Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
The CNS is protected from infections by a wide variety of pathogens by virtue of the blood-brain barrier (BBB), humoral immune factors, and resident and circulating immune cells.
Neurotropic pathogens possess specific features that allow them to overcome these protective mechanisms, invade the CNS, and cause disease (e.g., Streptococcus pneumoniae ).
Opportunistic pathogens are organisms that are normally unable to invade the CNS independently but may cause infection when protective mechanisms are impaired (e.g., Staphylococcus epidermidis ).
Infections of the CNS by these broad categories of pathogens are not mutually exclusive, but a discussion based on these categories illustrates the important features of host-pathogen interactions relevant to CNS infections.
A basic tenet of infectious disease pathogenesis is coined “the route of infection,” which is a major determinant in the development of all infections, including those of the CNS. The principle is simple: the host has a finite number of entry points for pathogenic organisms, some naturally present and some introduced iatrogenically. Those naturally present are predominantly mucosal surfaces, such as the nasopharynx, respiratory tree, and gastrointestinal tract, but also included is the cutaneous barrier; entry through this last structure is usually via damage to the watertight epidermis. Iatrogenic routes are more relevant to the neurosurgeon for obvious reasons. These routes include perioperative breeches in structural barriers protecting the CNS (scalp, cranium, meninges), implantation of foreign bodies (e.g., CSF shunts, dural implants, electrodes, spinal hardware), and breeches in mucosal defenses (e.g., intubation, vessel catheterization, urinary catheterization, stress ulceration in the gastrointestinal tract). Alterations involving mucosal barriers are a common route for pathogens to enter the CNS. Pathogens using mucosal routes gain indirect entry into the CNS by hematogenous spread or direct entry via contiguous anatomic structures such as cranial nerves, penetrating veins, or sinus structures. Generally, direct routes into the CNS bypass the blood-brain barrier (BBB), thus circumventing a major defense against CNS infection, whereas indirect routes (e.g., hematogenous spread) involve pathogens that must find pathways through or around the BBB to gain entry into the CNS.
The BBB generally consists of the blood-parenchymal barrier and the blood-CSF (or blood-ependymal) barrier, both referred to here collectively as the blood-brain barrier. The structural and functional properties of the BBB are addressed in more detail elsewhere in this textbook, but several salient features pertaining to infection and the BBB are highlighted here.
The BBB is composed of a specialized layer of microvascular endothelial cells, pericytes, and astrocyte foot processes (or ependymal cells in the case of the blood-ependymal barrier). Brain microvascular endothelial cells (BMECs) form monolayers with high transendothelial electrical resistance (TER) and highly selective macromolecular permeability, properties largely attributable to two features: (1) formation of highly organized intercellular tight junctions, and (2) a low rate of transcytosis relative to other endothelial subtypes. These features restrict the movement of pathogens from the intravascular space across the BBB into the brain parenchyma or CSF. Many pathogens have developed strategies to cross the BBB despite these elaborate defenses. Pathogens use the following three major pathways to gain entry to the CNS across the BBB: (1) transcellular passage (e.g., Escherichia coli , group B streptococci [GBS]), (2) paracellular passage (e.g., protozoa), and (3) carriage within a transmigrating leukocyte, known as the Trojan horse mechanism (e.g., Listeria monocytogenes, Streptococcus suis, Mycobacterium tuberculosis, HIV). Most of these pathways across the BBB are poorly characterized for the vast majority of pathogens associated with CNS infection. Among the best-characterized CNS-invasive pathogen with respect to passage across the BBB is E. coli . To highlight the complex interactions occurring between host and pathogen during the early stages of CNS infection, the following section discusses mechanisms involved in E. coli traversal across the BBB.
