Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Several methods are available for obtaining a urine specimen. They can be found in Table 67.1 and are listed in order of increasingly precise collection techniques. They come at the cost of increasing difficulty, patient discomfort, or both.
| METHOD | DESCRIPTION AND COMMENTS |
|---|---|
| Random voided | Any specimen provided by the patient. |
| Midstream voided | No skin preparation, container placed in the urinary stream 2–3 sec after initiation of micturition. |
| Clean catch | Same as above, plus antiseptic cleansing of the urethral area. This is performed by retraction of the prepuce in males and the labia in females and then cleansing the meatus in an anterior-to-posterior direction. Use three swabs soaked in povidone-iodine (or some other antiseptic solution). For female patients who are physically capable, the ideal position is sitting astride a toilet, facing backward. This helps separate the labia and position the cup for collection of the specimen. |
| Midstream clean catch | Cleansing as for clean catch, with midstream collection as for midstream voided. |
| Catheterized | Obtained from a newly placed catheter after cleansing of the meatus. |
| Suprapubic aspiration | See Chapter 55 . |
The advantages and disadvantages of each of the techniques listed in Table 67.1 can be determined only by the purpose of the urine test and the clinical context. The clinical context influences interpretation of the results. For the great majority of clinical scenarios, the basic dichotomy is between specimens obtained for infectious versus noninfectious reasons. With the exception of testing for red blood cells (RBCs) and white blood cells (WBCs), most of the noninfectious tests (e.g., ketones, glucose, bilirubin, protein) are not affected by the collection method. Urine specimens collected to diagnose infection can be contaminated in a number of ways, and the clinical scenario intricately influences the choice and interpretation of tests.
Urinary tract infections (UTIs) are either symptomatic or asymptomatic, and the symptoms determine which collection method is required ( Fig. 67.1 ). Symptomatic female patients without potential complications who present for the first time require no further urine testing, and should be treated empirically based on local patterns of susceptibility for urinary pathogens. However, in symptomatic patients who have failed a course of therapy or have potential complicating factors, testing is recommended and extremely low levels of bacteriuria (10 2 colony-forming units [CFUs]/mL) and pyuria are of clinical significance. This may be obscured in some clinicians' minds by an alternative, more widely promulgated fact: In asymptomatic patients the threshold for “significant bacteriuria” is 1000-fold higher at greater than 10 5 CFUs/mL. Symptomatic patients constitute a clinically distinct group who require a urine test that is much more sensitive and thus will render a false-positive result with much lower levels of contaminants. Although many studies do not show a statistically significant increase in contamination rates with less stringent urine collection techniques, most show increased accuracy with more meticulous or invasive collection methods. Because it takes only marginally longer, it makes sense to always strive for the highest-quality urine specimen available, especially in view of the delays, repeated testing, and additional cost entailed by false-positive results. A frequent misconception is that contaminated specimens are characterized by the isolation of multiple pathogens, but in fact, up to 50% of symptomatic women may have polymicrobial infections.
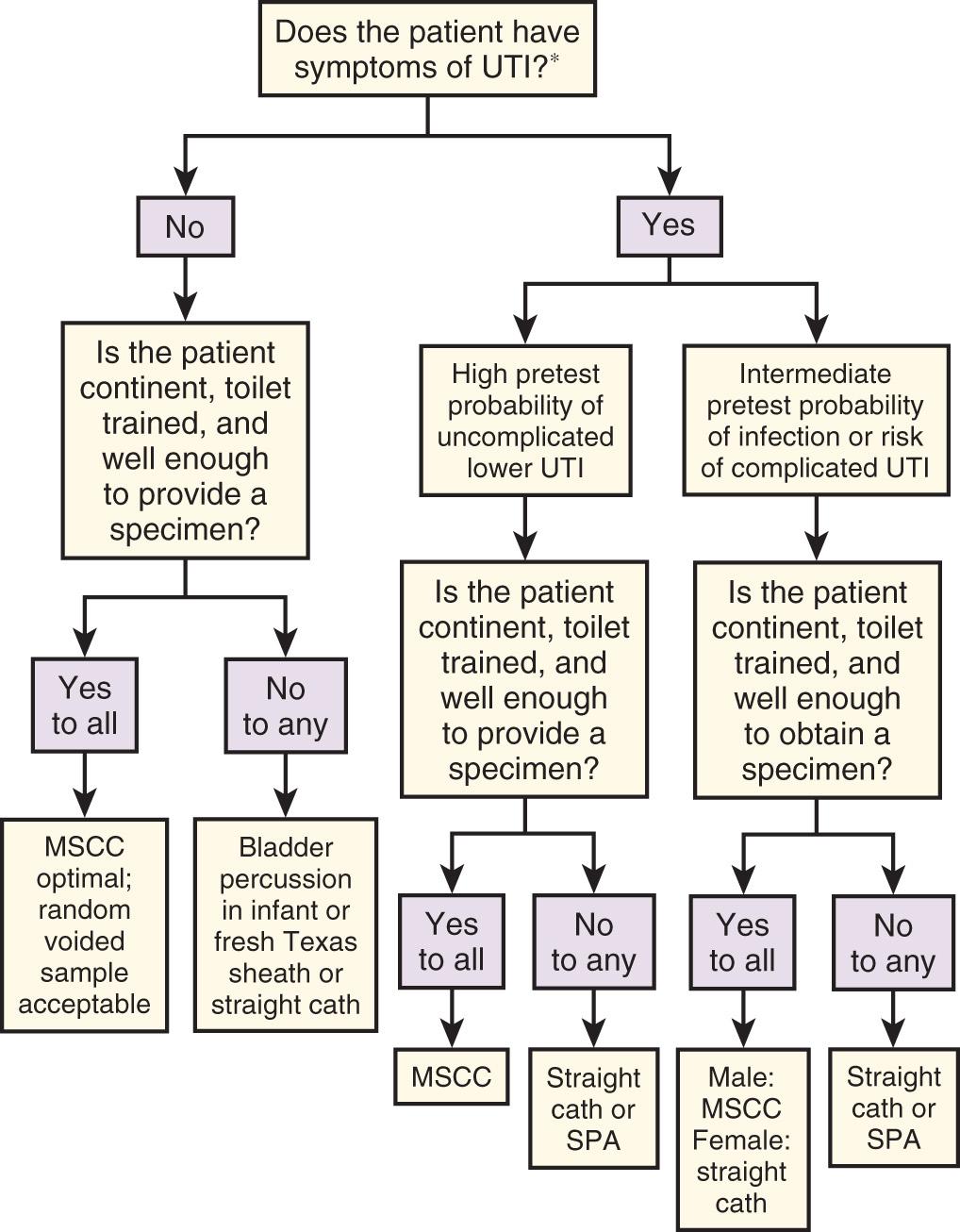
The issue of whether a patient is symptomatic might appear trivial, but the clinical practice of checking for UTI in most patients with any type of abdominal pain has important implications. Studies of urine collection and testing in symptomatic patients focus on the classic signs and symptoms of UTI (e.g., urgency, frequency, dysuria, flank pain, costovertebral angle tenderness) and do not include patients with nonspecific abdominal pain. Whether undifferentiated abdominal pain or fever constitutes a symptom of UTI has never been studied, and how to apply the results of studies done on patients with classic symptoms to those with nonspecific symptoms is unclear. Patients with classic symptoms need the most careful urine collection method because they have the most riding on the outcome of the test. This group of patients should include those with systemic signs of infection (e.g., fever and chills) who are unable to accurately report their symptoms and patients in whom failure to diagnose asymptomatic bacteriuria would be potentially dangerous (e.g., the immunocompromised, neonates and infants, pregnant patients, diabetics) or for whom urine cultures are going to be necessary because of a history of relapsing, recurrent, complicated, or childhood UTI.
