Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The diagnosis of bacterial infection is one of the many important services provided by the clinical microbiology laboratory. The past decade has resulted in a dramatic shift in the manner in which clinical microbiologists identify bacteria. With the widespread adoption of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and molecular methods such as 16S rDNA sequencing, clinical microbiologists are increasingly less reliant on traditional, growth-based, biochemical and metabolic methods.
This chapter describes bacteriology in its current state and as it will likely be practiced going forward rather than reiterating how it was traditionally practiced. By way of introduction, important preanalytical considerations, including specimen collection and submission, are discussed. Identification of bacteria is then detailed in addition to key principles in biochemical identification. Of note, organism categorization will be discussed in the context of modern diagnostic methods such as MALDI-TOF MS and sequence-based identification. In addition, this chapter includes a comprehensive discussion of bacterial infections with a focus on the laboratory methods required to diagnose them.
The discipline of microbiology has a long and rich history. Although the existence of “microorganisms” was entertained for centuries, the field of microbiology flourished with the design of the microscope and resulting observations of van Leeuwenhoek in the 17th century. Following this critical work, the discipline was further developed by the contributions of other “greats” in microbiology such as Robert Hook (17th century), Lazarro Spallanzani (18th century), Louis Pasteur, Ferdinand Cohn, Ilya Metchnikoff, Paul Ehrlich, Robert Koch (all of the 19th century), followed by Alexander Fleming, Kary Mullis, Oswald Avery, Maclyn McCarty (all of the 20th century), to name only a few. Thus clinical microbiology, as it is practiced today in the 21st century, has been shaped by the significant contributions made by these and other notable giants through the centuries. Their efforts formed the foundation of the field, proving that not only do bacteria exist, but that they can be cultured and characterized and can cause human disease.
In the last decade, practicing clinical microbiologists have witnessed significant changes in the approaches used to detect and identify bacteria. Specifically, there is a continued shift from conventional and traditional practices that employ phenotypic characteristics to identify bacteria (i.e., use of various carbohydrates, amino acids, enzyme production) to nonphenotypic methods such as DNA sequencing and mass spectrometry. As a result of the accelerated pace of technology evolution that has driven clinical microbiology, nonphenotypic methods, with or without total laboratory automation, have now become mainstream in clinical laboratory practice, as they are more accurate and faster than many of the more traditional approaches.
One of the primary roles of the clinical microbiology laboratory is to isolate and identify pathogenic bacteria or those with pathogenic potential in situations where the bacteria are likely playing a role in opportunistic infection. The laboratory can provide the treating clinician with information from a combination of direct smears and stains and cultures, as well as other information, such as antibiotic susceptibility testing results (discussed in Chapter 85 ) and serologic test results. In this chapter, we will review the key laboratory methods for detection and identification of bacteria, describe approaches to bacterial identification, and discuss bacterial infections in the context of the major organ systems. We will also note general considerations for each infection category, including resident microbiota, anatomic features, epidemiologic data, significant bacterial pathogens, types of infections, and methods of laboratory diagnosis. Rather than discuss the fundamentals of the traditional approach to the identification of bacteria from clinical specimens, we will instead focus only on those critical phenotypic tests that are likely to continue to play an integral role in laboratory diagnosis of bacterial infections.
The diagnostic process begins with the patient who seeks medical help because they are experiencing signs and symptoms suggestive of an infectious disease. Depending on the patient’s medical history, physical findings, imaging results, laboratory tests, and epidemiologic information (e.g., travel history, previous infections, exposures) that might indicate a possible bacterial infection, the clinician will obtain specimens from the patient to diagnose infection. Before laboratory testing begins, the clinician typically develops a differential diagnosis based on the infectious disease syndrome of the patient. This differential diagnosis guides the microbiological work up.
Multiple specimens may be obtained from the patient because it is important to recognize that many different organ systems may be involved in a specific infection. Because successful recovery of bacteria from culture is dependent on maintaining the viability of any bacteria present in the clinical specimen until it can be received and processed by the laboratory, appropriate collection and transport devices or containers and conditions must be used. These preanalytic aspects of the testing process are essential in ensuring successful recovery of bacteria from clinical specimens. Bacteria have numerous growth requirements for successful recovery from clinical specimens. It is therefore very important that physicians be apprised of specific bacteria that may not be cultivated by routine culture methods so that specialized or other appropriate orders can be made.
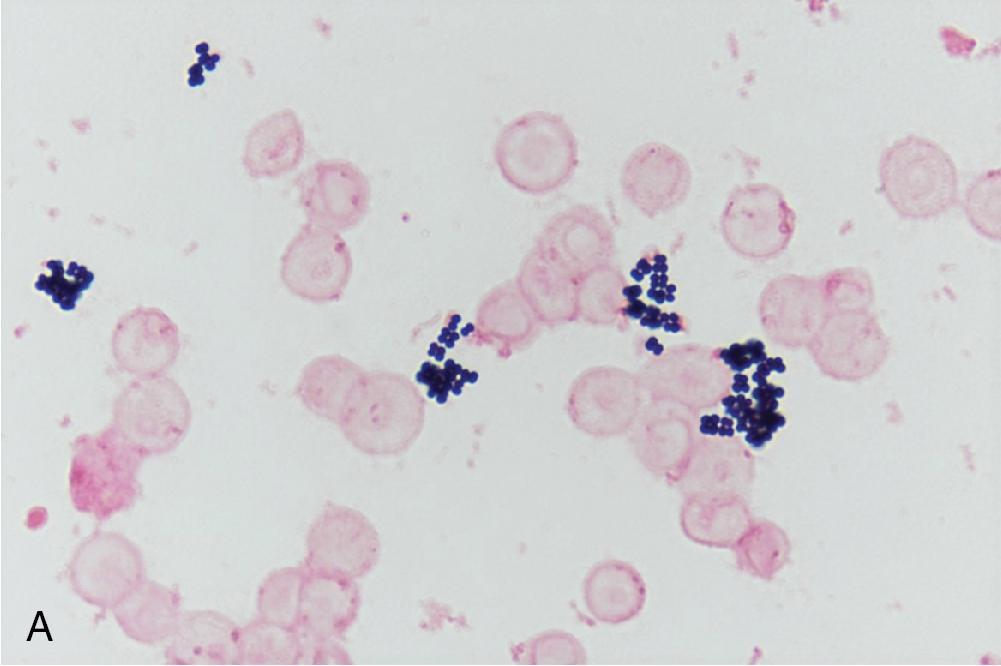
Upon arrival in the laboratory, information about patient specimens (e.g., specimen type, source, collection date/time) is recorded in the laboratory information system. Depending on the physician’s order and the type of specimen, wet mounts and smears can be prepared and stained for microscopic examination; the findings of these direct microscopic examinations can be immediately released and reported. Often, these preliminary results are used to establish a presumptive diagnosis and initiation of empiric therapy. Moreover, particularly with respect to direct Gram stains, results can help to assess specimen quality and provide guidance for interpreting subsequent culture results. For a summary of key microbiology stains, see Table 84.1 and Fig. 84.1 , and for common Gram stain results and example organisms associated with each, see Table 84.2 and Fig. 84.2 .