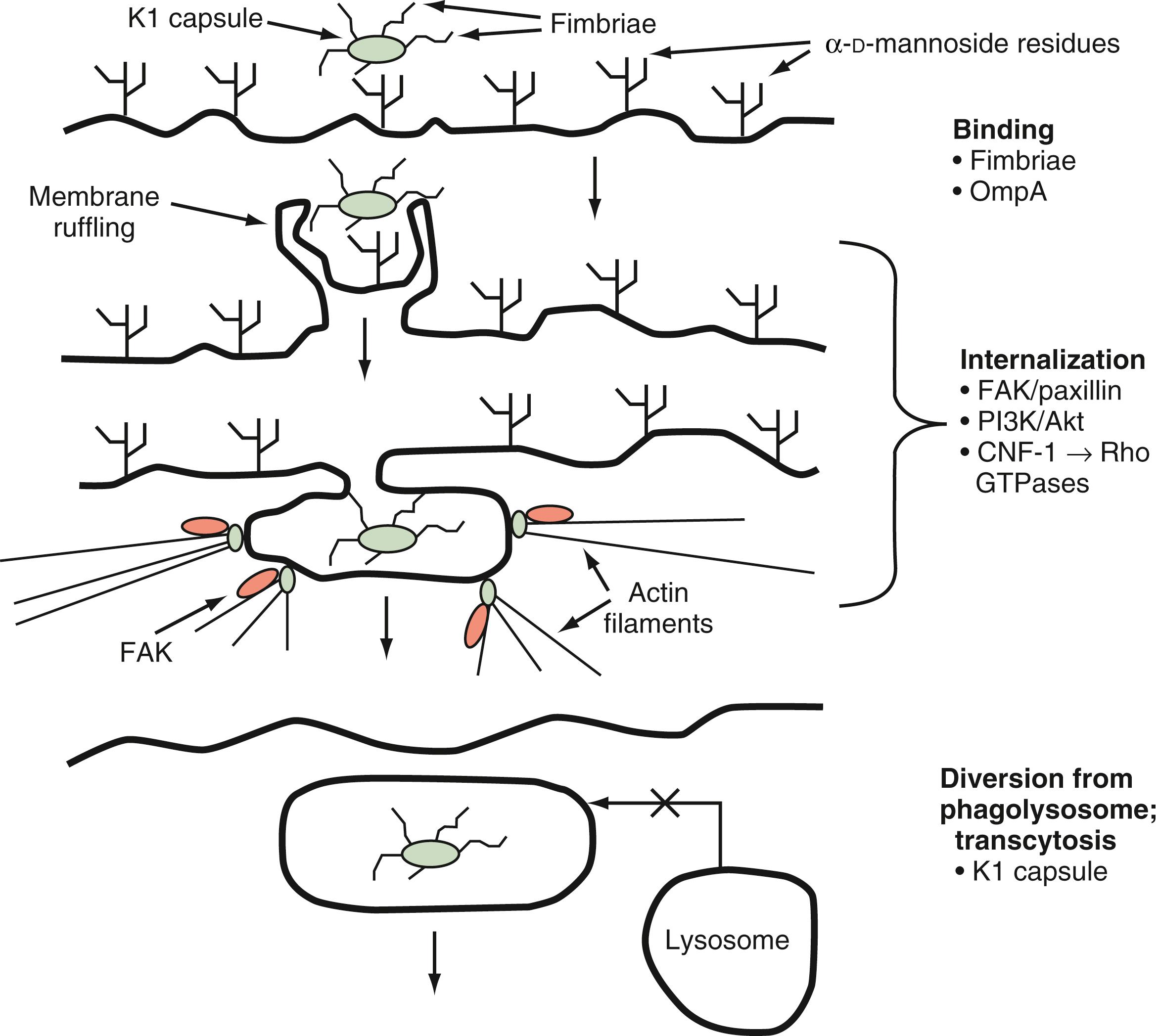
E. coli is a gram-negative bacterium implicated in many cases of neonatal meningitis. In vitro studies of infection of human brain microvascular endothelial cells (HBMECs) by E. coli K1, an encapsulated strain, have shown that E. coli K1 interacts with these cells in a unique manner involving both host- and pathogen-specific structures and signaling pathways. These interactions lead to alterations in the host actin cytoskeleton, membrane protrusion and ruffling around bacteria, and endocytosis of bacteria into membrane-bound vacuoles, where bacterial determinants act to prevent lysosome fusion and influence intracellular vacuole trafficking to achieve transcytotic passage. Transcriptome analysis of E. coli K1 binding to and invading HBMECs in vivo demonstrates a unique transcription profile involving 116 genes whose expression patterns were significantly different from those in E. coli interacting with a collagen-coated surface. Several bacterial determinants have been identified as part of the initial binding and invasion of HBMECs, including type 1 fimbriae, outer membrane protein A (OmpA), Ibe proteins, and cytotoxic necrotizing factor-1 (CNF-1). Type 1 fimbriae are adhesins that bind to α- d -mannosides on the surfaces of host cells, thereby allowing binding and interaction of a bacterium with the host cell. Type 1 fimbriae have been shown to play an important role in the binding of E. coli K1 to HBMECs; deletion of fimH, the gene for the major adhesin protein of type 1 fimbriae, significantly decreases binding of E. coli K1 to HBMECs, a finding that is reversed by genetic complementation of fimH in deletion mutants. Vimentin on the surface of HBMECs serves as a receptor for bacterial IbeA, and interaction of these surface proteins leads to caveolae/lipid raft-dependent entry of E. coli K1 into HBMECs. OmpA also facilitates binding of E. coli K1 to HBMECs via interaction with a host surface glycoprotein, Ecgp96, containing N -acetylglucosamine residues. In vivo studies using an experimental neonatal rat model of meningitis have shown that deletion mutants of ompA are impaired in their ability to enter the CNS in comparison with the parent K1 strain, and N -acetylglucosamine oligosaccharides are able to block penetration of the CNS by wild-type E. coli K1 in the same animal model. Infection of HBMECs with OmpA + E. coli induces translocation of Ecgp96 and Toll-like receptor 2 (TLR2), as a complex, to the host cell surface, where TLR2 appears to play an additional role in E. coli invasion via intracellular signaling (see later).
