or cystification are absent in benign lesions of this type.
The histologic images associated with benign PNSTs are varied. Prototypical neurofibromas are plexiform in NF1, putatively reflecting a neoplastic attempt to recapitulate a neural plexus. Constituent cells are serpiginous, with attenuated fusiform nuclei and delicately fibrillar cytoplasm ( Fig. 20.30 ). The supporting matrix is myxoedematous or collagenized, and it may contain scattered foam cells, mast cells, and lymphocytes. Mitotic activity is typically absent. Neurilemmomas—also termed schwannomas —are biphasic in their classic form, with compactly cellular (“Antoni A”) areas alternating with zones of loose cellularity and myxoid change (“Antoni B” foci; Figs. 20.31 and 20.32 ). Nuclei may be aligned in register in Antoni A areas, representing Verocay bodies ( Fig. 20.33 ). Intralesional blood vessels have thick walls in many neurilemmomas, and a well-defined peripheral tumor capsule may be identified in some instances. Variants of neurilemmoma include cellular schwannoma, which manifests intersecting bundles of densely apposed, focally mitotic spindle cells in the context of an encapsulated mass ( Fig. 20.34 ) ; glandular schwannoma; ancient schwannoma, showing scattered pleomorphic hyperchromatic nuclei in degenerative cells ( Fig. 20.35 ); plexiform schwannoma, in which broad plexiform fascicles of tumor cells show the substructure of neurilemmoma; and melanotic psammomatous schwannoma, exhibiting microcalcifications and melanin pigmentation ( Fig. 20.36 ). The last of these