Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
B-cell chronic lymphocytic leukemia (B-CLL) is a clonal disorder of mature B lymphocytes, which are characterized by small, round nuclei; highly condensed chromatin; inconspicuous nucleoli; scant cytoplasm; and unique immunophenotype. Admixed are occasional large prolymphocytes with prominent nucleoli, which usually account for less than 10% of leukemic cells in a typical CLL. CLL is heterogeneous and encompasses a diverse spectrum of morphologic, immunophenotypic, or genetic variants ( Table 14-1 ).
| Entity | Variants |
|---|---|
| CLL/SLL † |
|
| B-prolymphocytic leukemia | No variants described, but overlap with leukemic MCL is problematic |
† Borderline with monoclonal B lymphocytosis in blood, bone marrow, and lymph node problematic.
Peripheral blood and bone marrow are typically involved in CLL. Lymph node is by definition the primary site of involvement in small lymphocytic lymphoma (SLL). Liver and spleen are often involved. Other extranodal sites may be involved as well. Diagnostic criteria for CLL per the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues include that in the absence of extramedullary tissue involvement, there must be a sustained, persistent monoclonal lymphocytosis of greater than 5 × 10 9 /L with a CLL immunophenotype in the peripheral blood (PB). Although bone marrow lymphocytosis is also present, bone marrow examination is often not required for a diagnosis of CLL. Many CLL cases can be successfully diagnosed by morphologic review of blood smears with immunophenotypic confirmation.
The term SLL is used for non-leukemic cases with the tissue morphology and immunophenotype of CLL. The distinction between CLL versus SLL is based primarily on disease distribution and the number of circulating leukemic cells. Disorders with a predominant extramedullary disease distribution and less than 5 × 10 9 /L circulating leukemic cells in blood are termed SLL ( Box 14-1 ). Within the lymph node, larger cells (so-called prolymphocytes/paraimmunoblasts ) often form pale foci called proliferation centers in a background of monotonous small lymphocytes. Although CLL and SLL have been historically regarded as the same disease with different manifestation, recent data show that they may differ by the expression of chemokine receptors (reduced expression of CCR1 and CCR3 in SLL cells), integrins (lower expression of integrin αLβ2 in CLL cells), and genetic abnormalities (high incidence of trisomy 12 and lower incidence of del13(q) in SLL). These expression differences may underlie the different clinical presentations.
* Reference .
Sustained absolute mature, monoclonal lymphocytosis ≥5 × 10 9 /L
Monoclonal B cell with mature phenotype, CD5 and CD23 co-expression, weak CD20, weak SIg (IgM/IgD)
Extramedullary sites of disease predominate, especially lymph node
Diffuse infiltrate of small lymphocytes, proliferation foci
Monoclonal B cells <5.0 × 10 9 /L in blood
Monoclonal B cell with mature phenotype, CD5 and CD23 co-expression, weak CD20, weak SIg (IgM/IgD)
CLL, chronic lymphocytic leukemia; IgD, immunoglobulin D; IgM, immunoglobulin M; SLL, small lymphocytic leukemia.
Monoclonal B-cell lymphocytosis (MBL) is an asymptomatic hematologic condition characterized by the presence of less than 5 × 10 9 /L monoclonal B lymphocytes in the peripheral blood, bone marrow, or tissue. The majority of MBL cases show an immunophenotype that is indistinguishable from CLL, whereas a small subset has a non-CLL immunophenotype. MBL also shares similar genetic abnormalities with CLL.
Richter transformation is defined as transformation of CLL into a more aggressive lymphoma. It occurs in 2% to 10% of CLL/SLL patients. The vast majority of transformations are to diffuse large B-cell lymphoma (DLBCL), although transformation to Hodgkin's lymphoma sometimes occurs.
Chronic lymphocytic leukemia is the most common leukemia of adults in Western countries. It is also the most common familial leukemia. The incidence of CLL rises dramatically with age, and the incidence reaches 16.7 per 100,000 persons at age 65 years. CLL is more common in men than in women with a male : female ratio of 1.9 : 1 per SEER incidence report. It is most commonly diagnosed in the 70 to 79 years age group, with a median age at diagnosis of 73 years. A study on CLL in patients younger than 55 years reveals slightly different biology and clinical course than CLL in older patients. Younger patients are more likely to have Rai stage I or II disease, with IGHV (immunoglobulin heavy chain variable region genes) unmutated, with ZAP-70 expression. The time to treatment is shorter when compared with older patients. Although survival of CLL patients younger than 55 years is longer than patients older than 55 years, their survival is profoundly shortened when compared with the age-matched and sex-matched normal population. CLL is very rare in the Asian population, although recent data have shown an increase in incidence, suggesting an environmental role.