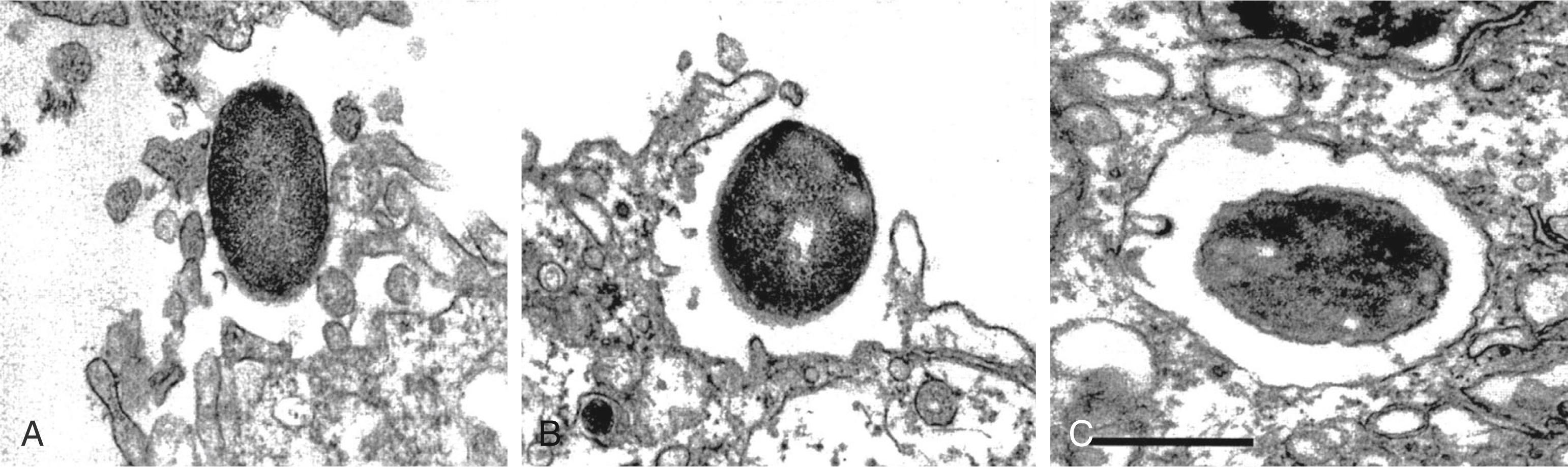
Once binding has occurred, the process of cellular invasion must take place for E. coli K1 to ultimately infect the CNS. Invasion of HBMECs depends on the host actin cytoskeleton; E. coli K1 invasion can be completely prevented in vitro with inhibitors of the actin cytoskeleton such as cytochalasin D. Entry of E. coli K1 into membrane-bound vacuoles in HBMECs involves the formation of membrane projections, described as “zipper-like” structures, and subsequent membrane ruffling before internalization ( Fig. 52.1 ). These events are related to signal transduction pathways in the host cell involved in the regulation of endocytosis, cell membrane interactions, and the actin cytoskeleton. In particular, E. coli K1 activates focal adhesion kinase (FAK) and phosphorylation of paxillin, a cytoskeletal protein that interacts with and regulates the actin cytoskeleton. The exact mechanisms underlying FAK activation by E. coli K1 are unknown but play a role in HBMEC invasion because overexpression of a dominant-negative form of FAK significantly inhibits HBMEC invasion by E. coli K1. Additionally, signal transducer and activator of transcription 3 (Stat3) interacts directly with Ecgp96 at actin condensation sites, and this interaction is required for E. coli invasion upstream of additional signaling cascades involved in this process.