In cooperative, motivated males and females with symptoms of uncomplicated lower UTI or pyelonephritis who are capable of diligently performing the necessary maneuvers, a midstream clean catch (MSCC) specimen is as accurate as a catheterized specimen, especially when the possibility of urethral or prostatic trauma and patient discomfort are considered. Lower resource utilization and avoidance of urethral or prostatic trauma and patient discomfort are additional advantages to an MSCC specimen. In patients who are unable to provide an MSCC specimen, or in children in whom thorough cleansing is more difficult, the increased accuracy afforded by a catheterized specimen is usually warranted.
If no symptoms of UTI are present, the urine examination can be considered a screening test. In asymptomatic patients, routine screening for bacteriuria is unwarranted in all but two clinical situations: pregnant women and all patients scheduled for urologic surgery. If only a urine culture is to be performed, some would argue that any spontaneously voided specimen would suffice because the diagnosis of asymptomatic bacteriuria depends on 10 5 or more CFUs of a single pathogen per milliliter of urine. With such criteria, contaminants are usually easily identified. Because performing cultures on all such patients is prohibitively costly, dipstick testing, urinalysis (UA), or both are commonly used for screening. With these tests, contamination by bacteria, leukocytes, or erythrocytes results in diagnostic confusion. The advantages of a less-contaminated specimen are worth the minimal, extra effort of asking the patient to provide an MSCC sample. The approach in Fig. 67.1 covers the vast majority of situations. A few circumstances and techniques deserve special mention.
The emergency clinician is familiar with how frequently a urine stream is generated in infants confronted by the alarming emergency department (ED) environment and a cold stethoscope. Rather than wasting a potentially perfect MSCC specimen on a laboratory coat with an ensuing delay in obtaining urine, the clinician can exploit the situation by approaching an infant with an open sterile urine container in hand in case the urine stream is spontaneously forthcoming. The process is facilitated by the application of cold povidone-iodine to the genitalia. Such an approach has been shown to generate a urine sample in a median time of 10 minutes. This is less than the typical time needed for straight catheterization or suprapubic aspiration (SPA), and it can be performed concomitantly with the history and physical examination and thereby circumvent an invasive procedure. If the urine specimen is not immediately forthcoming, a parent can be equipped with a sterile container and be instructed on collection of an ensuing specimen to free up ED staff for other tasks. Two techniques to actively induce voiding in infants have been described. The first, which is useful in newborns, exploits the Perez reflex. After cleansing the genitalia, hold the infant in one hand while stroking the paraspinal muscles in a cephalad to caudad direction. This causes extension of the back and flexion of the hips and induces micturition in less than 5 minutes in most cases. The second technique is known as “bladder tapping.” After urethral cleansing, if there is still no urine, use two fingers to tap on the suprapubic area at a rate of approximately once per second for a full minute, followed by a minute's rest. Repeat the cycle until urine is produced. The mean time before the production of a urine sample is approximately 5 minutes. This technique, though not practicable for the staff in a busy ED, can provide an infant's parents with a task that invests them in the clinical process. This clinical pearl may expeditiously furnish a specimen with significantly less investment of staff time than required for more invasive techniques. A combined method using finger-tap for 30 seconds alternating with lumbar stimulation with a light circular motion for 30 seconds after oral hydration achieved success in 86% of infants younger than 30 days old in less than 2 minutes.
The incidence of unsuspected UTI in a febrile neonate or infant is approximately 5%. Similar rates are found in asymptomatic children less than 5 years of age who present to the ED with nonspecific symptoms of acute illness. A true UTI in an infant or child requires subsequent evaluation for urinary tract pathology, and the disease may produce significant morbidity (e.g., hypertension, renal disease). One must be certain of the presence or absence of infection in this subgroup. The costs of overdiagnosis with a false-positive UA are significant and include bacterial resistance that leads to the use of increasingly expensive antibiotic medications. Numerous studies have demonstrated the disutility of urine specimens obtained for culture from a collection bag stuck to an infant's perineum. Bag specimens may be more sensitive than catheter specimens when used for UA or microscopy to identify infection in children at low or moderate risk for UTI. In this group it is acceptable to perform screening UA, microscopy, or both on a bag specimen. If negative, UTI is ruled out. If positive (leukocyte esterase or nitrite present, more than 5 WBCs/high-power field [HPF] on spun urine, or bacteria on an unspun Gram-stained specimen), it is followed by catheterization and culture, with treatment usually pending the results of culture. If a urine specimen is needed solely for chemical analysis (e.g., glucose, ketones, specific gravity), a bag specimen will suffice.
Urine obtained from any part of a chronic urinary drainage system is highly inaccurate for bacteriologic purposes. If UTI is suspected, insert a new catheter and obtain a fresh bladder urine specimen. A small study advocating replacement of a chronically applied Texas sheath catheter with a fresh one was performed on patients who did not have symptoms of UTI. Such a method might be sufficiently accurate for screening asymptomatic patients, but chronic asymptomatic bacteriuria is very common in such patients, and treatment is not recommended. In most cases, a Foley catheter should be used to obtain urine from patients with sheath catheters who have signs or symptoms of acute UTI and are unable to provide an MSCC specimen.
The low levels of bacteriuria found in 2% to 8% of patients after straight catheterization are generally below the threshold that defines the presence of UTI. Catheterization can cause minor local injury, as reflected by low-level hematuria in 15% of patients. SPA continues to be advocated by some for neonates in cases in which accurate diagnosis is essential and the risk for infection must be minimized. Both SPA (see Chapter 55 ) and catheterization have an approximately 25% failure rate as a result of an empty bladder. This problem and associated complications can be avoided by performing bedside ultrasound before the procedure. SPA may spuriously lower leukocyte or bacterial colony counts because of the necessity of filling the bladder before performing the procedure. A study in infants demonstrated that the discomfort associated with SPA is greater than that with catheterization. Surprisingly, however, older men who underwent both catheterization and SPA strongly preferred SPA.