| Stain | Protocol and Reagents a | Principle | Intended Use | Image Reference(s) |
|---|---|---|---|---|
| Gram stain |
|
Iodine cross-links crystal violet to peptidoglycan present in the cell wall. The gram-positive cell wall contains more peptidoglycan than gram-negative organisms and therefore appears purple owing to retained crystal violet stain. | Classifies bacteria based on retention of the crystal violet stain (purple = gram-positive) vs. those that do not retain crystal violet and counterstain with safranin (red = gram-negative). Useful for visualizing organisms in specimen and cultivated organism. | Refer to Table 84.2 and Fig. 84.2 |
| Acid-fast stain: Ziehl-Neelsen |
|
Acid-fast bacteria have long-chain fatty acids (mycolic acid) that retain the carbol fuchsin stain and are able to resist acid-alcohol decolorization. | Useful for the visualization of mycobacteria. | Ziehl-Neelsen in Fig. 84.1 A |
| Acid-fast stain: Kinyoun |
|
Acid-fast bacteria have long-chain fatty acids (mycolic acid) that retain the carbol fuchsin stain and are able to resist acid-alcohol decolorization. | Useful for the visualization of mycobacteria. Same overall performance as Ziehl-Neelsen, but due to omission of heating step is an easier stain to perform. | Kinyoun in Fig. 84.1 B |
| Modified acid-fast stain |
|
Employs a weaker decolorizing agent to differentiate acid-fast organisms from those that are partially or weakly acid fast. | Useful for the visualization of Nocardia, Rhodococcus, Gordonia, Tsukamurella, and several other organisms. | Modified acid-fast stain in Fig. 84.1 C |
| Acridine orange stain |
|
Stains nucleic acid with an intercalating fluorochrome. | Useful for visualizing organisms present in low concentrations. In addition, the stain can be useful in examination of positive blood cultures or other specimens with questionable results. | Acridine orange stain in Fig. 84.1 D |
| Spore stain |
|
Spores stain green while the remainder of the cell stains pink. | Stain is designed to detect the presence of spore formation in Bacillus spp. and Clostridium spp. Vacuolated gram-negative bacteria can falsely appear to produce spores with this stain. | Spore stain in Fig. 84.1 E |
a All stains require elements of washing and drying. These steps have not been included in this table to conserve space and because in many cases these steps are not standardized and vary by laboratory. In most cases, concentrations of reagents have been omitted because they can vary slightly by manufacturer. Concentrations that have been included are considered critical to the proper performance of the stain.
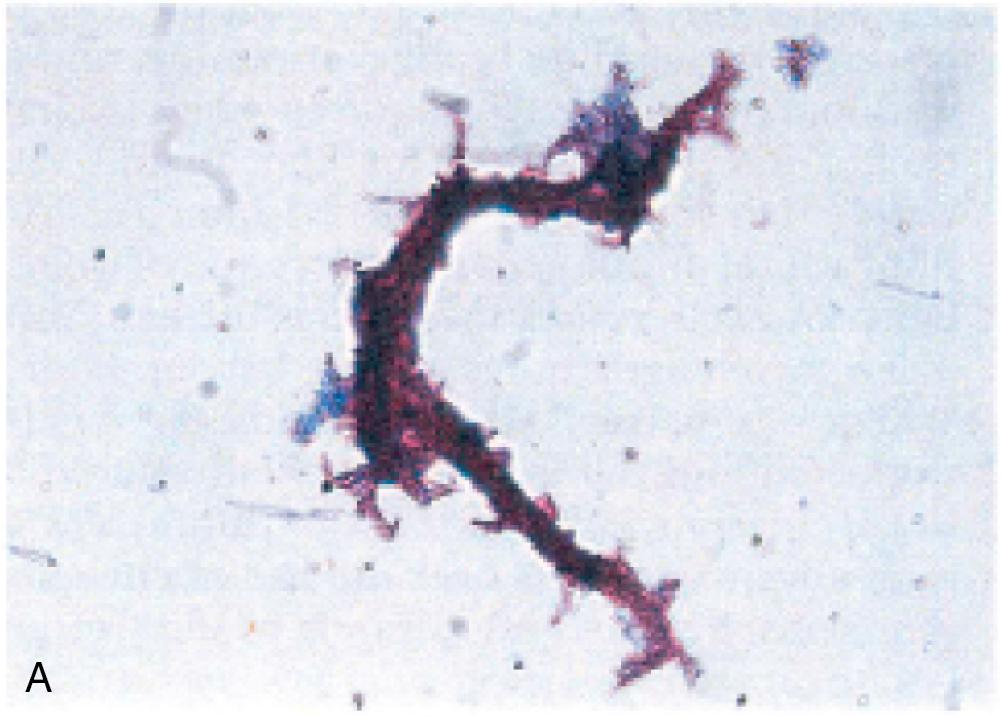
| Gram Stain Result | Possible Organisms | Gram Stain Example |
|---|---|---|
| Gram-positive cocci in clusters |
|
Fig. 84.2 A |
| Gram-positive cocci in pairs (lancet-shaped) |
|
Fig. 84.2 B |
| Gram-positive cocci in pairs and chains |
|
Fig. 84.2 C |
| Gram-positive rods—regular |
|
Fig. 84.2 D |
| Gram-positive rods—coryneform/pleomorphic |
|
Fig. 84.2 E |
| Beaded gram-positive rods |
|
Fig. 84.2 F |
| Branching, filamentous gram-positive rods (“beaded”) |
|
Fig. 84.2 G |
| Branching, filamentous gram-positive rods (regular/not beaded) |
|
Fig. 84.2 H |
| Gram-variable rods (although gram-positive, can stain gram-variable or gram-negative) |
|
Fig. 84.2 I |
| Gram-negative rods |
|
Fig. 84.2 J |
| Gram-negative rods—fusiform (long, thin rods) |
|
Fig. 84.2 K |
| Gram-negative rods—curved (Difficult to visualize in direct Gram stains. Acridine orange stain and/or prolonged staining with carbolfuchsin allows better visualization.) |
|
Fig. 84.2 L |
| Gram-negative diplococci |
|
Fig. 84.2 M |
| Gram-negative coccobacilli |
|
Fig. 84.2 N |
After receipt of the specimen into the laboratory information system, specimens are inoculated to appropriate primary plating media. Primary plating media are the nutritive media that a clinical specimen is first inoculated to in order to recover common bacteria that might be present. These media can be categorized as general purpose, selective (supporting the growth of one group of bacteria over another), enriched (contain particular nutrients that support the growth of fastidious bacteria), and differential (allow for phenotypic differentiation of bacteria via sugar utilization, hemolysis, etc.). These media categories are not mutually exclusive. Some common examples of primary media include 5% sheep’s blood agar (BAP; general purpose and differential—hemolysis), chocolate agar (CHOC; general purpose and enriched), and MacConkey agar (MAC; selective and differential—gram-negative organisms and lactose fermentation). The primary plating media used for any clinical specimen is based on the physician’s order in conjunction with the anatomic site from