Environmental, medical, and occupational risk factors for CLL have been extensively studied with inconclusive results. Studies linking CLL with radiation exposure have had inconsistent findings as well. Most studies on populations exposed to environmental, medical, or occupational sources of ionizing radiation have not found evidence of a connection. Chemical exposures appear to be associated with increased risk for developing CLL, particularly in farmers and other agricultural workers, rubber workers, and petroleum workers.
Familial history of CLL or other related diseases such as MBL and non-Hodgkin lymphoma, especially low-grade lymphoma, is one of the strongest risk factors for the development of CLL. In epidemiologic case-control and cohort studies, the relative risk for CLL in first-degree relatives of CLL patients is approximately 7.5. The risk for developing other lymphoproliferative disorders (LPDs) and MBL is also increased. Studies indicated that the familial cases present about 10 years earlier than sporadic cases and may imply a more aggressive clonal expansion.
CLL typically occurs in older adult patients and has an indolent clinical course. Up to 70% of patients are asymptomatic at presentation, and the disease is discovered incidentally by routine complete blood count (CBC) and smear review. Other patients may present with various signs and symptoms including peripheral lymphadenopathy, hepatosplenomegaly, autoimmune cytopenia, systemic symptoms (weight loss, night sweats), and/or cytopenias-related symptoms (weakness, fatigue, or bleeding). Low-level monoclonal paraprotein may present in up to 50% of CLL patients.
Cytopenia is often related to leukemic infiltrate of the bone marrow leading to compromised hematopoiesis. Cytopenia can also be secondary to autoimmune phenomenon, hypersplenism, treatment, or non–CLL-related etiology. Autoimmune complications are seen in 4% to 25% of CLL/SLL patients and include autoimmune hemolytic anemia (AHA), immune thrombocytopenic purpura (ITP), pure red cell aplasia, and autoimmune agranulocytosis. AHA is reported in 2% to 25% of CLL patients, whereas ITP is seen in approximately 2% of CLL patients.
CLL is associated with a profoundly impaired immune system characterized by recurrent infections and failure of antitumor immune response. CLL patients have a decreased number and function of B cells, resulting in defective humoral immunity. They also have defective cell-mediated immunity with decreased T-cell subsets and natural killer (NK) cells. Abnormalities in complement activity, in monocytes, and in neutrophils are also common. CLL patients may also have an increased number of regulatory T cells and nurselike cells. Hypogammaglobulinemia is common in CLL and is a contributing factor to infections. Its severity increases with duration and stage of the disease. Although there is still limited understanding of the initiating events of immunodeficiency in CLL, recent literature has discovered progressive loss of plasmacytoid dendritic cell (pDC) function, which underlies the major immunodeficiency affecting CLL patients. pDCs are highly specialized immune cells that play a major role in promoting innate and adaptive immune responses. Decreased pDC function results in decreased interferon alpha (IFNα) production, and the latter is a cytokine that is critical in immunity and has well-described anti-tumor activity.
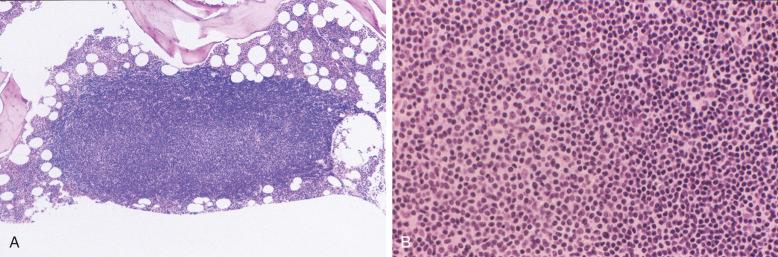
Small lymphocytic lymphoma (SLL) accounts for about 5% to 10% of CLL/SLL overall. Lymph node involvement in CLL/SLL is generally characterized by diffuse effacement of the architecture with variably preserved sinuses. The infiltrate consists of small, round lymphocytes with condensed chromatin and scanty amounts of cytoplasm, imparting a dark color on low magnification. Mitotic activity is virtually absent in these areas. Interspersed among these diffuse sheets of small, round lymphocytes are proliferation foci that produce a vague, pale, nodular pattern ( Fig. 14-1 ). On high magnification, proliferation foci are composed of larger lymphoid cells (so-called prolymphocytes and paraimmunoblasts ) with more abundant cytoplasm and more conspicuous nucleoli (see Fig. 14-1, C ). By assessment of DNA synthesis such as Ki67 staining, these proliferation foci represent the mitotically active portion of the neoplastic clone. Occasionally, proliferation foci may be very large and confluent, raising concerns of transformation to large cell lymphoma ( Fig. 14-2 ). Recent studies link adverse outcome to lymph node sections with expanded and highly active proliferation foci.