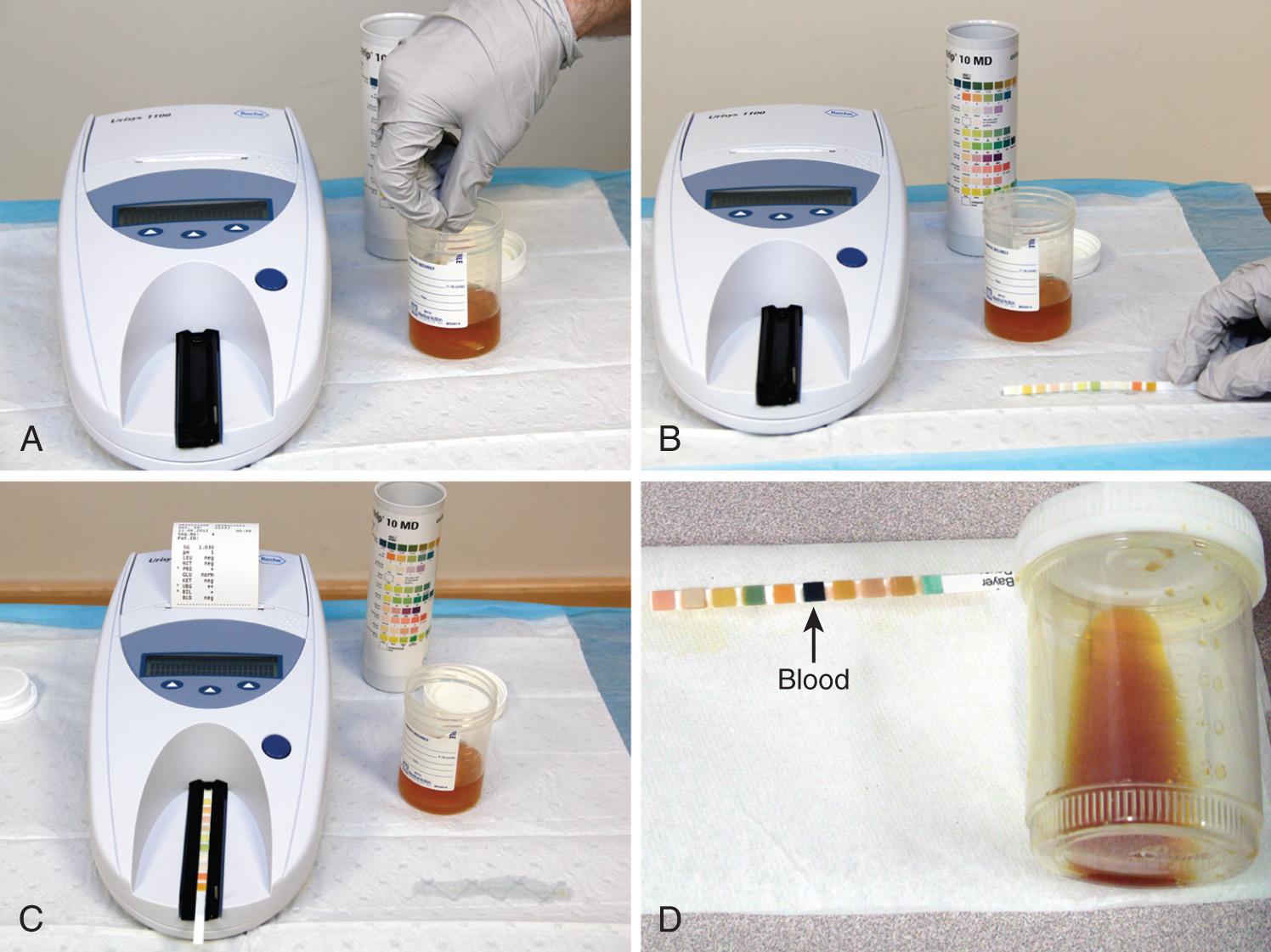
Urine dipstick tests are available to test 10 separate parameters. The unassuming appearance and commonplace use of the urine dipstick might lead one to mistakenly underestimate its technical sophistication. Each colored square on a urine dipstick involves a biochemically complex assay, and therefore it is essential to meticulously follow the manufacturer's instructions for storage and use. Even with optimal storage and testing conditions, the false-negative and false-positive rates of these tests are problematic. In addition, most of the tests are susceptible to interference from a variety of substances ( Table 67.2 ).
| SOURCES OF ERROR AND ARTIFACT | COMMENTS | |
|---|---|---|
| Glucose | False positive with peroxide, hypochlorite, ketonuria, levodopa, and the dipstick exposed to air False negative with ascorbate, ketones, uric acid, and high specific gravity |
Hypothermia may cause glycosuria despite hypoglycemia Glycosuria without hyperglycemia suggests renal tubular dysfunction |
| Ketones | False positive with ascorbate, low pH urine, high specific gravity, levodopa, valproate, phenazopyridine, N -acetylcysteine, high-protein diet, phenylketonuria, phthalein compounds | Very susceptible to deterioration with humidity and delay in analysis, which can cause false-negative results |
| Nitrites | False positive with phenazopyridine False-negative with high specific gravity, frequent urination, ascorbate, high urine pH, and urine standing in the specimen cup >2 hr |
75% false-negative rate when exposed to air for 15 days Does not detect reductase-negative bacteria |
| Protein | False positive with pH >7 and chlorhexidine False negative with low pH, very dilute urine |
Only reliable for albumin (glomerular proteinuria) Does not detect Bence-Jones protein Positive with pyuria, rarely with hematuria |
| Blood | False positive with povidone-iodine, certain (peroxidase-producing) bacteria, hypochlorite False negative with high specific gravity and high concentrations of urinary nitrites, ascorbate, or captopril |
Positive test with speckles or dots implies nonhemolyzed blood Positive test with a diffuse pattern implies hemolyzed red blood cells or high levels of myoglobin |
| Bilirubin | False positive with iodine, stool contamination, chlorpromazine, mefenamic acid False negative after prolonged standing |
Hard to read with agents causing marked urine discoloration |
| Urobilinogen | False positive with phenazopyridine, sulfisoxazole, sulfonamides, porphyrin, methyldopa, procaine, aminosalicylic acid, 5-hydroxyindolacetic acid False negative with sulfisoxazole and phenazopyridine |
Use a fresh specimen: rapidly broken down by light and in acidic urine |
| Leukocyte Esterase | False positive with vaginal contamination, oxidizing agents, eosinophils in urine, Trichomonas False negative with high glucose, ketones, protein (especially albumin), pH, and specific gravity, as well as with cephalexin, tetracycline, oxalates, ascorbic acid, neutropenia |
Sterile pyuria seen with tuberculosis, nephrolithiasis, interstitial nephritis |
| pH | Urea-splitting bacteria elevate pH Runoff from the protein strip can falsely lower pH |
Use a fresh specimen: standing raises pH by loss of CO 2 |
| Specific Gravity | Overestimates specific gravity with low pH, ketoacidosis, and protein Underestimates specific gravity with glucose, urea, or pH >7 |
Not reliable at specific gravity >1.025 Elevated with use of dextran, intravenous contrast material, proteinuria |
Test urine specimens as soon as possible after they are collected. If the urine has been standing, stir or shake the specimen well because cells sink rapidly in a container. Immerse the test strip completely for 1 second or less. Draw the edge of the strip along the rim of the specimen container and lightly tap it to remove excess urine, thus avoiding mixing the reagents between different test patches. Next, hold the strip horizontally or place it on a clean gauze pad until the recommended time has elapsed. Most strips are designed so that all the test results can be read together after 1 to 2 minutes ( Fig. 67.2 ).
The urine glucose test is normally negative. Urine glucose testing has limited usefulness in quantitative testing because the serum glucose level at which spillage occurs varies (although in most patients it starts at between 180 and 200 mg/dL). Changes in urine glucose lag behind changes in blood glucose by approximately half the interval between voids. Glycosuria in the absence of hyperglycemia suggests renal tubular dysfunction. Glycosuria may occur in hypothermic patients in the absence of hyperglycemia and indeed may actually occur with hypoglycemia in such patients.