subtypes may be associated with myxomas of the skin, heart, and breast; the presence of cutaneous ephelides; and overactivity of the endocrine glands.
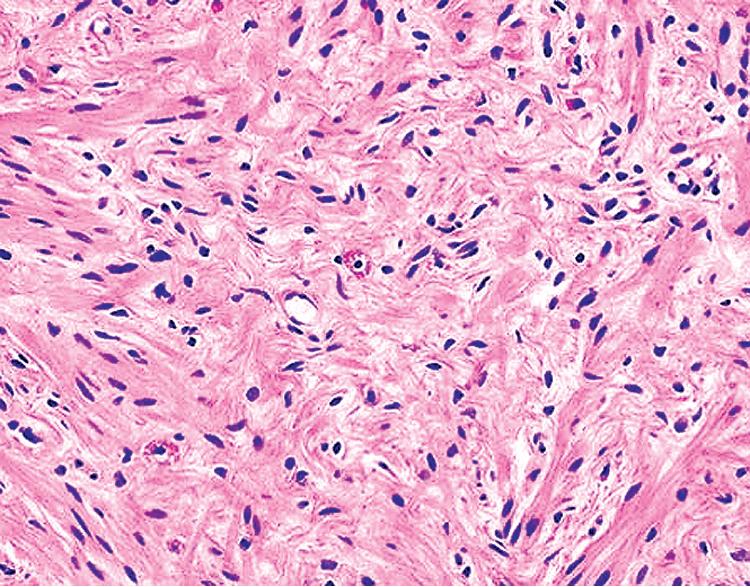
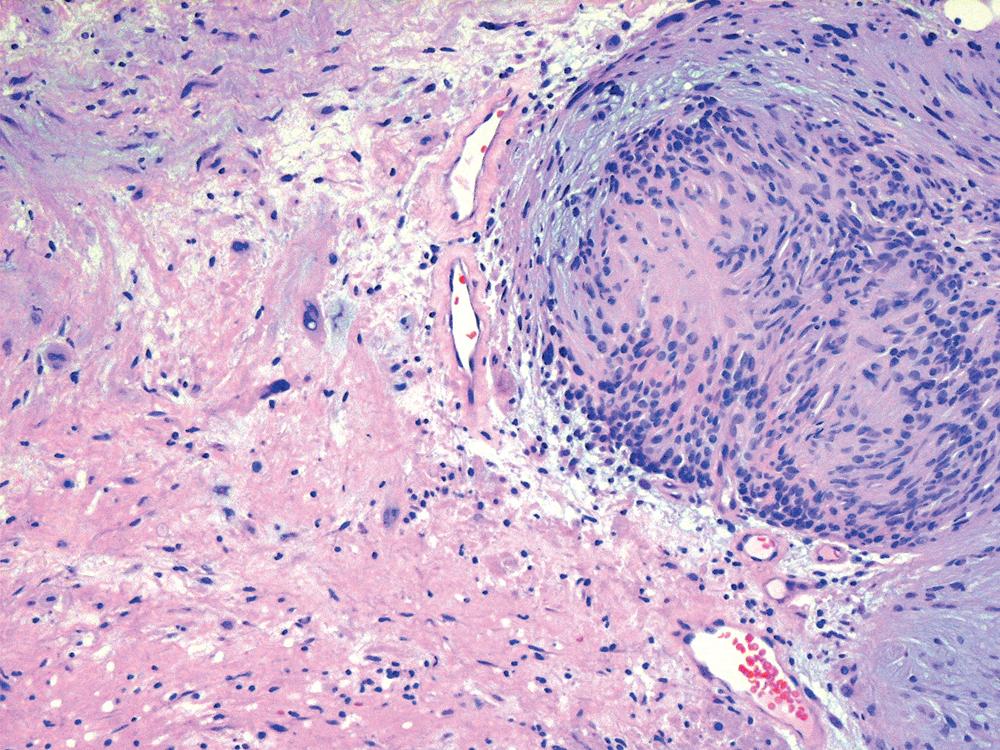
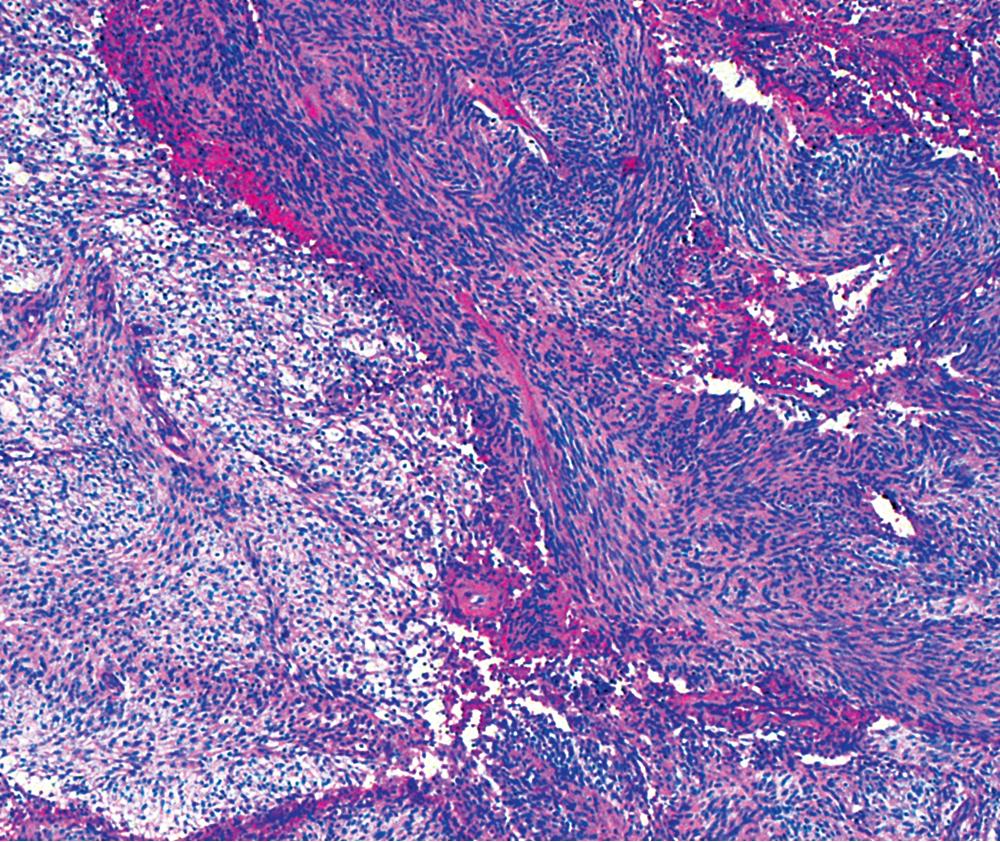
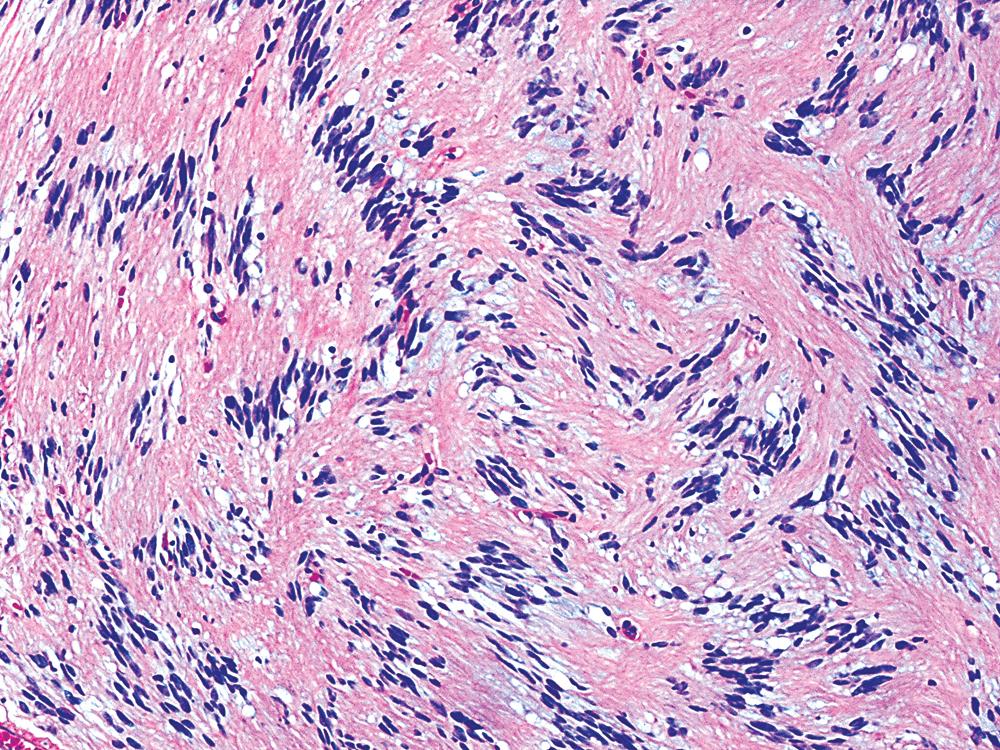

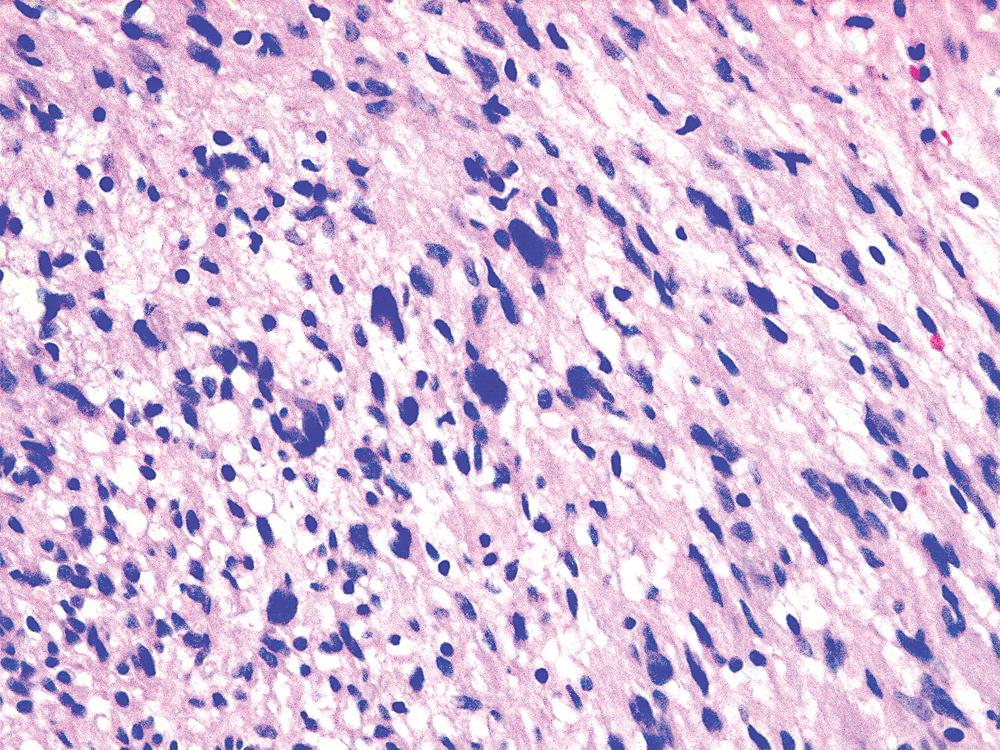
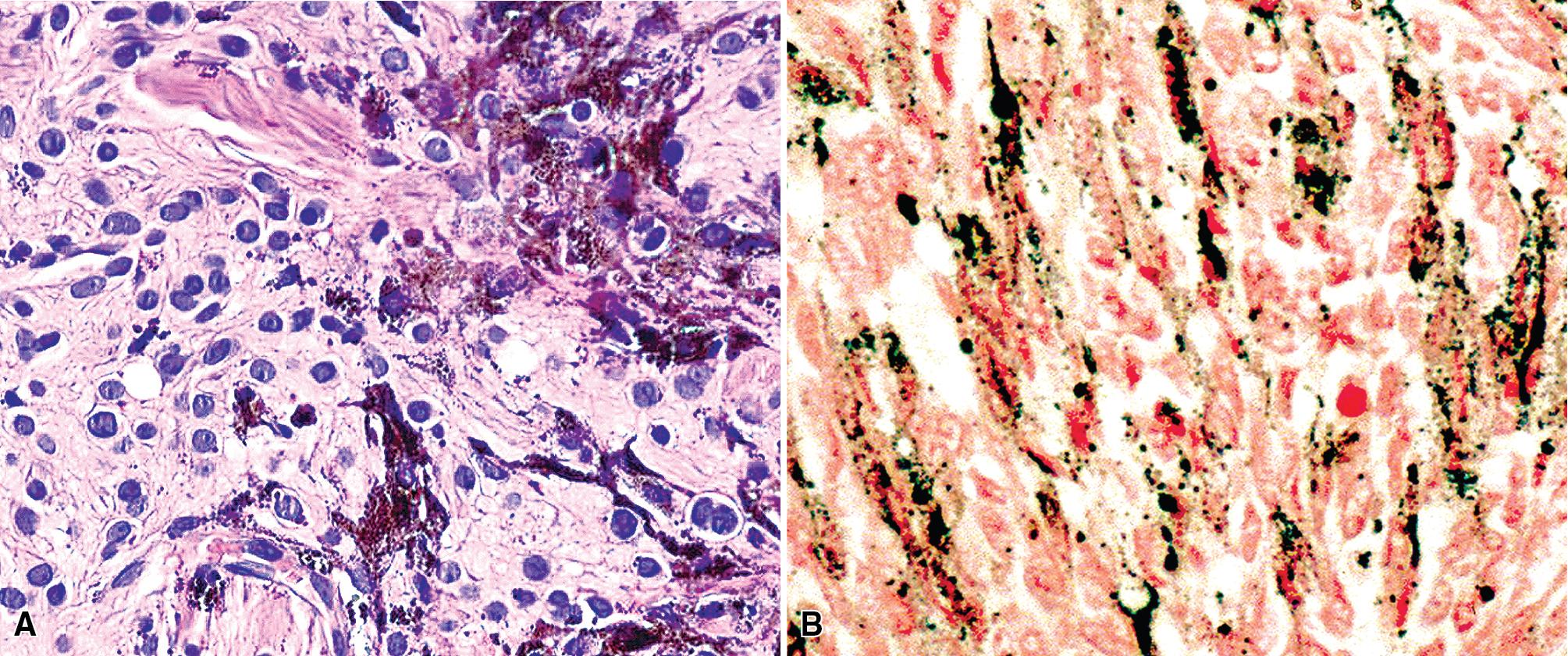
The immunophenotype of PNSTs features reactivity for vimentin and variable labeling for S-100 protein ( Fig. 20.37 ), CD56, CD57, glial fibrillary acidic protein, and EMA. The last of these markers is believed to represent perineurial differentiation in this context. Keratin positivity is absent, except in the glands of glandular schwannoma, and myogenous markers should be negative.