which the specimen was obtained. Certain specimens may require additional processing prior to culture inoculation. For example, cerebrospinal fluid (CSF) and other body fluids can be centrifuged or otherwise concentrated if sufficient volume is available, as bacteria isolated from this source are typically found in low numbers. Furthermore, decontamination methods may be required to assure that slowly growing pathogens, such as Mycobacterium spp., are not outcompeted by normal microbiota (i.e., contaminants) when specimens are collected from nonsterile sites. Once inoculated, plates are incubated under conditions that support the growth of potential bacterial pathogens suspected from the particular anatomic site of the specimen. Typically, these conditions include incubation at 35 ± 2 °C, in either room air or 5% CO 2 .
Once placed into major organism groups, based on Gram stain characteristics and growth on media, an approach to identify the bacteria can be delineated. There are numerous approaches to the identification of bacteria which are recovered from clinical specimens using routine culture media. As previously mentioned, the main media used routinely for most clinical specimens submitted for routine bacterial culture are BAP, CHOC, and MAC. In addition, a large number of primary plating media are used by the clinical microbiology laboratory for certain patient populations and specimen types to recover potential bacterial pathogens. For example, Burkholderia cepacia selective agar (BCSA) or similar can recover B. cepacia group isolates from respiratory specimens obtained from patients living with cystic fibrosis (CF). Another example is the specific recovery of Salmonella or Shigella species from stool with xylose-lysine-dextrose (XLD), Hektoen Enteric (HE), or Salmonella-Shigella (SS) agars. If a specimen is submitted in which anaerobic bacteria may play an important role in a disease process, anaerobic primary plating media are also inoculated and incubated under anaerobic conditions. These media include Brucella blood agar (BBA), Brucella laked blood agar with kanamycin and vancomycin (LKV), Bacteroides bile esculin agar (BBE), and phenylethyl alcohol agar (PEA). Table 84.3 and Fig. 84.3 compiles both nutritive and differential primary plating media. The potential pathogens and their recovery by bacterial culture are discussed in their respective clinical syndromes later in this chapter.
| Media Type | Primary Components a | Description of Use | Notable Organisms b |
|---|---|---|---|
| Blood agar plate (BAP) |
|
|
|
| Chocolate agar plate (CHOC) |
|
|
|
| MacConkey agar (MAC) |
|
|
|
| Bacteroides bile esculin (BBE) agar |
|
|
Bacteroides spp., Parabacteroides spp., Fusobacterium mortiferum, some enterococci ( Fig. 84.3 E) |
| Brain heart infusion (BHI) agar |
|
|
|
| Brucella agar |
|
|
|
| Buffered charcoal yeast extract (BCYE) agar |
|
|
|
| Burkholderia cepacia selective agar |
|
|
|
| Campylobacter blood agar |
|
|
C. jejuni , C. coli. Growth of other Campylobacter spp. is dependent on the specific formulation of the media |
| Cefsulodin-irgasan-novobiocin (CIN) agar |
|
|
Yersinia spp. and Aeromonas spp. will produce similar colony morphologies on this media ( Fig. 84.3 G). |
| Columbia colistin nalidixic acid (CNA) agar |
|
|
|
| Eosin-methylene blue (EMB) agar |
|
|
|
| Hektoen enteric (HE) agar |
|
|
Salmonella spp. ( Fig. 84.3 H) and Shigella spp. ( Fig. 84.3 I) |
| Laked sheep blood agar with kanamycin and vancomycin (LKV) |
|
|
Anaerobic gram-negative bacilli |
| MacConkey agar with sorbitol (SMAC) |
|
|
E. coli O157:H7 |
| Mannitol salt agar (MSA) |
|
|
Staphylococcus spp.S. aureus appear yellow ( Fig. 84.3 J). |
| Mueller-Hinton agar |
|
|
Sheep blood supplementation required for the testing of S. pneumoniae. NaCl supplementation required for the testing of Staphylococcus spp. |
| Phenylethyl alcohol agar (PEA) |
|
|
|
| Salmonella-Shigella (SS) agar |
|
|
Note: This is a highly selective medium and some strains of Shigella are inhibited, including Shigella dysenteriae. |
| Thayer-Martin agar |
|
|
Neisseria meningitidis and Neisseria gonorrhoeae |
| Thiosulfate citrate bile salt sucrose (TCBS) |
|
|
Vibrio spp. ( Fig. 84.3 K) except for V. hollisae and V. cincinnatiensis. |
| Xylose-lysine-deoxycholate (XLD) agar |
|
|
Salmonella and Shigella |
a The list of reagents for each media is not exhaustive. Only those reagents which are most critical to the performance of the media have been included. Media within each category will vary slightly from manufacturer to manufacturer. For lists of specific reagents and concentrations, please consult manufacturer package inserts.
b These lists are not exhaustive and are only meant to identify representative and key organisms for each media.