Ketones are found in the urine of patients with starvation, inadequate carbohydrate intake, diabetic and alcoholic ketoacidosis, isopropyl alcohol poisoning, or glycogen storage disease. Tests for urine ketones are 5 to 10 times more sensitive to acetoacetate than to acetone, similar to the serum tests. Dipsticks do not detect 5-hydroxybutyrate, which accounts for 80% to 95% of the three ketone bodies and is the predominant form in the setting of ketoacidosis. Urine ketone testing is significantly more sensitive than serum ketone testing. There is generally no need to obtain serum acetones to diagnose or manage diabetic ketoacidosis when urine ketone monitoring is coupled with blood gas and anion gap analysis.
This portion of the dipstick test is designed to detect enzymes from the azurophilic granules in neutrophils. Normally the test is negative. Studies report a wide and clinically important range of thresholds for the sensitivity of dipstick testing, from 10 to 100 WBCs/µL urine. Studies suggest that the test is between 50% and 96% sensitive in detecting infection. Its specificity for the presence of WBCs is between 91% and 99%. The most common cause of a false-positive leukocyte test is vaginal contamination.
Normally, urine does not contain nitrites. Nitrites are specific (≈ 95%), but not sensitive (≈ 45%) indicators of UTI. Urinary nitrates are converted to nitrites most strongly by enteric coliform bacteria, thus explaining the nitrite test's 90% sensitivity in detecting UTI caused by Escherichia coli . Enterococcus , a moderately frequent urinary pathogen, Pseudomonas species, and Acinetobacter lack the reductase enzyme and are not detected by nitrite testing. False-negative results also occur because of the lack of dietary nitrate, frequent voiding, and diuresis. Early morning–voided specimens are ideal because they allow time for conversion of nitrate to nitrite, but they are rarely available in the ED. If possible, a specimen obtained more than 4 hours after the last voiding is preferred.
Proteins with a molecular weight below 50,000 to 60,000 daltons can pass through the glomerulus to be reabsorbed in the proximal tubule. Normal passage of protein in urine is less than 150 mg/24 hours, or approximately 10 mg/dL of urine. Approximately 10% to 33% of urinary protein is albumin, 33% is Tamm-Horsfall glycoprotein (secreted by renal tubular cells), and the balance is made up of a variety of immunoglobulins and other proteins. Proteinuria is a finding noted in approximately 5% of routine urine screens in men. This may represent a normal variant as 3% to 5% of healthy adults have postural proteinuria (proteinuria when standing but not when recumbent). Proteinuria is rarely clinically significant unless 3+ or greater is seen on the dipstick, although lower levels are still indicative of some degree of renal dysfunction (see later). The dipstick detects negatively charged proteins more strongly than positively charged ones; it is therefore most sensitive to albumin. A study of ED patients with severe acute hypertension identified renal dysfunction, defined as elevated serum creatinine, with 100% sensitivity when using urine dipstick detection of 1+ proteinuria or hematuria. Along similar lines, in patients being considered for radiographic contrast-enhanced studies who do not have a serum creatinine measurement, a negative dipstick test for protein or blood combined with the absence of prior renal disease, hypertension, diabetes, congestive heart failure, and age younger than 60 years effectively excludes renal insufficiency. The urine dipstick test is positive for protein with pyuria of greater than 6 WBCs/HPF. This is a false-positive finding for protein but is helpful when the urine dipstick is being used to screen for UTI because the threshold for leukocyte esterase is often significantly higher. Hematuria only slightly elevates urine protein levels.
In assessing a patient with proteinuria, it is helpful to divide the list of causes into those that are and those that are not associated with hematuria. These are listed in Box 67.1 . Elevated urinary protein is more commonly due to renal than systemic causes. The source is either glomerular, with passage of normally unfiltered proteins, or tubular, with failure to reabsorb physiologically filtered, low-molecular-weight globulins. The former condition causes albuminuria. Renal tubular proteinuria is characterized by low levels of urinary albumin and is therefore more likely to be missed.
Proteinuria Usually With Hematuria
Infectious Diseases
Multisystem Diseases
Primary Glomerular Diseases |
Proteinuria Usually Without Hematuria
Systemic Conditions
Medications, Drugs, and Toxins
Renal Diseases
|
The blood section of the urine dipstick is positive if exposed to RBCs, hemoglobin (Hb), or myoglobin. Urine in healthy volunteers contains fewer than 7 RBCs/mL. Studies have shown that the urine dipstick is very sensitive to 10 RBCs/mL. False-negative results are confined to clinically insignificant hematuria. The dipstick pad should be inspected for discrete positive “dots,” indicative of nonhemolyzed RBCs. Moderate intravascular hemolysis does not cause hemoglobinuria because Hb is tightly bound to haptoglobin and is therefore not filtered. Massive intravascular hemolysis gives rise to free plasma Hb with a molecular weight of 32,000 daltons, which easily passes through the glomerulus. Myoglobin has a molecular weight of 17,000 daltons, which also allows easy glomerular passage, but the dipstick has been shown to have a sensitivity of only 14% with heat-induced rhabdomyolysis, as reflected by serum creatine phosphokinase levels of up to 1000 U/L. Guidelines for distinguishing hematuria, hemoglobinuria, and myoglobinuria are outlined in Table 67.3 . In asymptomatic men older than 50 years, significant disease can be signaled by intermittent hematuria, thus mandating follow-up of patients with this incidental finding.
| HEMATURIA | MYOGLOBINURIA | INTRAVASCULAR HEMOLYSIS | |
|---|---|---|---|
| Serum Findings | Color: clear | Color: clear | Color: pink |
| Haptoglobin: normal | Haptoglobin: low | ||
| Urine Appearance | Color: clear to brown; clears with centrifugation | Color: clear to red/brown; no clearing with centrifugation | Color: clear to brown; no clearing with centrifugation |
| Urine Microscopy | RBCs, RBC casts, and protein: glomerular source | Possible occasional RBCs and tubular cells secondary to rhabdomyolysis-induced renal damage | Usually unremarkable |
| RBCs, no RBC casts, tubular cells, small protein: nephron source | |||
| RBCs alone: source distal to the nephron (e.g., ureterolithiasis) |
Dipsticks are vitiated by humidity and air, which can cause false-negative results after improper storage. Because RBCs may lyse rapidly, delays in performing UA may misleadingly suggest myoglobinuria or hemoglobinuria. Microscopy or dipstick testing of a freshly obtained specimen can clarify this issue. Conversely, high specific gravity or low pH can inhibit lysis of erythrocytes, which is necessary for the dipstick chemical reaction to occur, thus causing false-negative results. A study reproducing clinical conditions has demonstrated that povidone-iodine does not cause false-positive results on the dipstick. Iatrogenically caused trace positive results may occur after catheterization in 15% of cases.
Urine bilirubin represents the filtered, soluble, conjugated form of bilirubin. Unconjugated bilirubin is bound to protein and does not pass through the glomerulus. Bilirubinuria is therefore due to intrahepatic or extrahepatic cholestasis. Bilirubinuria will be detected significantly earlier than clinical jaundice. Urinary bilirubin excretion is enhanced by alkalosis. A fresh sample of urine should be tested because bilirubin glucuronide is hydrolyzed when exposed to light. Ascorbic acid and high levels of urinary nitrites decrease the sensitivity of the test to bilirubin.