Another important signaling pathway involved in E. coli K1 invasion of HBMECs is the family of phosphatidylinositol-3′-kinases (PI3Ks), which lie downstream of FAK activation. Pharmacologic inhibition of PI3Ks with LY-294002 significantly limits HBMEC invasion by E. coli K1. Akt/protein kinase B, a downstream effector of PI3K, is increased during E. coli K1 invasion of HBMECs. Vascular endothelial growth factor receptor 1 (VEGFR1) is involved in E. coli K1 invasion of HBMEC via increased association with the p85 subunit of PI3K; VEGFR1 appears to be required for E. coli K1–induced Akt activation via PI3K during invasion of the HBMEC. Another host endothelial surface receptor that associates with Ecgp96 is the type 1 angiotensin II receptor (AT1R); inhibition of this receptor using telmisartan or knockdown of the receptor using small interfering RNA significantly reduced HBMEC invasion by E. coli K1, and pretreatment of neonatal mice with telmisartan prevents the development of meningitis caused by this strain.
Host cytosolic phospholipase A 2α (cPLA 2α ), an enzyme that liberates free arachidonic acid (AA) from host cellular membranes, also plays an important role in facilitating E. coli invasion of HBMECs. Inhibition of cPLA 2α prevents HBMEC invasion by E. coli K1; this inhibition results from prevention of the formation of cysteinyl leukotrienes downstream of AA liberation.
Rho family guanosine triphosphatases (GTPases) have been shown to regulate a diverse array of processes that affect the actin cytoskeleton, cell motility, and cell-cell/cell-matrix interactions. E. coli strains associated with urinary tract infections and meningitis have been shown to produce CNF-1, an AB-type toxin with deaminase activity that targets this family of GTPases. CNF-1 activates Rho GTPases and increases the in vitro uptake of bacteria by nonprofessional phagocytes such as epithelial and endothelial cells. Deletion of the cnf1 gene from E. coli K1, which significantly decreases the invasion of HBMECs, is associated with reduced activation of RhoA and Cdc42, GTPases that are activated during E. coli K1 invasion of these cells.
Once E. coli K1 bacteria have been internalized in membrane-bound vacuoles within HBMECs, these organisms must survive the internal hostile milieu and avoid destruction in the lysosome to gain entry into the CNS. The K1 capsule appears to play a very important role in preventing the normal maturation of endosomes and fusion of vacuoles with the lysosome. K1 isogenic deletion mutants have been shown to traffic through the endosomal system and colocalize with cathepsin D, thus confirming fusion of the lysosome with the vacuoles containing these bacteria. K1-containing vacuoles acquire the late endosomal marker Rab7, and it is possible that interference by the capsule with this lysosome fusion–promoting GTPase underlies the enhanced intracellular survival of encapsulated bacteria.
In summary, a neuroinvasive strain of E. coli uses specific tools to gain entry into the CNS by binding to or uptake into HBMECs and subsequent diversion of the normal protective trafficking of endosomal compartments to the lysosome ( Fig. 52.2 ). These events allow E. coli K1 strains to cross the highly selective BBB and cause meningitis. The following section addresses the host response to pathogens attempting to invade the CNS.
Once pathogens have entered the CNS, they encounter cellular and humoral elements of the innate immune system present within the CNS. This section addresses the interactions of pathogens with this “inner defense.” Despite the common perception of the CNS as an immunologically inert compartment, an array of resident cells, including microglia, astrocytes, perivascular macrophages, and meningeal macrophages, participate in initiating a rapid, but relatively nonspecific response to invading pathogens. In addition to providing an initial defense, these cells recruit immune effectors from outside the CNS and provide a bridge to a more specific adaptive immune response. It is the evolution of this immune response in the CNS that largely dictates the ultimate clinical outcome for a patient with a CNS infection.
Santiago Ramón y Cajal (1852 to 1934) divided the histology of the brain into “elements,” as follows: neurons were termed the first element, astrocytes were the second element, and a category of non-neuronal, nonastrocytic cells were the third element . This third element contained cells later identified as oligodendrocytes as well as a group of small, highly branched cells that were morphologically distinct from other cells in the CNS. These cells were eventually termed microglia by Pio del Rio Hortega (1882 to 1945), who further characterized the cells as a distinct entity in the brain parenchyma. Like monocytes and macrophages, microglia are derived from the bone marrow and share many features of monocytes/macrophages with respect to immune modulation.