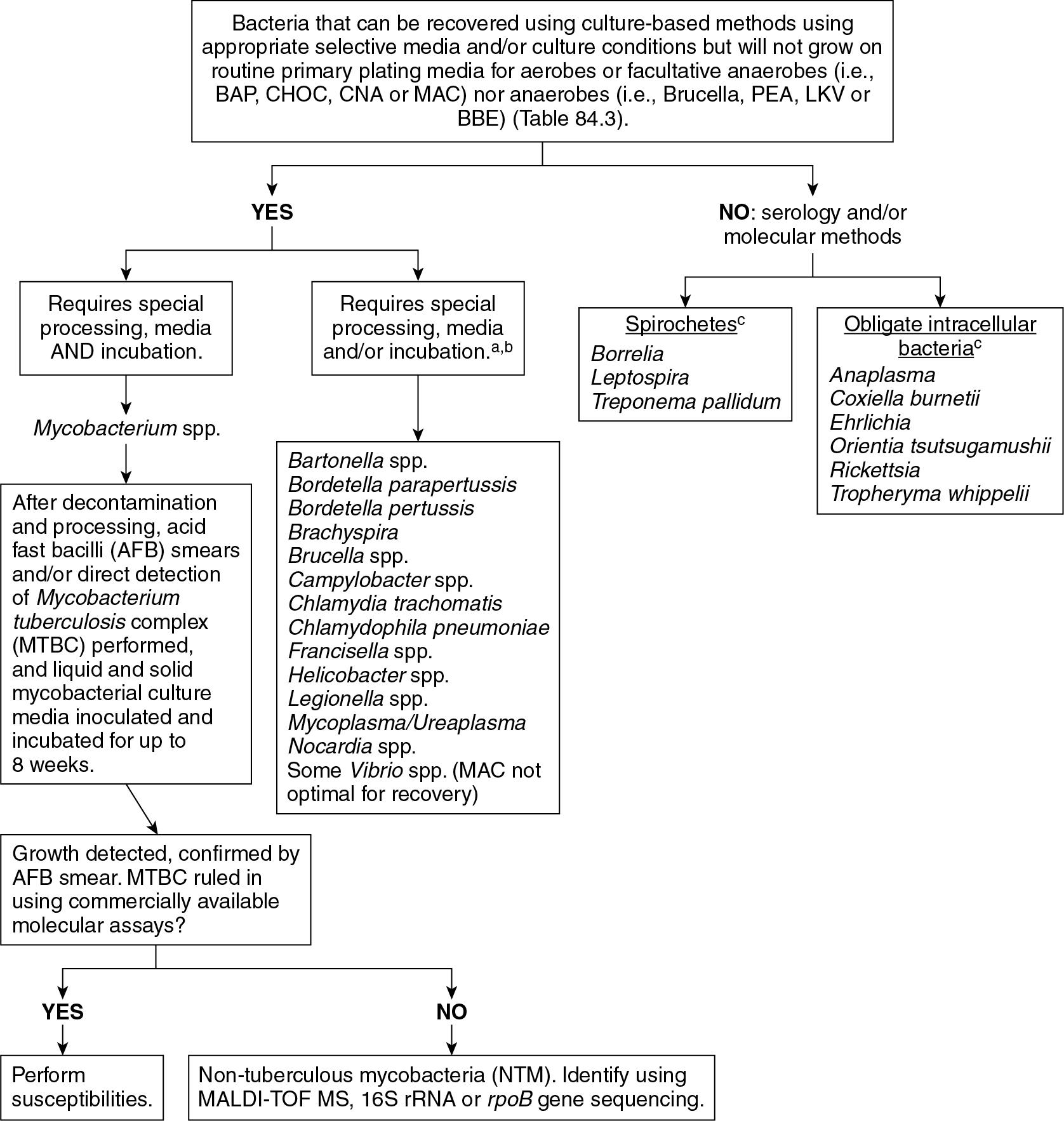
Some bacteria can be recovered by culture but require special media and/or growth conditions, while others cannot be cultured at all. Detecting organisms that are difficult to grow relies on the prior request of the clinician for specific bacteria or groups of bacteria. In other words, some bacteria will not be detected using routine primary plating media, and their presence can be detected only through use of special selective media, special incubation conditions, serologic methods, or direct molecular detection methods ( Fig. 84.4 ).
In recent years, laboratory automation has been introduced into some clinical microbiology laboratories. Whereas each of the available laboratory automation systems can be used for the majority of the culture workflow (processing, plating, incubation, and reading), many laboratories use only the processing modules to automate just the processing and plating of specimens onto primary culture media. The two most commonly used systems are the Walk-Away Specimen Processor (WASP) by Copan Diagnostics, Inc. (Murrieta, CA), and the Kiestra InoqulA by Becton, Dickinson and Company (Franklin Lakes, NJ). The WASP system uses disposable calibrated loops, whereas the Kiestra InoqulA system uses a pipette to dispense a known volume, followed by streaking of the plate via magnetic beads. Both systems are able to vortex specimens, de-cap/re-cap specimen containers, and streak plates according to a number of customizable patterns. As not all specimens are liquid, both systems allow for some manual specimen preprocessing, and even preinoculation of media.
A huge benefit of using laboratory automation systems for incubation of cultures is the ability of these systems to image each individual plate of an associated culture while never removing those plates from their incubation conditions. This allows for faster growth and more robust yield of microorganisms from cultured specimens, especially for specimens that may contain fastidious organisms. For additional information on automation in the clinical laboratory, refer to Chapter 29 .