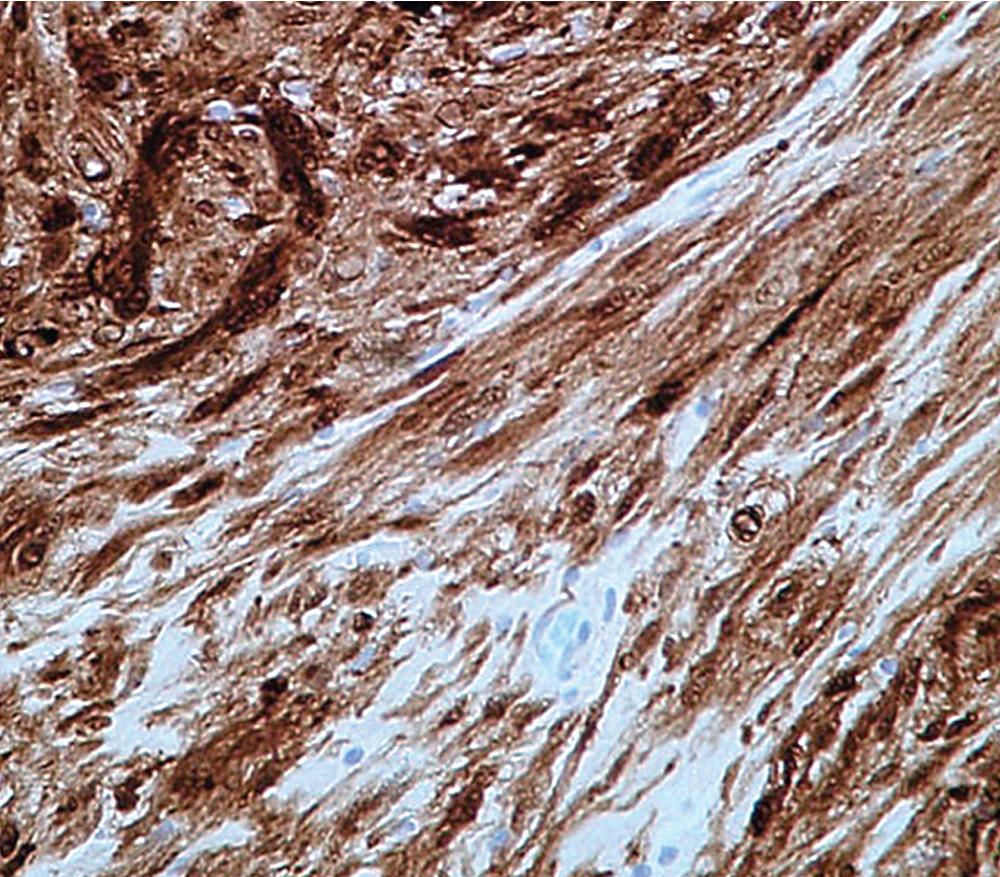
As is true of all spindle cell lesions of the lung, it is necessary to exclude sarcomatoid carcinoma before making a final diagnosis of PNST; immunohistologic evaluation is the most expeditious means of doing so. In addition, other mesenchymal lesions of the lung—especially leiomyoma and leiomyomatous hamartoma—must be considered as alternatives to an interpretation of neurofibroma or neurilemmoma. The immunoprofiles of those lesions are dissimilar as well.
If the identification of PNST can be established in evaluations of bronchial biopsy specimens and radiographic findings support a benign diagnosis, the surgeon can undertake a conservative approach to the removal of such lesions. , However, small samples of neurogenic neoplasms are notoriously unreliable in predicting the biologic potential of PNSTs, and they should not be used in isolation to govern decisions on therapy.