In a healthy person, conjugated bilirubin is excreted in bile. In the colon it is broken down into a number of compounds, including urobilinogen. Most of these compounds are excreted in stool, which is the source of its characteristic color. A small amount of urobilinogen is absorbed from the colon, and if it is not taken up on the first pass through the liver, it enters the circulation. Ultimately, some of this urobilinogen may enter the urine, so it is normal to have zero to moderate levels of urinary urobilinogen on dipstick testing. Most diseases that cause hepatocyte dysfunction (hepatitis, cirrhosis, passive liver congestion, etc.) increase urinary urobilinogen excretion by impairing hepatic uptake of urobilinogen. As a qualitative test with a wide range of normal values it is rarely helpful, but in evaluating a patient with jaundice it can have diagnostic significance ( Table 67.4 ).
| HEALTHY NORMAL | COMPLETE BILIARY OBSTRUCTION | INTRAVASCULAR HEMOLYSIS | HEPATOCELLULAR DISEASE | |
|---|---|---|---|---|
| Urinary Bilirubin | None | Elevated | None | Elevated |
| Urinary Urobilinogen | None or present | None | Present, sometimes large | Normal early, increased late |
| Stool Color | Normal | Acholic | Normal | Normal |
The average daily excretion of 50 to 100 mmol of H + in urine gives rise to a typical urine pH of approximately 6 with a range from 4.5 to 8. Dietary protein lowers urinary pH, whereas fruit (especially citrus) and vegetables tend to raise it. The significance of pH testing is in the assessment of normal renal function. In most states of alkalosis and acidosis, healthy kidneys maintain homeostasis by conserving or excreting H + . Failure to do so suggests renal disease, especially renal tubular acidosis. An exception is the paradoxical aciduria of hypokalemic alkalosis secondary to volume contraction, hypercorticism, or diuretics, where the highest priority of the renal tubule is to conserve sodium. pH is elevated by the action of urea-splitting bacteria, especially Proteus species. This can occur with stasis of urine, either in the bladder or in specimen cups. A persistently alkaline urine is seen in patients with struvite (triple phosphate) urolithiasis.
The dipstick test for urine specific gravity assays for the primary urinary cations, sodium and potassium. True specific gravity, which is also dependent on anions, albumin, proteins, urea, and glucose, is therefore not measured. Artifactually low specific gravity readings are obtained with alkaline urine, whereas acidic urine and albumin falsely elevate the specific gravity reading. Consequently, some investigators believe that these strips on the dipstick test are of marginal clinical utility. Other clinical indicators of a patient's hydration status are probably more reliable. If necessary, a refractive specific gravitometer or a hygrometer should be used.
Microscopic UA is performed to identify cells, bacteria, and other microbes, as well as formed elements such as casts and crystals ( Fig. 67.3 ). The following discussion focuses on the findings of significance in diagnosing UTI: WBCs, bacteria, and WBC casts. The presence of WBCs with bacteria distinguishes infection from colonization (bacteriuria without pyuria). Some authorities state that significant infection without pyuria occurs in less than 5% of cases, thus making pyuria alone a sensitive marker of infection. Other studies do not support reliance on pyuria by itself as an indicator of infection. The presence of WBC casts distinguishes pyelonephritis (“upper UTI”) from cystitis (“lower UTI”).
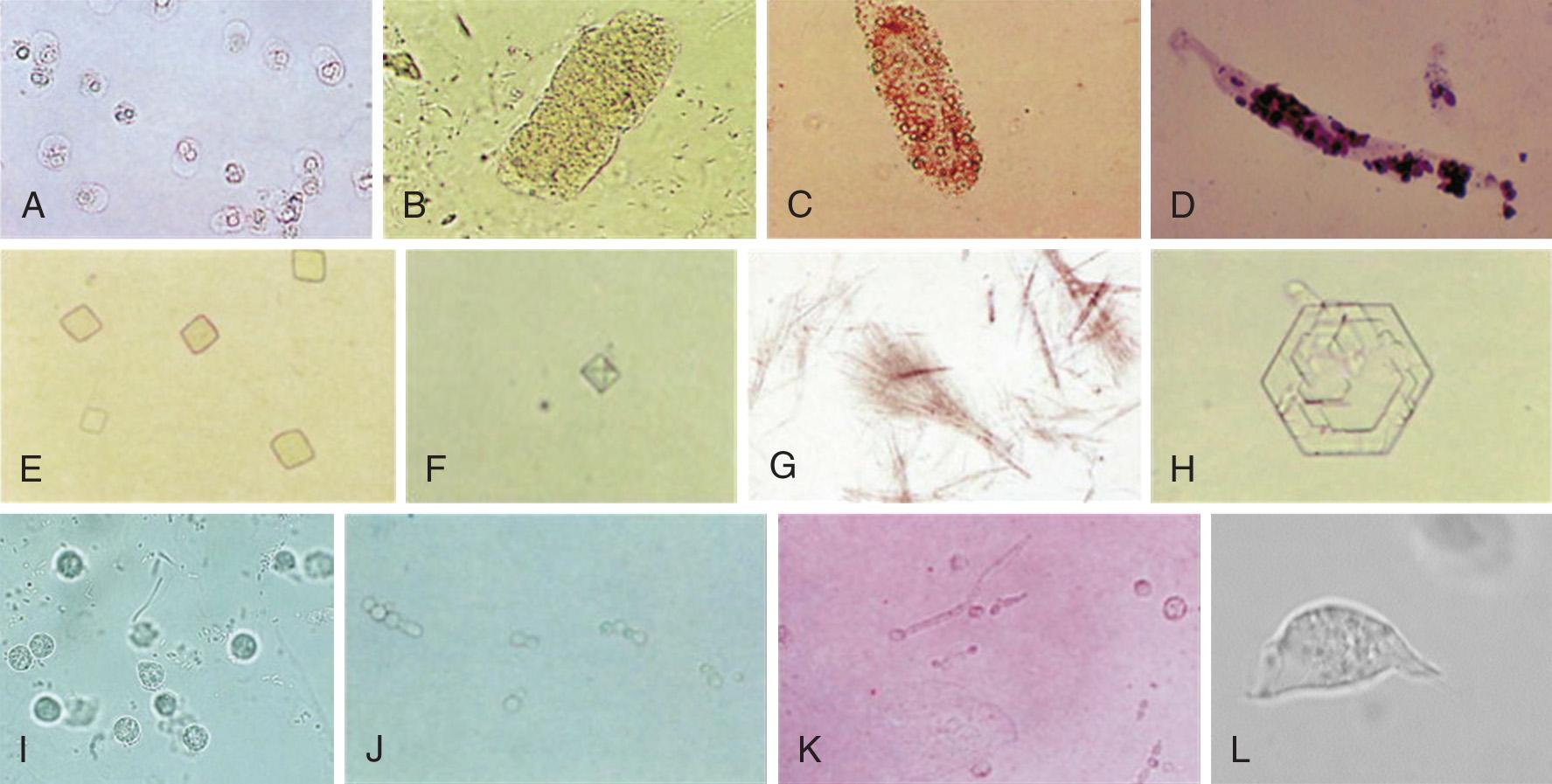
Microscopic UA is performed by one of five methods. Traditionally, the most common technique has been examination of unstained centrifuged urine. It has the advantage of concentrating formed elements that might otherwise be missed. Its disadvantage is that the presence and quantity of elements in a specimen will depend on many uncontrolled factors: the volume of the specimen, the duration and speed of centrifugation, the fragility of the formed elements, the volume of the “drop” in which the pellet is resuspended, and the size of the microscope's HPF.