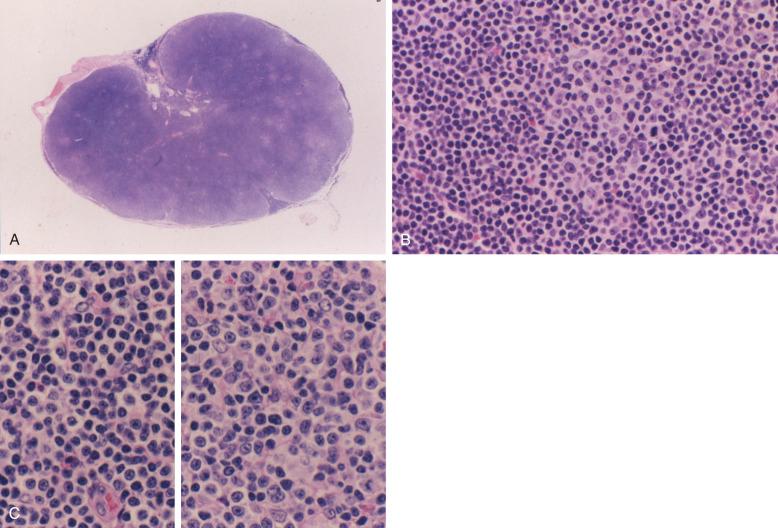
In a minority of CLL/SLL cases, the pattern of lymph node infiltration is interfollicular or exhibits a perifollicular/marginal-zone–type pattern resulting in potential differential diagnostic confusion with mantle cell lymphoma, marginal-zone lymphoma, or other B-cell neoplasms. Likewise, some cases exhibit greater nuclear irregularity of the small lymphocytes, reminiscent of mantle cell lymphoma or even follicular lymphoma. However, the presence of proliferation foci, the distinctive immunophenotypic profile including LEF1 expression, and the absence of cyclin D1 and SOX11 expression support a diagnosis of CLL/SLL (see the section on differential diagnosis later in the chapter). Some authors suggest a link between atypical features of circulating lymphocytes and this lymph node picture with greater cytologic atypia than is encountered in prototypic CLL/SLL cases (see the section on atypical/mixed CLL later in the chapter). Recent studies suggest that lymph node specimens that contain lymphocytes with a “CLL-immunophenotype” may represent nodal monoclonal B lymphocytosis if the node is small and there are no proliferation foci (see the section on monoclonal B-cell lymphocytosis later in the chapter).
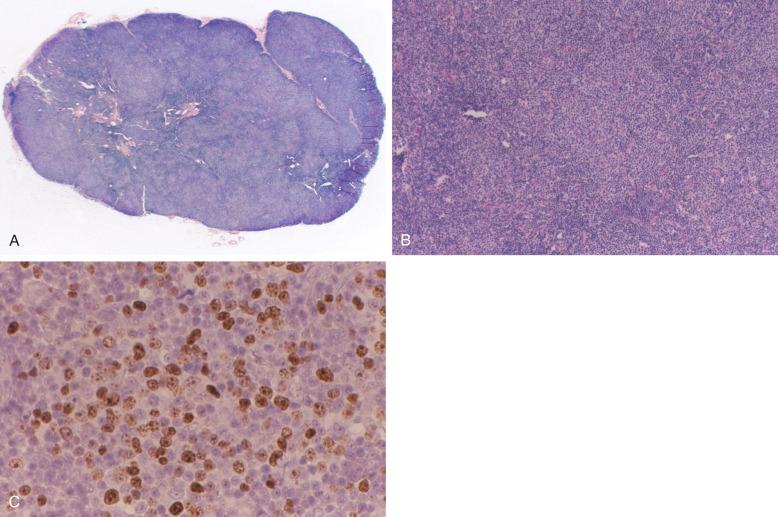
Although the degree of splenomegaly is highly variable in CLL patients, some patients exhibit significant and symptomatic splenic involvement. Splenic involvement by SLL/CLL is characterized by white pulp expansion producing a miliary micronodular appearance grossly ( Fig. 14-3 ). The white pulp infiltrates consist of small, round lymphocytes with scant cytoplasm similar to those seen in lymph node ( Fig. 14-4 ). Although occasional prolymphocytes and paraimmunoblasts may be present, typical proliferation foci generally are not readily apparent. Extension of the lymphoid infiltrate into the periarteriolar lymphoid sheath, along the splenic trabeculae, and into both the red pulp cords and sinuses is common.