Microglia can exist in several different states, as defined by surface markers, morphology, migration status, and function. Resting microglia are small cells with few surface markers and prominent thin branches that are constantly reorganizing and sampling the microenvironment of the brain parenchyma. A large number of stimuli activate microglia, with the nature of the stimulus influencing the structural and functional changes that occur during activation. Molecules released from cellular damage, particularly free adenosine triphosphate (ATP), activate microglia and induce morphologic changes in microglia from small, ramified cells to amoeboid cells capable of phagocytosing cellular debris like canonical macrophages. Microglia sense extracellular ATP through P2Y receptors, leading to both maturation and migration of the microglia to the site of CNS damage.
Activated microglia express a large number of receptors associated with various mediators of the inflammatory response to tissue damage, pathogens, and immune stimuli. A major family of pattern recognition receptors (PRRs) expressed by microglia known as the Toll-like receptors (TLRs) recognize pathogen-associated molecular patterns (PAMPs). Ten TLRs (TLR1 to TLR10) have been identified to date in humans. Microglia express TLR1 to TLR9, allowing them to detect and respond to a huge array of PAMPs; lipoteichoic acid (LTA) (TLR2), double-stranded RNA (TLR3), lipopolysaccharide (LPS; TLR4), flagellin (TLR5), single-stranded RNA (TLR7), and unmethylated CpG DNA (TLR9) are examples. Engagement of these receptors by cognate ligands triggers signal transduction of multiple intracellular pathways responsible for the inflammatory response. LPS-induced activation of TLR4 has been well characterized in microglia and leads to activation of nuclear factor κB (NF-κB), cytokine production (interferon-β [IFN-β], tumor necrosis factor-α [TNF-α]), signal transducer and activator of transcription 1α (STAT1α), production of reactive oxygen species (ROS), and production of nitric oxide (●NO, or NO). Flagellin-mediated activation of TLR5 on microglia has been shown to upregulate expression of TLRs 1, 2, 4, and 5, as well as interleukin-6 (IL-6). TLR3 activation in microglia has been demonstrated in response to HIV, and TLR9 activation in microglia leads to the production of multiple cytokines and chemokines (TNF-α, IL-1β, IL-6, IL-12, macrophage inflammatory protein-1α [MIP-1α], MIP-1β). , Prestimulation of TLRs 1/2, 4, and 9 in murine microglia results in substantially enhanced phagocytosis and intracellular killing of pneumococci.
In addition to TLRs, microglia express several cytosolic “alarm systems” that allow for sensing of intracellular pathogens. One such receptor is nucleotide-binding oligomerization domain 2 (NOD2), which recognizes a common motif in bacterial peptidoglycan from both gram-negative and gram-positive bacteria. NOD2 has been shown to augment microglial activation in the presence of whole pneumococci, but not pneumococcal cell lysates, and engagement of this cytosolic receptor is associated with astrogliosis and demyelination in vivo.
For the detection of intracellular viruses, microglia harbor several RNA- and DNA-sensing intracellular PRRs, which allow microglia to exert an antiviral response through the production of type 1 interferon. Membrane-bound TLR 3 and 9 sense RNA and unmethylated CpG dsDNA, respectively. Cytosolic receptors RIG-I and melanoma-associated differentiation antigen 5 (MDA5) sense viral RNA and signal through the adaptor protein mitochondrial antiviral signaling (MAVS) to allow for interferon-β production during RNA virus infection. Cyclic GMP–AMP synthase (cGAS) functions as yet another more recently described sensor of DNA viruses such as herpes simplex virus (HSV)-1 and signals through the adaptor protein stimulator of type I IFN genes (STING) to induce an interferon response in microglial cells. , Mice deficient in cGAS or STING have increased susceptibility to HSV-1 infection leading to rapid development of severe encephalitis and death. The redundancy of these nucleic acid sensors highlights the adaptation of mammalian cells to multiple viral pathogens capable of circumventing any particular host mechanism of pathogen detection.
Activation of microglia by invading pathogens has many consequences, some beneficial to the host and some detrimental. Microglia are capable of NO synthesis and a respiratory burst that both cause oxidative damage to the offending pathogen. Microglia produce NO via inducible nitric oxide synthase (iNOS, encoded by the NOS-2 gene), leading to the formation of peroxynitrite (ONOO − ), a highly toxic product capable of damaging both the host and pathogen.