Granular cell tumor (GCT; also known as Abrikossoff tumor ) may be seen in many anatomic locations. Fewer than 200 have been reported in the trachea and lungs. Patients of any age may have such lesions, and multiple tumors in the same individual have been reported. , , , ,
Endoscopic examination often reveals a sessile polypoid endoluminal component, with intact overlying mucosa ( Fig. 20.38 ). Radiographic studies confirm that characteristic, but also demonstrate an infiltrative aspect to many GCTs that may lead to a mistaken preoperative diagnosis of malignancy. GCTs in the peripheral lung parenchyma are unusual, but these likewise may assume a spiculated appearance that closely simulates that of adenocarcinoma. This situation is further complicated by occasional case reports of GCTs that coexisted with malignant neoplasms. ,
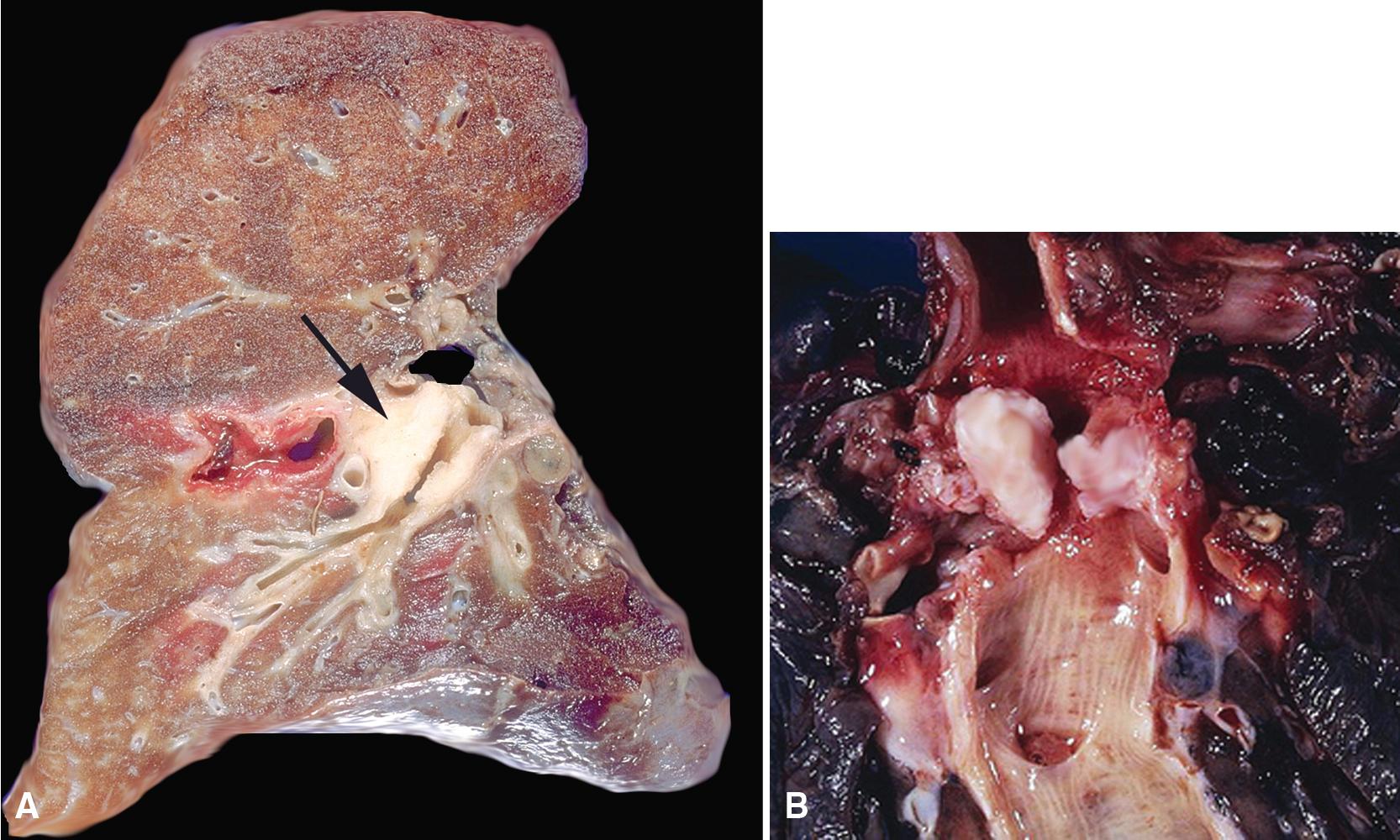