Nonphenotypic methods, such as target-specific nucleic acid amplification or DNA sequencing, require different preanalytical considerations than those typically observed in the routine clinical microbiology laboratory. One of the greatest advantages of molecular testing is its ability to detect low numbers of target-specific DNA or RNA molecules, but this enhanced sensitivity can also contribute to false-positive results if steps to avoid contamination are not enacted. To minimize contamination, molecular testing has historically required dedicated laboratory space that is divided into two to three rooms, where testing proceeds in a unidirectional workflow. The first area or room is dedicated to reagent preparation and must have positive air pressure to prevent the entry of aerosols. The next area should be devoted to sample preparation and be under negative air pressure to retain aerosols that may be generated when working with the specimen. Laboratories with limited space may combine these two areas into one, but doing so will require barrier containment, such as a dead air box or biological safety cabinet, for reagent preparation. The final area in the unidirectional workflow is the amplification detection room. This area is considered “dirty” and should be under negative pressure to minimize amplicon contamination.
Another consideration, beyond the physical space, is the use of dedicated instrumentation and testing materials, such as pipettes, tubes, and vortexes. Consumables or equipment should never be moved to another area without being decontaminated first. The movement of testing personnel between rooms should also be limited to minimize aerosolization and the spread of amplicons. If staff need to move between rooms, there should be dedicated personal protective equipment (PPE) in each area, sticky mats at the entrance of each room, and gloves should be changed periodically, especially in between specimens. Work areas and equipment should also be cleaned at the beginning and end of each day and between each test.
Once plates have been inoculated and incubated, usually for 18 to 24 hours, primary cultures are evaluated by the clinical microbiologist. In this initial assessment, which requires extensive training and experience, the microbiologist examines colony growth and determines, depending on the site from which the specimen was obtained and the potential pathogens associated with that particular site, whether identification is required. Features included in this initial assessment are shown in Box 84.1 .
Visual inspection of bacterial growth, noting the gross characteristics and relative number of each colony type recovered on various primary plating media, including:
Colony morphology: size, presence of pigment, shape, hemolysis, sugar utilization
Gram stain reaction, morphology and arrangement: gram-positive, gram-negative, or gram-variable; cocci, rods/bacilli, coccobacilli; arrangement in pairs, clusters, chains
Growth characteristics: growth rate, media and atmospheric conditions required for growth, nutritive requirements
If a decision is made to identify bacteria, some preliminary phenotypic testing is usually performed in order to place the bacteria into major groups for identification. Depending on the organism and site of infection, these preliminary findings may be reported to the clinician to provide guidance for empiric therapy until definitive identification and susceptibility testing results become available. Once the bacteria are phenotypically assessed, the specific organism can then be identified using the most appropriate means. This entire process is summarized in the At a Glance feature.
The approach to bacterial identification has changed drastically as new technologies have become mainstream in many clinical microbiology laboratories. However, these technologies are not available in every laboratory, and some laboratories still use traditional microbiologic methods for primary identification of clinical isolates. A detailed discussion of traditional methods is outside the scope of this chapter. There has been a gradual shift away from traditional phenotypic and metabolic approaches toward nonphenotypic methods, including mass spectrometry for bacterial identification (discussed in detail later). Nevertheless, several phenotypic and metabolic tests remain vital for bacterial identification ( Table 84.4 ). These phenotypic tests remain important because: (1) They are a means to verify that final bacterial identification by MALDI-TOF MS or sequencing correlates with these initial tests, thereby ensuring that the isolate was correctly processed for identification; (2) some of these tests are faster, less expensive, and as accurate for identification as the newer technologies; and (3) these tests are required for identification of some bacteria that cannot be adequately differentiated by the newer methods. The intent of this delineated approach to bacterial identification is to shift microbiological paradigms from those using largely phenotypic methods to those using largely nonphenotypic methods (MALDI-TOF MS and/or sequencing) with only a few supplemental phenotypic methods. For discussions regarding more traditional approaches to bacterial identification, the interested reader is referred to a selection of excellent current textbooks and reviews.
| Test | Principle | Primary Use in Identification |
|---|---|---|
| Catalase | Enzyme that mediates the breakdown of hydrogen peroxide (H 2 O 2 ) to water and oxygen. Transfer colony to surface of glass slide. Add 1 drop 15% H 2 O 2 . Observe for oxygen bubbles. Test is positive if bubbles are produced. | Differentiates Staphylococcus spp. (positive) from Streptococcus spp. (negative) |
| Coagulase | SLIDE (clumping factor): A suspect colony is emulsified in a drop of rabbit plasma. Bacterial clumping occurs within 2 min, thereby indicating the presence of bound coagulase. TUBE (free coagulase): Several colonies of a suspect isolate are emulsified in 0.5 mL of rabbit plasma with sterile EDTA and incubated at 35 °C. Read at 4 h and 24 h; if a clot forms, isolate is most likely Staphylococcus aureus . |
Differentiates S. aureus from coagulase-negative staphylococci Commercial latex agglutination kits can also be used |
| Bile solubility | 1−2 drops 10% sodium desoxycholate added to suspect colony. Incubated at 35 °C for 30 min. Examine for colony lysis. | Helps differentiate alpha-hemolytic streptococci (not lysed) from Streptococcus pneumoniae (lysed) |
| Pyrrolidonyl arylamidase (PYR) | A heavy inoculation of the pyrrolidonyl substrate (L-pyrrolidonyl-β-naphthylamide) with a suspect Enterococcus is incubated for 4 h. N,N -methyl-aminocinnamaldehyde is added. If a red color is produced, it is considered positive. | For colonies >1 mm, nonhemolytic on BAP, and catalase negative, gram-positive cocci, a positive test identifies Enterococcus species. |