Examination of unspun urine in a hemocytometer counting chamber. A hemocytometer is a precisely milled slide etched with measured squares, which allows exact enumeration of the cells in each square. Because the distance between the etched surface and the coverslip is known exactly, it is possible to determine the number of cells per unit volume of specimen. Enough fresh unspun urine is placed on the slide to fully cover the counting area, and the cells are counted. There should usually be less than 1 WBC/µL, although more than 10 WBCs/µL and more than 5 RBCs/µL are unequivocally abnormal. The threshold for diagnosing UTI is usually set at 8 or more WBCs/µL. Bacteria do not sink to the surface of the hemocytometer, so counting them through the many focal planes of the chamber is not possible, although methods to estimate the bacterial count per unit volume have been described. The hemocytometer is accurate, fast (time need not be spent on staining or centrifugation), and relatively easy to master. Its major drawbacks are cost (approximately $150 for the slide and cover slip) and fragility (easily destroyed if dropped). These characteristics are problematic given the conditions and resources in many EDs, although their use has been advocated by some. Formed elements other than WBCs and RBCs (e.g., casts) occur in such low concentrations that they are encountered in the hemocytometer only by chance, and therefore microscopy of spun urine is needed for their identification.
Examination of unspun, unstained urine placed on a regular microscope slide. This qualitative method is sometimes used for the diagnosis of UTI. Using 1 organism/HPF as a positive result, the sensitivity and specificity of detecting 10 5 CFUs/mL are between 60% and 90%. This method identifies only 1 WBC/HPF with the highly pyuric state of 250 WBCs/µL, which has led some to advocate the use of more than 1 WBC per low-power field as a criterion for infection.
Examination of unstained, centrifuged urine. With this method, 10 mL of urine is centrifuged at approximately 450 g (1000 to 4000 rpm) for 3 to 5 minutes. Roughly 9 mL of supernatant is poured off and the pellet is resuspended in the remaining fluid. This suspension is placed on a slide with a coverslip and examined. The larger formed elements, especially casts, tend to migrate to the edge of the coverslip, and they can be seen with low magnification. One or two casts, depending on the clinical context, may be normal; more are not. The morphology of the more commonly encountered formed elements of urine sediment is shown in Fig. 67.3 . The significance of each is beyond the scope of this text, but this information can be found in a standard textbook of clinical laboratory procedures and diagnostic testing. When examining centrifuged urine, more than 5 WBCs/HPF in the middle of the coverslip has traditionally been taken as being indicative of abnormal pyuria. Most authors have estimated that 10 WBCs/µL is equivalent to approximately 1 WBC/HPF; thus, this oft-cited threshold for diagnosing UTI is actually equivalent to 50 WBCs/µL in unspun urine. As the gold standard for the diagnosis of UTI is more than 8 WBCs/µL of unspun urine, many infections will escape detection with this method. Various numbers of bacteria per HPF have been used as criteria for the diagnosis of UTI. A threshold of 10 to 20 organisms/HPF has been recommended to rule out bacteriuria at the 10 5 -CFU/mL level. As noted previously, this threshold would not exclude infection in symptomatic patients.
Examination of Gram-stained, uncentrifuged urine. Also a semiquantitative measurement, it is estimated that 1 bacterium/HPF is equivalent to 10 5 CFUs/mL in bacterial culture. A drop of urine is placed on the slide, allowed to air-dry, and then heat-fixed and Gram-stained.
Examination of Gram-stained, centrifuged urine. This method is probably the optimal technique, short of culture, for the assessment of bacteriuria. It is more than 95% sensitive and more than 60% specific at 10 4 CFUs/mL, a concentration of bacteriuria that is an order of magnitude lower than the previously described methods. Detection of 1 organism per oil-immersion field constitutes a positive result. Specificity is increased to 95% if 5 organisms/HPF are seen.
The three tests commonly used to evaluate a patient for the presence or absence of UTI are urine dipstick, microscopic UA, and urine culture. Each represents an increasing degree of expense, delay, and resources. A brief discussion of their relative strengths and weaknesses ensues.
Dipstick testing of urine is faster than microscopic UA, is less labor-intensive, cheaper, and circumvents multiple sources of potential and proven error. Is it sufficiently accurate to replace UA; however, if either leukocyte esterase or nitrites are used to indicate infection, the dipstick is only 50% to 90% sensitive for culture-proven infection. This is not adequate to rule out infection in symptomatic patients, in whom the prevalence of disease is high, but may be acceptable in asymptomatic patients, in whom the test has a serviceably high negative predictive value of 95% to 99%. A large retrospective series demonstrated laboratory-performed UA (with microscopy) in children to be 8% more sensitive than dipstick testing in the ED, with similar specificity of approximately 80% for both tests. In symptomatic men, sensitivity is enhanced by taking a positive result in any one (or more) of either leukocyte esterase, nitrites, protein, or blood as an indication of UTI. This process can be augmented by allowing extra time before reading the strip. For women with classic symptoms of UTI, empirical treatment is recommended because no test can rule out infection. A negative dipstick test in a patient with a high pretest probability of UTI should prompt a search for an alternative source of the patient's symptoms. Maneuvers to enhance dipstick sensitivity diminish its specificity, which may be as low as 26%. This has led some to advocate microscopic UA on all urine specimens that are found to be abnormal by dipstick, but this probably adds little to a carefully performed dipstick test.
If the urine dipstick has such poor specificity when it is used as a test with adequate sensitivity, should it be discarded altogether and microscopic UA be relied on instead? Apart from the hemocytometer, the most reliable method for identifying significant bacteriuria is oil-immersion microscopy of Gram-stained, centrifuged urine. Nonetheless, the practice in most hospital laboratories is to examine a resuspended pellet of unstained, centrifuged urine. The problems with this method have been discussed. In various studies a range between 1 and 10 organisms/HPF or 5 WBCs/HPF has been considered a “positive” test (as the threshold number rises, so does specificity, at the price of sensitivity). In aggregate, the accuracy of microscopy in the diagnosis of UTI is similar to that of the dipstick alone, with 22% false-positive and 23% false-negative rates when compared with culture. Microscopic UA, like the dipstick, cannot rule out infection in symptomatic patients. The specificity of pyuria is improved when viewed as a marker of all genitourinary infections, including urethritis, prostatitis, epididymitis, vaginitis, and cervicitis. These diagnoses should always be entertained in patients with urinary symptoms, especially those with sterile pyuria.