A mature lymphocytosis is a diagnostic requirement for CLL, with at least 5000/mm 3 as a minimum absolute monoclonal lymphocyte count with CLL immunophenotype (see Box 14-1 ). These lymphocytes are typically monotonous with relatively homogeneous features including small, round nuclei with highly condensed nuclear chromatin and inconspicuous nucleoli ( Fig. 14-5 ). The exaggerated chromatin clumping may give a “cracked mud” appearance to these nuclei. These leukemic lymphocytes are easily disrupted during smear preparation creating smudge cells, which may be numerous ( Fig. 14-6 ). Larger lymphoid cells or lymphocytes with irregular nuclear contours may be evident, but these cells usually compose less than 2% of the leukemic population ( Fig. 14-7 ). In cases in which these “atypical” forms are conspicuous, a diagnosis of atypical/mixed CLL or CLL/PL may be preferred to convey to the clinician these aberrant morphologic features (see the section on morphologic variants later in the chapter). In general, the agranular cytoplasm is scant, but some CLL cases will demonstrate moderate amounts of cytoplasm. Distinctive cytoplasmic vacuoles, crystals, or globules are noted occasionally.
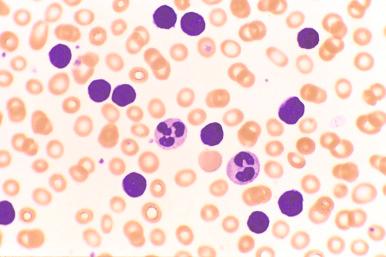
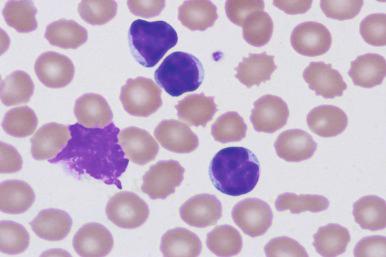
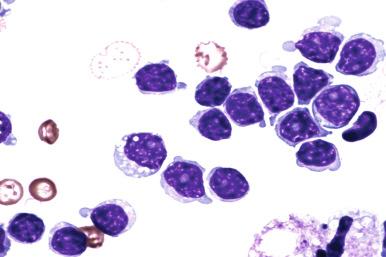
The number of prolymphocytes, clonal cells with a larger nuclear size and a single, distinct nucleolus, should be assessed (see Fig. 14-7 ). In some patients, an increasing proportion of prolymphocytes may be a harbinger of clonal transformation, whereas other stable CLL cases demonstrate consistently high numbers of prolymphocytes. Any significant change in the blood picture should prompt re-evaluation of the patient's disease status.
Although not necessary for diagnosis, bone marrow examination in CLL patients may be useful in assessing residual hematopoiesis and in determining the pattern and extent of bone marrow effacement. On aspirate smears, CLL cells exhibit similar cytologic features to circulating clonal cells ( Box 14-2 ). These small, mature lymphocytes are generally abundant on aspirate smears, although variability is expected due to the multifocal nature of neoplastic infiltration of the bone marrow. The extent of bone marrow replacement by CLL generally parallels the proportion of lymphocytes on differential cell counts, although the extent of disease is best assessed on core biopsy specimens. In most cases, there is substantial residual preserved hematopoiesis, reflecting the typical blood finding of normal red blood cell, neutrophil, and platelet counts.