| Optochin (ethylhydrocupreine) disk | Streak 2−3 suspect colonies onto half of a BAP. Place an optochin disk (6 mm) in the middle of the lawn. Gently tap the disk to ensure adequate contact with the agar surface. Incubate plate 18–24 h at 35 °C. Measure zone of inhibition: if zone is ≥14 mm, organism is S. pneumoniae . | Helps differentiate viridans streptococci from S. pneumoniae |
| Lactose fermentation | MacConkey agar has a peptone base with a sole carbohydrate, lactose, crystal violet, bile to inhibit gram-positive bacteria, and a pH indicator, neutral red. Bacteria that use the lactose turn the pH indicator pink, while those that do not use lactose remain colorless. | Nonfastidious gram-negative organisms can be initially divided into two major groups, based on those that do and those that do not ferment lactose. |
| Oxidase | Determines the presence of bacterial cytochrome oxidase using the oxidation of the substrate tetramethyl- p -phenylenediamine dihydrochloride to indophenol. A portion of a colony is smeared onto an impregnated substrate strip. Development of a blue color within 10 s is considered positive. | Helpful to exclude Enterobacterales. Also, helpful with identification of other gram-negative rods such as Pasteurella spp., Eikenella , Cardiobacterium spp., Pseudomonas spp., Vibrio spp., and others. |
| Spot indole (lactose fermenters from urine) | Tryptophanase converts tryptophan to indole. A piece of filter paper is moistened or impregnated with 5% p -dimethylaminocinnamaldehyde and a portion of the colony is rubbed onto the paper. Rapid development of a blue color within 20 s is considered positive. | Sufficient to report the identification of Escherichia coli from urine cultures with appropriate colony morphology (i.e., β-hemolytic lactose fermenters). |
| Rapid grower versus nontuberculous mycobacteria | A rapidly growing mycobacteria is determined by appearance of growth in less than 7 days after subculture. | Helpful to determine preliminary identification of a rapid grower from other nontuberculous mycobacteria. If sequencing for identification, determination of a rapidly growing mycobacteria will help select appropriate target (e.g., rpoB gene) |
This chapter provides an overview of placement of bacteria that grow on routine primary plating media into major groups for identification. The extent of the culture workup is dictated by the specimen source and disease presentation.
Most microbiology laboratories routinely identify bacterial pathogens using one of two identification systems: (1) growth-based systems that evaluate biochemical reactions and (2) systems that evaluate protein profiles using MALDI-TOF MS. Historically, growth-based systems have been the most common methodology for organism identification, but with improved speed and accuracy, MALDI-TOF MS is now the dominant technology. The following sections will describe these two important systems and discuss their performance for the identification of commonly encountered bacteria.
Growth-based organism identification systems evaluate biochemical and enzymatic reactions for an organism of interest. These reactions yield a series of positive and negative results, which are then compared to a database of reactions for known organisms that can then be used to produce an identification. There are several commonly used growth-based systems for organism identification with varying workflows and performance characteristics.
Despite being based on foundational principles in microbiology, these systems all suffer from the same limitation, namely, that they require an organism to be growing well and in the same way as those organisms that were used to generate the reference database. A multitude of variables (antibiotic exposure, the impact of immune system response, biofilm growth, and heterogeneous morphologies) can impact the growth of an individual patient isolate and therefore system performance. With that said, these systems generally work very well for commonly isolated, nonfastidious pathogens, but have difficulty differentiating closely related organisms, uncommonly isolated organisms, and organisms that are metabolically inert.
Phenotypic organism identification can be performed using either automated or manual systems. The automated systems require that the user inoculate an identification card or plate, which is placed in an instrument that incubates, analyzes, and interprets the biochemical reactions to produce an identification. The manual systems rely on many of the same principles, but are manually incubated, read, and interpreted. Historically, most laboratories have relied on both methods, with the automated systems serving as the primary identification platform and the manual method serving as the backup or to aid in discrepant resolution.
Because there are numerous manufacturers of both automated and manual identification systems, a comprehensive review of their performance characteristics is beyond the scope of this chapter. A few commonly used automated identification systems include the bioMérieux VITEK 2, the BD Phoenix, and the Beckman Coulter MicroScan. Among manual identification systems, the most common are the series of bioMérieux API identification panels and the Beckman Coulter MicroScan identification panel. The following is a brief overview of some of the key general reactions that are used to generate organism identifications using these systems.
Carbohydrate utilization is the backbone of both automated and manual phenotypic identification systems. These reactions are growth-based and require up to a 24-hour incubation in order to register the reaction. The reactions utilize a pH-based indicator that results in a color change. Carbohydrate utilization results in an acidic condition, while protein utilization results in an alkaline condition, both of which are indicated by color changes that are either read automatically or with the naked eye, depending on the system being used.
Enzymatic profiles can be assessed much more quickly than other reactions because they rely on the presence of preformed enzymes, which is not a growth-requiring reaction. The reactions can be read within 6 hours and are indicated by a color change that results from the hydrolysis of a colorless complex.
Some manual systems utilize the simple observation of growth to determine a reaction. These systems are not conducive to automation as the reaction requires observation with the naked eye. Growth-based reactions assess the dependence of an organism on a variety of different substrates and can be read within 24 hours.
MALDI-TOF MS has now largely replaced biochemical organism identification in the clinical microbiology laboratory. This technology has enjoyed widespread adoption due to its ability to rapidly identify organisms for a fraction of the cost of conventional, growth-based systems.