Grossly, GCT is a white-tan, ill-defined mass with a gritty cut surface, again simulating the features of an infiltrating carcinoma. However, necrosis and hemorrhage are distinctly unusual. Maximum tumor size approximates 5 cm.
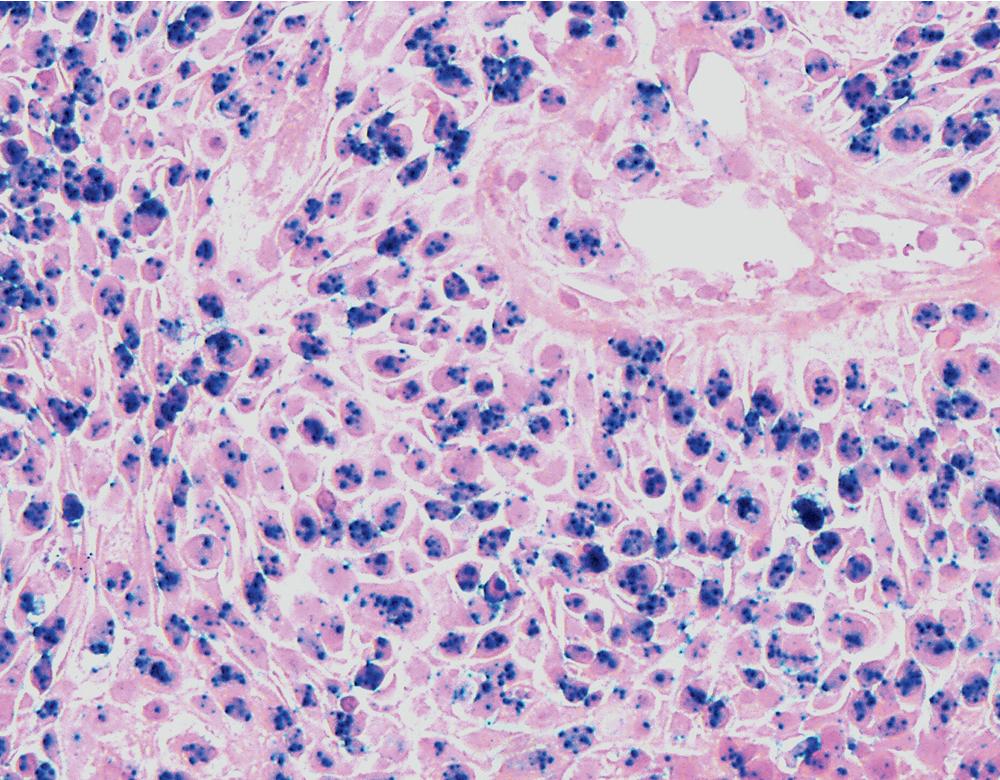
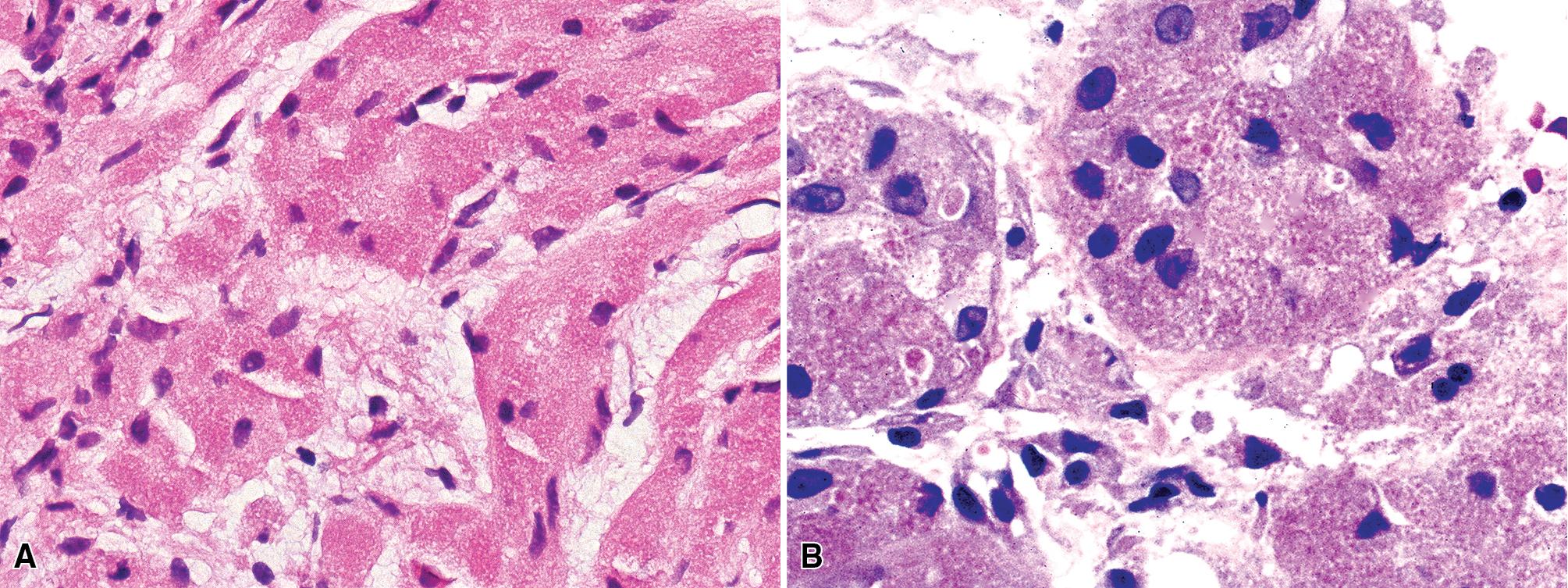
Microscopically, these tumors comprise a uniform population of polygonal or fusiform cells that contain small ovoid hyperchromatic nuclei with indistinct nucleoli ( Fig. 20.39 ). The cytoplasm is abundant, eosinophilic, or amphophilic, and coarsely granulated, often with the additional presence of rounded inclusions that superficially resemble Michaelis-Gutman bodies ( Fig. 20.40 ). Like Michaelis-Gutman bodies, these may also have a targetoid configuration. Mitotic figures are typically scarce and physiologic; necrosis and vascular invasion are absent. The advancing border of