Small lymphoid cells with round nuclear contours, condensed chromatin, and inconspicuous nucleoli analogous to appearance in blood
Extent of BM involvement variable; ≥30% lymphocytes is considered characteristic
Non-paratrabecular lymphoid nodules with closely packed round nuclei, scant cytoplasm, and minimal mitotic activity
No predilection for paratrabecular localization
May also see a diffuse increase in interstitial lymphocytes admixed with hematopoietic elements
Diffuse solid bone marrow effacement relatively uncommon
Proliferation foci may be present, but true germinal centers only rarely described (2 cases)
Reference .
CLL, chronic lymphocytic leukemia.
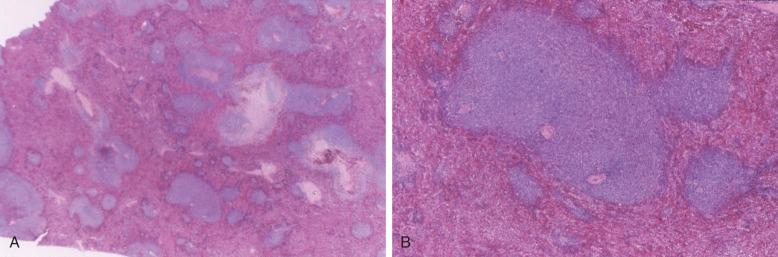
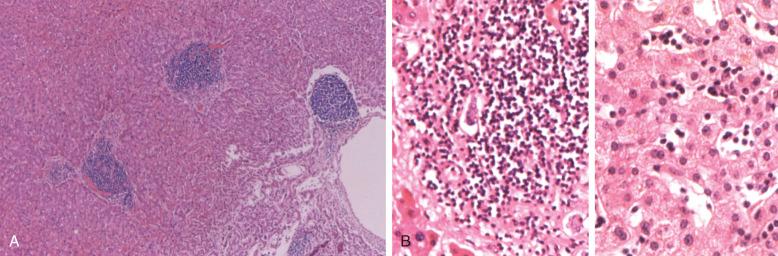
On core biopsy sections, the pattern of bone marrow infiltration may be useful for both distinction of CLL from other differential diagnostic considerations and in possibly providing prognostic information. In CLL, the bone marrow core biopsy may exhibit a range of infiltration patterns including focal, non-paratrabecular nodules; an interstitial infiltrate in which CLL cells are admixed with hematopoietic elements; and diffuse solid lesions ( Figs. 14-8 and 14-9 ). Multiple patterns may be evident in a single core biopsy specimen. The typical nodular infiltrates of CLL are readily apparent on low magnification in which their dark appearance is quite distinct from paler normal hematopoietic cells. This dark appearance is due to closely packed nuclei with scant cytoplasm that typifies this disorder (see Box 14-2 ). On high magnification, the densely packed nuclei exhibit round contours and minimal, if any, mitotic activity. The borders of these non-paratrabecular nodules may show infiltrative margins with diffusion of lymphocytes into adjacent hematopoietic tissues. Likewise, diffuse interstitial infiltrates of CLL may be evident throughout the bone marrow widely separated from discrete nodular lesions. In these areas, adipose cells and hematopoietic elements are at least partially preserved, although the CLL infiltrates impart a darker appearance on low magnification than uninvolved bone marrow (see Fig. 14-8 ). The most extensively effaced bone marrow core biopsy sections are characterized by solid areas of complete replacement of both hematopoietic and fat cells by CLL filling the entire hematopoietic cavity between bone trabeculae. The presence of extensive diffuse, solid infiltrates would predict for cytopenias and, consequently, higher clinical stage. Similarly, a diffuse pattern of bone marrow infiltration has also been linked to ZAP-70 expression. The extent of bone marrow effacement in CLL is best estimated from technically optimal core biopsy specimens of at least 10 mm in length that do not consist of larger portions of hypocellular subcortical regions.