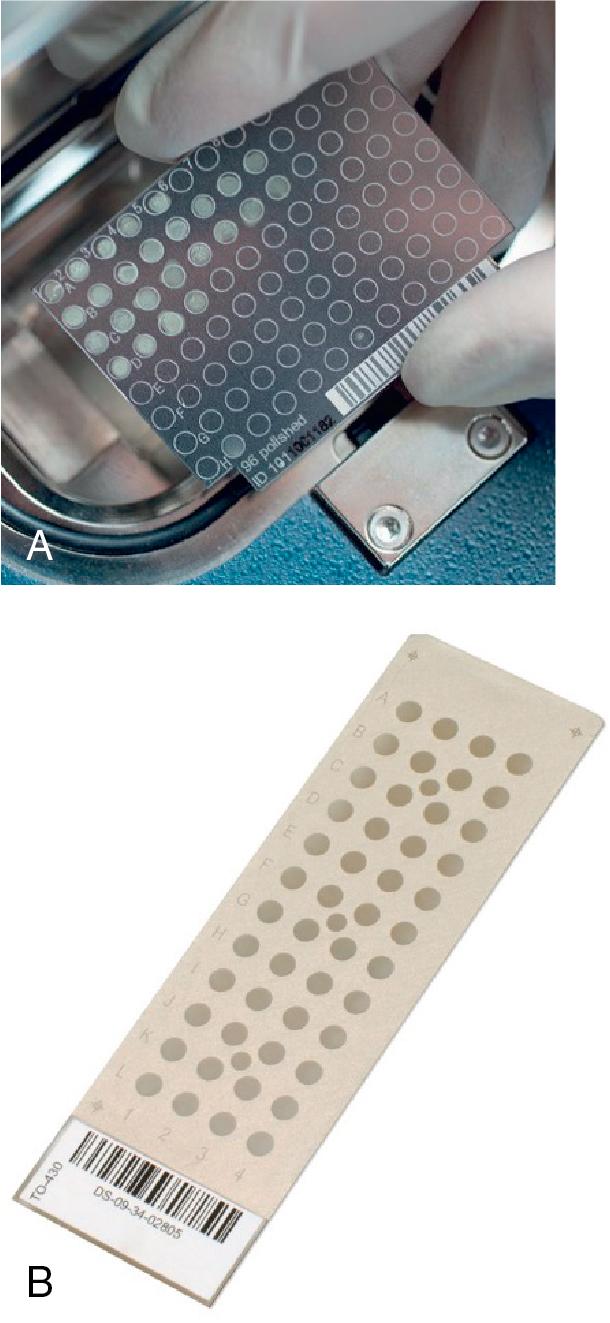
The first step in identifying an organism by MALDI-TOF MS is harvesting at least 10 5 colony forming units (CFUs) of pure (single, isolated, well-sized colony), cultured organism. A single colony is usually selected from solid agar growth and applied to a target plate with a toothpick or a plastic loop ( Fig. 84.5 ). A matrix of α-cyano-4-hydroxycinnamic acid (usually 1 μL) is applied to the colony using a calibrated pipette and allowed to dry. This matrix is critical to the performance of MALDI-TOF MS, because it absorbs laser energy and facilitates the soft ionization process of proteins prior to their migration through the flight tube. An alternative procedure includes adding formic acid (70 to 100%) to the colony prior to the addition of the α-cyano-4-hydrocinnamic acid as an “on-plate extraction.” This procedure has been shown to improve identification rates for some organisms, most commonly gram-positive organisms. For the Bruker platform, if the routine workflow outlined above fails to produce an identification, a “tube extraction” may be performed. In this tube extraction, an organism suspension is first made in approximately 75% ethanol, pelleted, and resuspended in 70% formic acid and an equal volume of acetonitrile. This suspension is centrifuged leaving protein in the supernatant, which can then be applied to the target plate for analysis by the MALDI-TOF MS for organism identification.
Once the organism or protein suspensions have been applied to the target plate, the target plate is entered into the mass spectrometer and analyzed. Through this process, organism proteins are ionized and migrate through the vacuum chamber. The rate at which they migrate is measured (time-of-flight) and is a product of both mass and charge (mass to charge ratio; m/z) . A detector at the end of the MALDI-TOF MS flight tube measures the ionized proteins and generates a spectral profile or “protein fingerprint.” This fingerprint is unique to the organism and can be compared with a database of previously defined spectra of known organisms (see Chapter 22 for more detail). The algorithm for establishing a match between the spectra of the unknown organism and the database spectra differs by manufacturer. After this comparison, an organism name is reported. Although the instrumentation plays an important role in performance, a high-quality database is the most critical factor for reliably identifying an organism.
In contrast to growth-based identification methods, MALDI-TOF MS identifies organisms by analyzing protein fragments between 2000 and 20,000 Daltons. The protein profiles generated are less susceptible to environmental conditions that can confound biochemical identification. Further, in order for biochemical reactions to produce reliable patterns that accurately predict identity, the organism must be grown in a reproducible and robust manner. This is particularly problematic for fastidious and/or anaerobic organisms. In contrast, MALDI-TOF MS is less affected by these constraints to produce a reliable identification. Studies have shown that MALDI-TOF MS reliably identifies organisms grown on a variety of media.
One of the strengths of MALDI-TOF MS is that it provides high-confidence identification results, with some known exceptions. This characteristic is particularly useful for the identification of rarely encountered or fastidious organisms. In contrast, biochemical systems not infrequently generate confident but erroneous results, requiring significant experience to know when to trust a result. It is important to note that MALDI-TOF MS does have difficulty distinguishing some species of bacteria from one another. For these key organisms, laboratories must implement confirmatory testing to ensure that accurate identifications are generated. Table 84.5 for a list of select organisms that may be misidentified by MALDI-TOF MS along with biochemical reactions that may be used to confirm organism identification.
| Organism | Possible Misidentification | Supplementary Identification Methods | Comments |
|---|---|---|---|
| Streptococcus mitis/oralis | Streptococcus pneumoniae |
|
Some MALDI-TOF MS systems are unable to accurately identify S. pneumoniae and S. mitis/oralis |
| Shigella spp. | Escherichia coli |
|
Reliable differentiation not possible because Shigella and E. coli are very closely related. |
| Brucella spp. a | Ochrobactrum anthropi, Prevotella disiens |
|
Most systems do not include Brucella spp. in commercial databases |
| Streptococcus pyogenes | Streptococcus dysgalactiae |
|
|
| Aeromonas hydrophila | Aeromonas caviae |
|
|
| Bacillus anthracis a | Bacillus cereus |
|
Most systems do not include B. anthracis in commercial databases |
| Francisella tularensis a | Streptococcus constellatus, Streptococcus pluranimalium, Acinetobacter johnsonii, Kytococcus sedentarius |
|
Most systems do not include F. tularensis in commercial databases |
| Burkholderia mallei/pseudomallei a | Burkholderia thailandensis, Burkholderia multivorans |
|
Most systems do not include B. mallei/pseudomallei in commercial databases |
| Yersinia pestis a | Yersinia pseudotuberculosis |
|
Most systems do not include Y. pestis in commercial databases |
| Clostridium botulinum | Clostridium sporogenes |
|
|
| Pathogenic Neisseria spp. ( N. meningitidis and N. gonorrhoeae ) | Nonpathogenic Neisseria spp. | Pathogenic Neisseria spp.: Colistin resistant, growth on modified Thayer-Martin or GC-Lect agars, acid production from carbohydrates ( N. meningitidis = maltose and glucose; N. gonorrhoeae = glucose only) |
a Suspected biothreat pathogens, such as Francisella tularensis , Brucella spp., Bacillus anthracis , Burkholderia mallei/pseudomallei , and Yersinia pestis should undergo rule out/refer testing in hospital clinical laboratories. Please refer to local and national guidelines for this process, such as the Association of Public Health Laboratories guidelines for the recognize/rule out/refer process.