GCTs may be “pushing” or irregular and permeative. A proportion of these lesions infiltrate deeply into the bronchial wall or even through it into the adjacent parenchyma. In nonpulmonary sites, such a growth pattern has been linked to a greater risk of recurrence, but that correlation does not seem to apply to GCTs in the airways or lungs. The existence of primary malignant GCT of the respiratory tract has never been convincingly documented, and recurrent neoplasms are typically those that have never been completely excised.
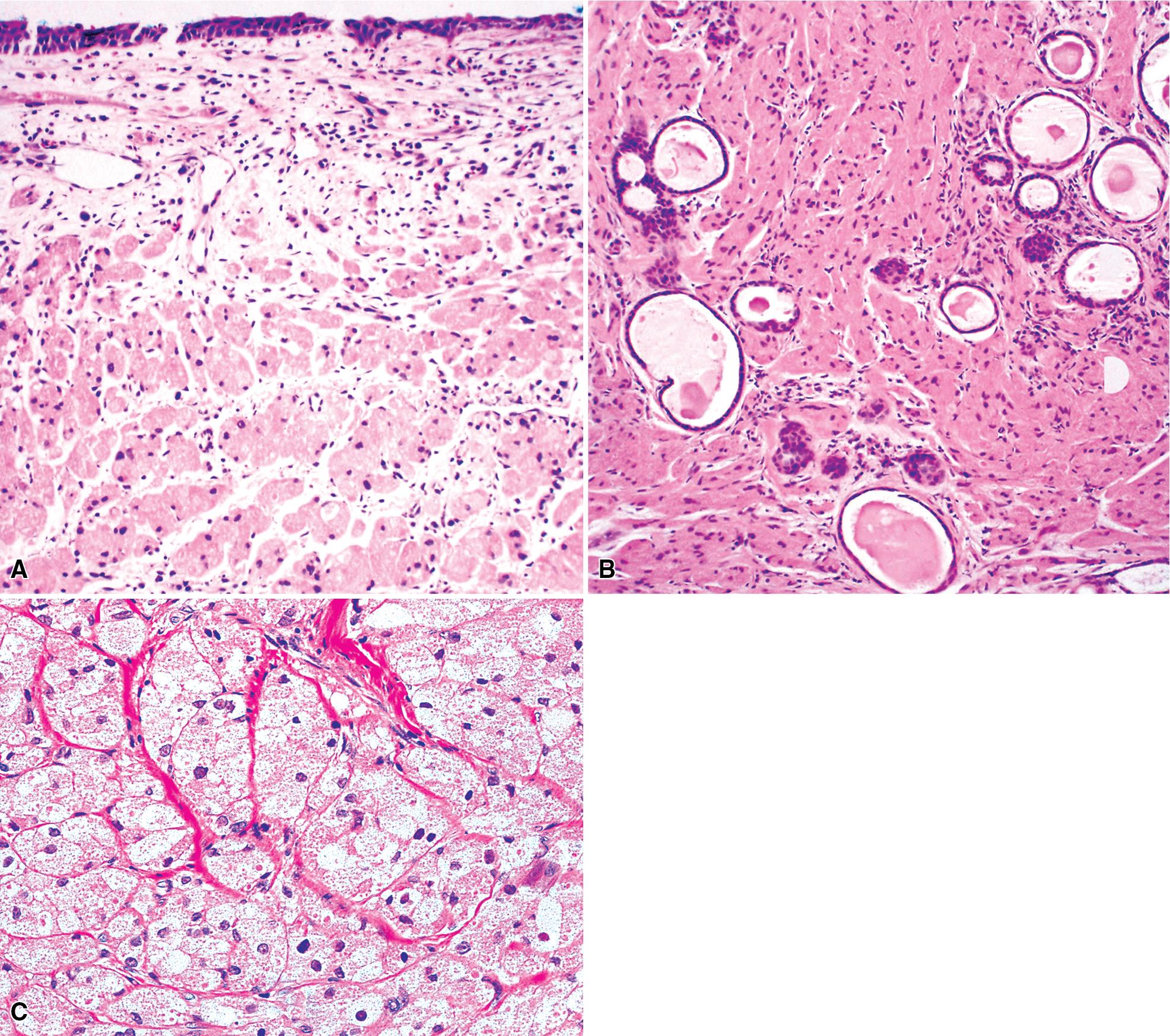
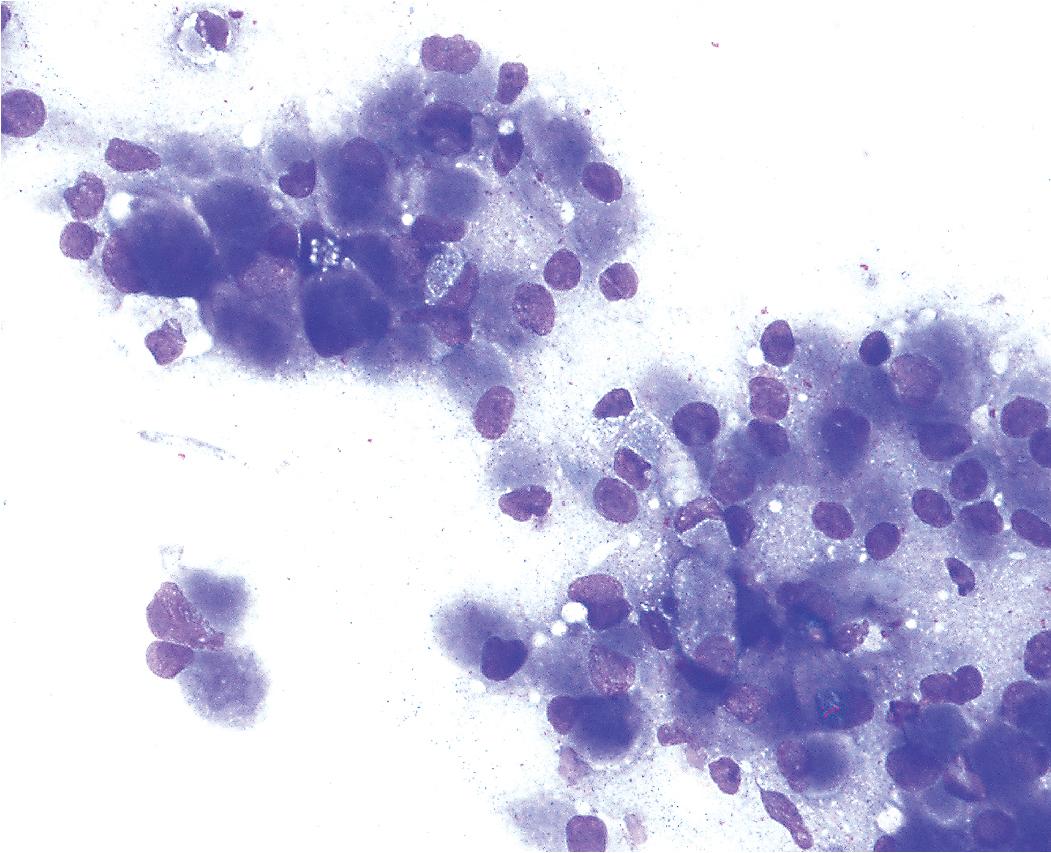
FNA biopsy of GCT yields a monotonous population of large epithelioid cells that are variably cohesive. They contain ovoid nuclei, distinct chromocenters, and abundant granular cytoplasm that are best seen in Romanowsky-stained slides ( Fig. 20.41 ). A plasmacytoid appearance is common.