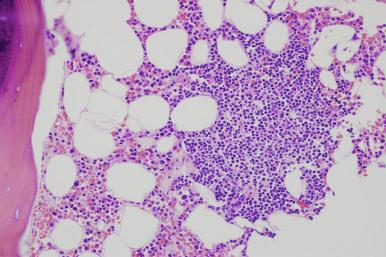
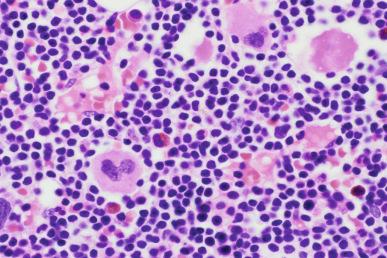
Although proliferation foci are a typical feature of nodal infiltrates of CLL/SLL, these foci of larger transformed prolymphocytoid cells are not generally as conspicuous in bone marrow sections, possibly reflecting the relatively limited sample size of core biopsy specimens ( Fig. 14-10 ). Only very rarely are true germinal centers evident in bone marrow specimens from CLL patients; the presence of these germinal centers should prompt systematic exclusion of other B-CLPN (B-chronic lymphoproliferative neoplasms). In rare CLL patients, foci of large cell lymphoma transformation will be first appreciated in bone marrow specimens. Due to the large overall cell size and the presence of moderate amounts of cytoplasm, these large cell lymphoma infiltrates are quite distinct, even on low magnification in which they are a pale pinkish color (see the section on transformation later in the chapter).
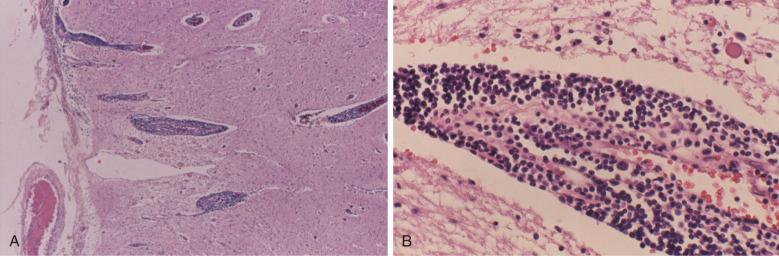
Liver involvement is common in CLL, but clinically significant hepatic dysfunction is encountered in only a small subset of patients ( Fig. 14-11 ). Portal infiltrates of neoplastic cells predominate, and fibrosis may also be noted. Likewise, a small subset of CLL patients present with cutaneous manifestations of disease including generalized papules, or isolated plaques, nodules, and discrete masses. The dermal infiltrates of CLL range from patchy perivascular/periadnexal infiltrates to solid dermal masses. In some patients, the cutaneous lesions are a manifestation of large cell lymphoma transformation (Richter syndrome) and show a predominance of large cells with high Ki67 activity. Although widespread infiltrates of small lymphocytes are common in autopsy specimens from CLL patients, clinically significant infiltrates of CLL/SLL in organ systems such as the central nervous system and gastrointestinal tract are very rare, being encountered at essentially the “case report” level in clinical practice ( Fig. 14-12 ).
The designation of atypical/mixed CLL is suggested for those cases that exhibit some, but not all, features of prototypic CLL, especially in terms of cytologic features of the clonal cells. In addition, some CLL cases may exhibit immunophenotypic properties that are different than prototypic CLL/SLL ( Box 14-3 ). * Other types of B-CLPN should be systematically excluded before a diagnosis of atypical CLL is made. The term atypical/mixed CLL has been applied to cases that resemble CLL morphologically except for the presence of subsets of neoplastic cells (usually 10% to 15%) that are larger, exhibit greater nuclear irregularity, have a prominent nucleolus of prolymphocytes, or have plasmacytoid features ( Fig. 14-13 ). * These cases with atypical morphology also often exhibit an immunophenotypic profile that deviates from prototypic CLL ( Fig. 14-14 ) (see the section on immunophenotype later in the chapter). Studies document other important associations with atypical morphology in CLL including various specific cytogenetic aberrations, more profound leukocytosis, more pronounced cytopenias, either advanced disease stage at presentation or more rapid disease progression, and overall worse outcome compared with prototypic CLL/SLL.
* References .
Term generally applied to CLL cases in which nuclear irregularity is fairly prominent and/or increased numbers of lymphocytes with distinct nucleoli are present or lymphoplasmacytic cells are noted
Cases exhibit morphologic and immunophenotypic spectrum with some, but not all, features of classic CLL/SLL; overlap with other B-CLPN is common
Atypical features noted in both blood and lymph node specimens
May be linked to distinct genotypic subtypes
Linked to more advanced stage, adverse outcome, and rapid disease progression
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here