Cultures are indicated for any potentially complicated UTI, including infections in children; men; women with recurrences or relapses; immunocompromised individuals; patients with urinary tract pathology, including stones and possible pyelonephritis; and pregnant patients. Cultures are usually recommended at approximately the 16th week of gestation. Every effort should be made to obtain a high-quality specimen because of the difficulty in distinguishing contamination from significant low-count bacteriuria in symptomatic patients and in view of the expense of cultures and treatment if the culture is positive.
It is well established that female patients with symptoms of uncomplicated lower UTI can be treated without culture because the prevalence of disease is 50% or greater in women with classic urinary symptoms. Treatment is generally benign, and 95% of urine cultures that are positive will yield a limited number of organisms with predictable antibiotic susceptibilities. Some authors recommend dipstick or microscopic UA (or both) because a negative test might prompt more careful consideration of alternative diagnoses in this group, with a high incidence of sexually transmitted disease. If none is found, such patients can be treated empirically.
Fluoroquinolone antibiotics are no longer recommended as empiric treatment for uncomplicated UTI. Traditional choices have included trimethoprim-sulfamethoxazole or macrodantin, but both of these agents have high resistance rates in many locations, so choices should be based on local microbial susceptibility patterns, if available.
Urine testing is more likely to influence clinical management in patients with an intermediate pretest probability of infection. Urine microscopy may also help in distinguishing pyelonephritis (WBC casts), vaginitis (absence of pyuria or hematuria in a meticulous MSCC or catheterized specimen), and urethritis (pyuria, rarely hematuria) from cystitis (pyuria and often hematuria). In the ED, the ease of performing a dipstick test argues for its use without UA because it is equally sensitive unless formed elements such as casts, crystals, Trichomonas, or other parasites are suspected. In patients with pyelonephritis, urine cultures are warranted because they alter therapy in approximately 5% of cases. In most asymptomatic patients, a negative dipstick or UA result has sufficient negative predictive value to rule out disease unless the patient is pregnant or undergoing urologic surgery. Special clinical considerations in some patients (e.g., the immunocompromised, diabetic females at high risk for UTI) may mandate adjustments to this approach. Recommendations vary regarding infants. Cultures are recommended for all febrile infants younger than 2 months and for children who appear sick or have a high pretest probability of infection. In a 2009 study, combined microscopic and dipstick UA was only 64% sensitive (and 91% specific) for culture-proven UTI in febrile infants younger than 24 months, thus suggesting the use of culture unless an alternative source of infection is clear.
Some investigators have found female patients reliable in determining their own pregnancy status. Others have not. With respect to ordering radiographic studies on women of childbearing age, clinicians should determine from their own practice experience the reliability of patient history in this regard. If in doubt and with the potentially lethal outcome of an ectopic pregnancy, it might be prudent to use an objective test. Pregnancy tests are based on the detection of β-human chorionic gonadotropin (β-hCG) in serum or urine. β-hCG is secreted by trophoblastic cells of the placenta starting from the time of implantation of the blastocyst. Qualitative serum and urine tests can detect β-hCG levels of between 15 and 25 mIU/mL ( Fig. 67.4 ). The concentration of β-hCG is usually lower in urine than in serum, which accounts for the slight advantage of serum tests in detecting early pregnancy. Optimal results on urine pregnancy tests are obtained with first-voided, concentrated morning specimens. It has recently been established that whole blood is a reliable specimen for the bedside dipstick tests used in most EDs. The same volume of blood is placed in the testing well(s) as when performed with urine. This alternative may be lifesaving in timely recognition of an ectopic pregnancy in a hypotensive female with pelvic complaints and unknown pregnancy status when a urine specimen is unavailable or results in significant delay.
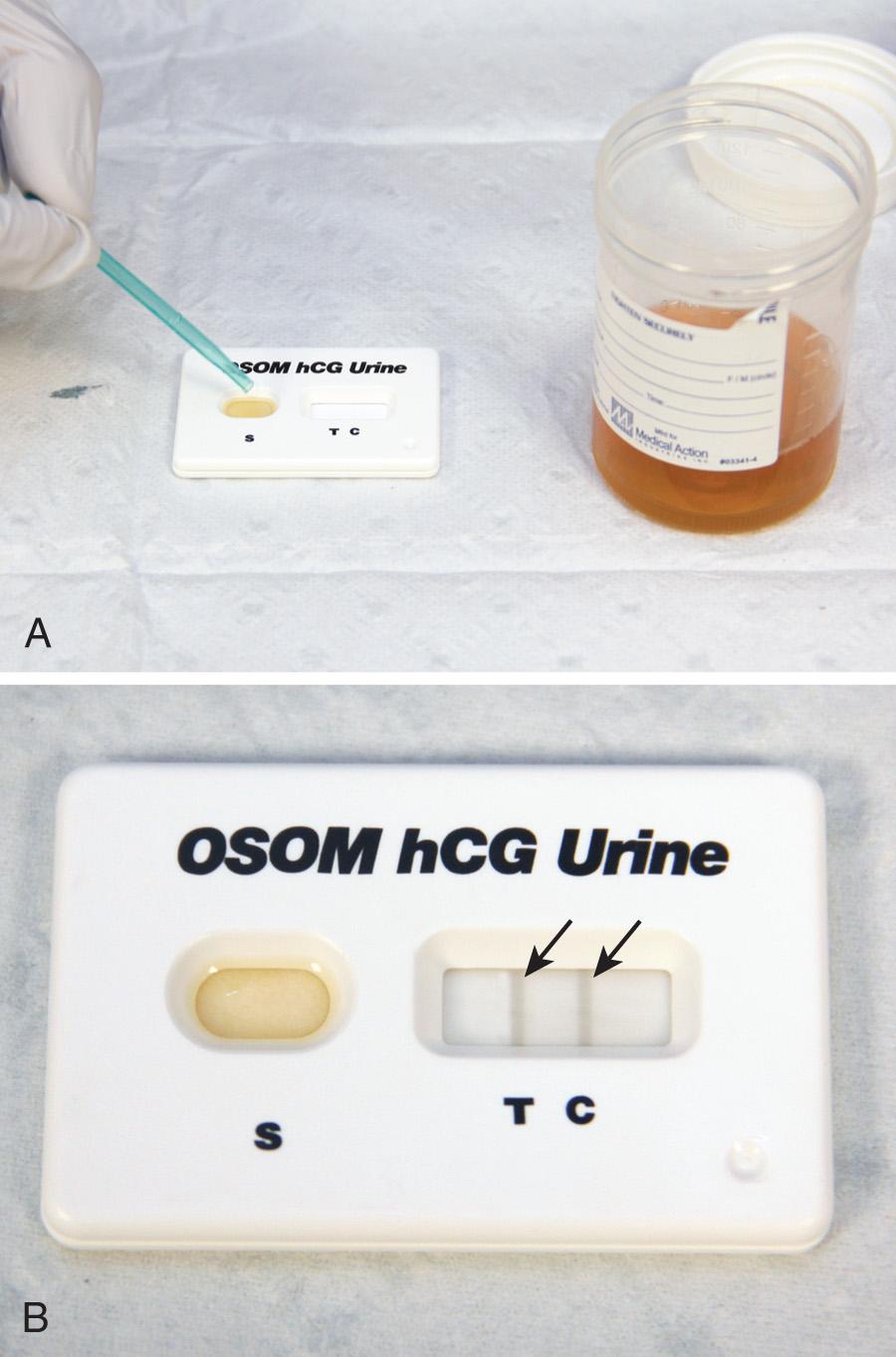
If fertilization has occurred, β-hCG levels of 5 to 8 mIU/mL (the threshold of the quantitative serum test) correspond to the 9th to 11th day after ovulation (23 to 25 days after the first day of the last normal menstrual period). In a viable intrauterine pregnancy (IUP), the β-hCG level doubles approximately every 2.5 days during the first 4 weeks of gestation and reaches a serum level of greater than 25 mIU/mL, which is detectable by virtually all pregnancy tests on the first day of the missed menstrual period. The doubling rate declines to every third day thereafter. β-hCG reaches a peak of between 100,000 and 200,000 mIU/mL between the 10th and 14th gestational weeks and declines to 10,000 to 20,000 mIU/mL for the rest of the pregnancy ( Table 67.5 ). There is a wide range of β-hCG levels in different women at the same stage of gestation, thus making definite clinical determination on the basis of a single quantitative test impossible. False-positive test results have been described in association with molar pregnancy, choriocarcinoma, teratoma, occasional malignancies outside the genitourinary tract, and very high levels of proteinuria. There is one report of a positive urine β-hCG finding (but normal serum β-hCG) associated with a tuboovarian abscess.