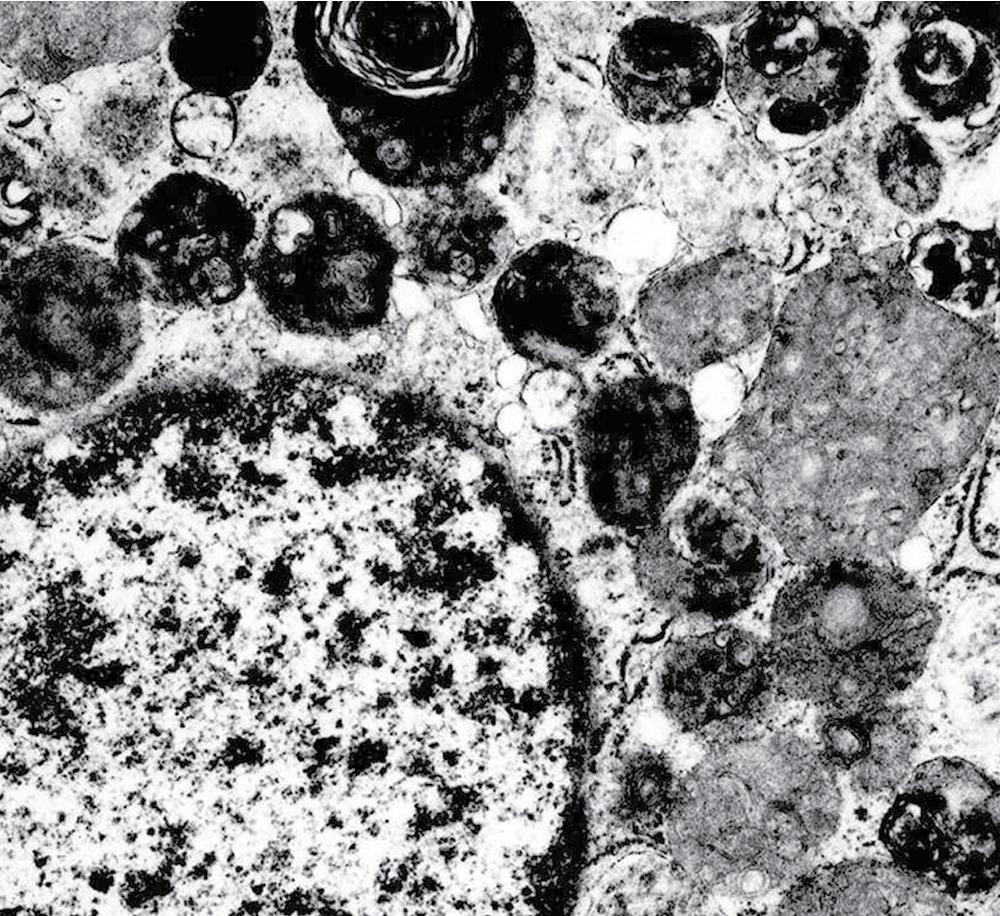
Electron microscopy of GCTs shows a distinctive multiplicity of secondary and tertiary lysosomes in the cytoplasm of the tumor cells, virtually to the exclusion of other organelles ( Fig. 20.42 ). , Immunohistologic evaluation most often reveals evidence of schwannian differentiation, with reactivity for S-100 protein, CD56, CD57, myelin basic protein, and combinations thereof, in 80% to 85% of cases ( Fig. 20.43 ). The abundance of lysosomes is reflected by their CD68 reactivity. Recently, the presence of calretinin and alpha-inhibin has also been reported in GCT.
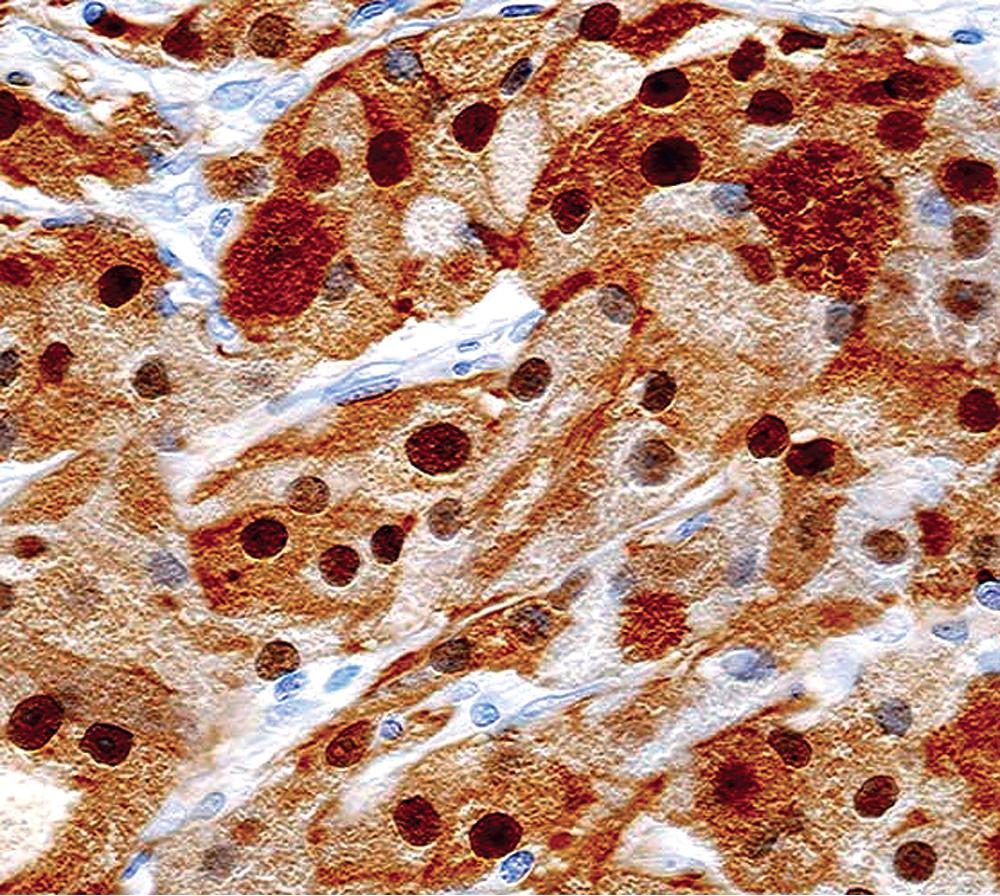
An important caveat regarding the immunophenotype of this tumor type is that nonschwannian lesions containing granular cells are a heterogeneous group. Carcinomas (both neuroendocrine and nonendocrine), , smooth muscle tumors, endothelial proliferations, and neoplasms of uncommitted lineage have all been described with a granular cell phenotype. Therefore it is important to include immunohistologic evaluations to address these possibilities in differential diagnosis, with or without ultrastructural studies. Oncocytoid granular cell grade 1 neuroendocrine carcinoma (carcinoid) of the lung is the most common simulator of GCT, , making chromogranin-A and synaptophysin important markers in this context.
As discussed earlier, aggressive behavior by bronchopulmonary GCT has not been observed. Therefore conservative therapy for this neoplasm is warranted.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here