Microglia also possess the metabolic machinery necessary for generation of the superoxide anion (●O 2 − ), an extremely reactive oxygen species that can damage nucleic acids, lipids, and proteins. Pathogens expressing superoxide dismutase are able to neutralize this effective defense. Microglia also function as phagocytes in the CNS under both physiologic and pathophysiologic conditions. Microglia are the major scavengers of cell debris in the CNS and interact, in part, with apoptotic bodies expressing externalized phosphatidylserine. The orphan receptor triggering receptor expressed on myeloid cells-2 (TREM-2) is important in transforming microglia into phagocytes, and activation of TREM-2 enhances phagocytosis while suppressing the production of proinflammatory cytokines, events that may be important for prevention of the autoimmune responses to the autoantigens present in apoptotic bodies.
Microglia also regulate the response of other CNS cells to injury or infection and recruit cells from outside the CNS via the production of a wide variety of cytokines, chemokines, and lipid mediators. Table 52.1 lists some of the cytokines and chemokines known to be generated by microglia in response to a number of activating stimuli. Microglia also produce factors that support glial and neuronal cells in their microenvironment, including nerve growth factor, neurotrophin-3 (NT-3), brain-derived neurotropic factor, glial-derived neurotropic factor, and basic fibroblast growth factor. Many potent lipid mediators are synthesized by microglia, including prostaglandins (PGs D 2 , E 2 , and F 2α , thromboxane B 2 ), leukotriene B 4 , and platelet-activating factor. , Several of these lipid mediators serve an autocrine role; the EP2 receptor on microglia participates in the activation of microglia, and antagonism of this receptor may exert a neuroprotective effect by preventing excessive microglial neurotoxicity. Microglia also produce antimicrobial peptides, such as rat cathelicidin-related antimicrobial peptide (rCRAMP), the rat homologue of the human cathelicidin LL-37, and these peptides can be found in the CSF of humans with meningitis. The critical role of microglia in orchestrating the innate immune response has been established, and experimental evidence for this role is expanding rapidly; an extensive review of this evidence can be found elsewhere. , ,
| Cytokines | IL-1α/IL-1β IL-1 receptor antagonist IL-3 IL-4 IL-6 IL-10 IL-12 IL-13 IL-15 IL-18 TNF-α TGF-β M-CSF |
| Chemokines (chemoattractant cytokines) | CXCL1 (growth-regulated oncogene-α) CXCL2/3 (MIP-2) CXCL8 (IL-8) CXCL10 (IP-10) CCL2 (MCP-1) CCL3 (MIP-1α) CCL4 (MIP-β) CCL5 (RANTES) CCL22 (macrophage-derived chemokine) |
Microglia also function as antigen-presenting cells and are able to prime CD4 + T cells to initiate the adaptive immune response. Various TLR ligands (i.e., LPS) and infection with the Theiler murine encephalomyelitis virus result in increased expression of major histocompatibility class II complexes and costimulatory molecules on microglia, events that favor efficient antigen presentation and development of an adaptive immune response. Cytomegalovirus (CMV) elicits CXCL10 (IFN-γ–inducible protein-10 [IP-10]) production from primary microglia, but not from astrocytes. CXCL10 is an important chemokine involved in IFN-γ–induced T-cell recruitment, a critical process for control of CMV infection. Remarkably, astrocytes infected with CMV produce the viral homologue of IL-10, UL111a, which suppresses the production of CXCL10 from activated microglia. Thus CMV is able to suppress T-cell recruitment in the CNS by subverting microglial production of an important antiviral chemokine.
Microglia, as potent regulators of the proinflammatory response to CNS injury and infection, are also able to downregulate these proinflammatory responses. Microglia express anti-inflammatory cytokines (IL-4, IL-10, IL-13, and transforming growth factor-β [TGF-β]) and actively phagocytose apoptotic T cells after stimulation by IFN-β and IFN-γ. , , Production of IL-4 and IL-13 ultimately triggers microglial apoptosis via autocrine receptor engagement, thereby providing a means of balancing inflammation in response to a specific CNS insult.
Microglia are clearly multifunctional cells that serve as central regulators of the innate and adaptive immune responses in the CNS. However, these cells do not act in a vacuum, instead interacting with both immune and nonimmune cells to coordinate the events surrounding CNS injury or invasion. One important cell type included in this response is the astrocyte. The following section briefly addresses the importance of astrocytes in the CNS during various stages of infection.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here