| TIME ELAPSED FROM THE FIRST DAY OF THE LAST NORMAL MENSTRUAL PERIOD | Qβ-hCG LEVEL (mIU/mL) USING THE IRP | ULTRASOUND FINDINGS |
|---|---|---|
| < 28 days | 5–50 | |
| 4–5 wk | 50–500 | From approx. 4.5 wk and Qβ-hCG level of 1000–1500 |
| EVU can show a nonspecific intrauterine sac | ||
| 5–6 wk | 100–10,000 | Definitely abnormal if no DDS seen on TVU with a Qβ-hCG level >2000 or by TAU with a Qβ-hCG level >6500 |
| Abnormal if no YS with an MSD >10 mm | ||
| 6–7 wk | 1000–30,000 | FP, cardiac activity 5.5–7 wk, Qβ-hCG level >10,000 |
| Abnormal if no FP with an MSD >18 mm | ||
| 7–8 wk | 3500–115,000 | |
| 8–14 wk | 12,000–270,000 | |
| >10 wk | 270,000–15,000 |
Although a previous quantitative β-hCG level is rarely available to the emergency clinician, doubling rates are an important part of the assessment of a healthy first-trimester pregnancy. Fetal nonviability, ectopic pregnancy, and intrauterine demise are signaled by abnormalities in the predicted rise in quantitative β-hCG. A serum quantitative β-hCG level that does not increase by 66% every 48 hours has a 75% chance of being a nonviable pregnancy. β-hCG levels in a healthy IUP and associated sonographic findings are listed in Table 67.5 .
The rate of decline in quantitative β-hCG after gestation depends on the reason for the conclusion of the pregnancy. After a term delivery, β-hCG falls to zero in 2 weeks; after surgery for an ectopic pregnancy, the range is 1 to 31 days, with a median of 8.5 days; after a first-trimester spontaneous abortion, the range is 9 to 35 days (median, 19 days); and after a first-trimester elective abortion, the range is 16 to 60 days (median, 30 days).
The association between β-hCG levels and gestational dates has led to the concept of the “discriminatory zone.” In a normal pregnancy, a quantitative β-hCG level of 1000 to 2000 mIU/mL should be reflected by the presence of a double decidual sac (at the least) on transvaginal ultrasound (and on transabdominal ultrasound with levels higher than 6500 mIU/mL). The clinician should beware of several potential pitfalls in applying this concept. First, it applies only to the double decidual sign, itself subject to interobserver variation among sonographers. The discriminatory zone cannot be extrapolated to the expected levels at which any of the more definitive sonographic signs of IUP such as yolk sac, fetal pole, or cardiac motion should be seen. Second, although it is true that if the double decidual sign is absent at these thresholds, the pregnancy is almost certainly abnormal with a significant possibility of being ectopic, the converse—that it is pointless to perform ultrasonography if the quantitative β-hCG level is lower than 1000 mIU/mL—is not true. This is because the discriminatory zone does not preclude the possibility of identifying an ectopic pregnancy (or an IUP) before the quantitative β-hCG level reaches 1500 mIU/mL. Ectopic gestational sacs are pathological and therefore do not display β-hCG levels according to nomograms established for normal IUPs, and advanced ectopic pregnancies may have low β-hCG levels. One percent of ectopic pregnancies have a quantitative β-hCG level of less than 10 mIU/mL, and approximately a third of ectopic pregnancies are diagnosed in patients with a quantitative β-hCG level lower than 1000 mIU/mL. Even if no definitive diagnosis can be made, the clinician should be aware that pregnant ED patients with pelvic complaints and a β-hCG level of 1000 mIU/mL or lower have a fourfold increased risk for ectopic pregnancy in comparison to those with the same symptoms and a β-hCG level higher than 1000 mIU/mL.
In summary, the discriminatory zone provides a basis for interpreting the ultrasound image, but not a basis for deciding whether to perform it. Although the percentage of patients below the discriminatory zone who will receive a definitive diagnosis by ultrasound is much lower than the percentage in those above the discriminatory zone (25% vs. 90%), even a 25% diagnosis rate would seem to merit pursuit for a potentially lethal condition, especially as follow-up for patients whose evaluation is indeterminate on their ED visit is time-consuming and resource-intensive.
Blood cultures are indicated when the clinical findings suggest an otherwise unidentifiable bacteremic state ( Box 67.2 ). Twenty-five percent of patients with documented bacteremia have periods without fever. In the elderly the proportion is even higher, with 50% of bacteremic patients older than 65 having a temperature between 97.1°F (36.2°C) and 100.9° F (38.3°C) and at least 13% with no documented temperature higher than 99.1°F (37.3°C) at any time. In the elderly, increasing age, vomiting, altered mental status, urinary incontinence, presence of a Foley catheter, or greater than 6% band forms is predictive of a positive blood culture. The subjective impression of “having fever” in adults is not a reliable indicator of the presence of fever, although the subjective impression of “no fever” is much more likely to be accurate. Prediction models to optimize the use of this costly test have been explored but are cumbersome, add little to educated clinical judgment, and lack widespread validation or acceptance. Many studies have shown that blood cultures in patients with uncomplicated pneumonia or pyelonephritis are of very limited clinical value, although a 2010 prospective investigation of patients with upper UTI found that malignancy, an indwelling urinary catheter, and ongoing antimicrobial treatment were clinical conditions that made it significantly more likely that blood cultures would be discordant with urine cultures and that they would reveal clinically important additional information. Blood cultures are obtained during 2.8% of all ED visits in the United States, and in a single 3-year period this rate increased by 33%. As disease prevalence is unlikely to have changed so rapidly, this increase is probably due to changes in practice. These changes have been coincident with regulatory agency–mandated blood cultures in patients being evaluated for pneumonia and have led to calls for more stringent criteria for obtaining blood cultures.
Unexplained alterations in mental status, functional status, or autonomic status in a previously healthy patient between the ages of 5 and 65
No source found and younger than 2 years, older than 65, or immunocompromised
Age younger than 2 months
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here