Though there are many genera of gram-positive cocci, the most commonly encountered genera in clinical specimens are Staphylococcus , Streptococcus, and Enterococcus ( Fig. 84.6 ). MALDI-TOF MS is a reliable method for the identification of both staphylococci and enterococci. Studies have shown that MALDI-TOF MS accurately identifies more than 90% of these organisms, while incorrect identifications are very rare. ,
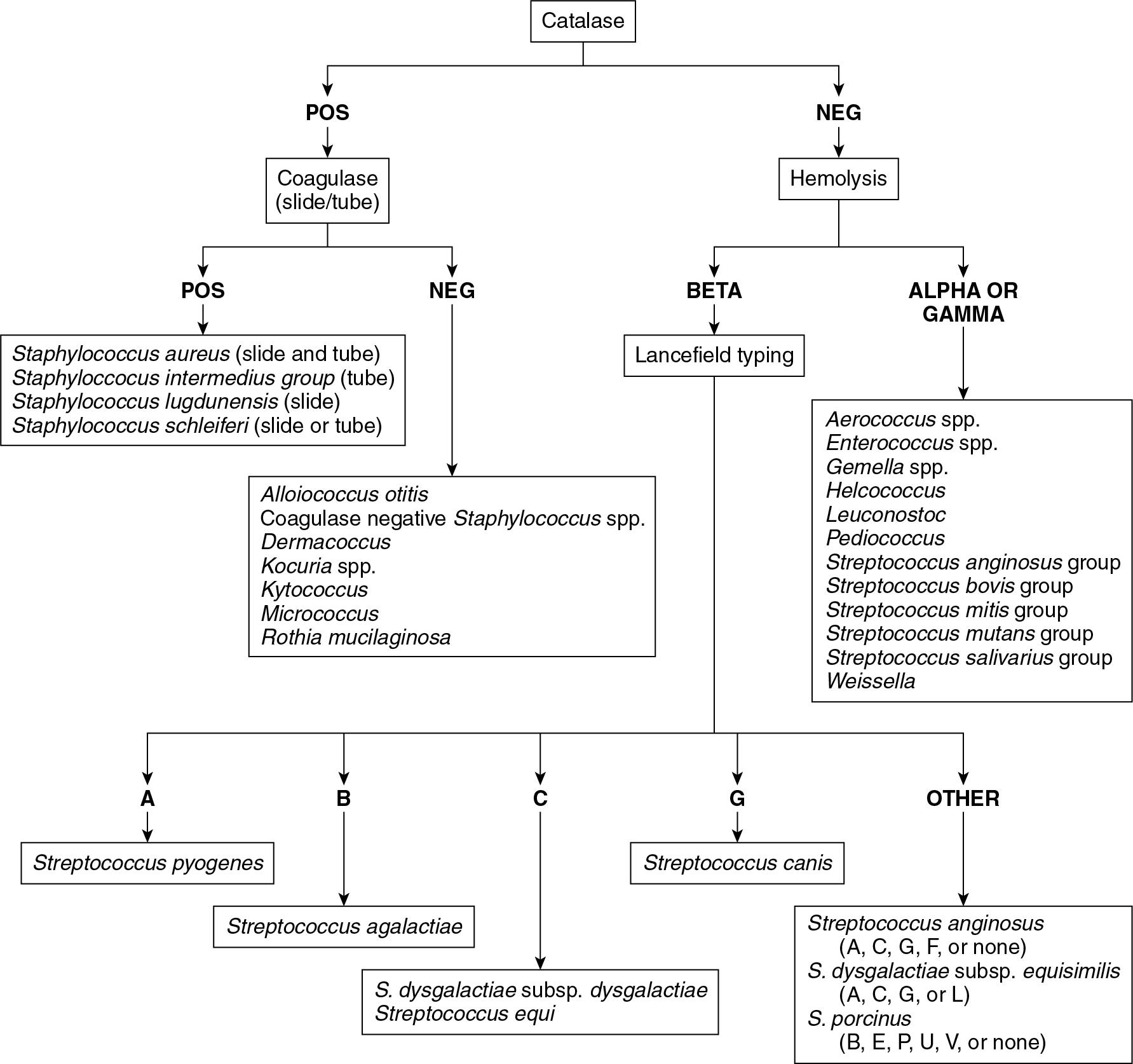
Some streptococci are more challenging for MALDI-TOF MS to identify accurately. A well-known limitation for Bruker Biotyper systems is the inability to discriminate between Streptococcus pneumoniae and the S. mitis/oralis group of organisms. As a result, laboratories using a MALDI-TOF MS system that is unable to differentiate these species must rely on biochemical reactions to supplement their testing. Isolates identified by MALDI-TOF MS as either of these two organisms should be tested with optochin and/or bile solubility.
The ß-hemolytic streptococci are also problematic for bioMérieux VITEK MALDI-TOF MS. Rychert and colleagues were unable to differentiate between Streptococcus pyogenes (Group A Streptococcus , GAS) and Streptococcus dysgalactiae with only 51% of S. dysgalactiae correctly identified.
Prior to the implementation of MALDI-TOF MS, clinical microbiology laboratories did not have a reliable and efficient method to identify most gram-positive rods ( Fig. 84.7 ). This was particularly problematic for the Corynebacterium spp. and to a lesser extent, the Bacillus spp. These organisms are increasingly recognized as causes of opportunistic infections in some patient populations, including immunocompromised patients.
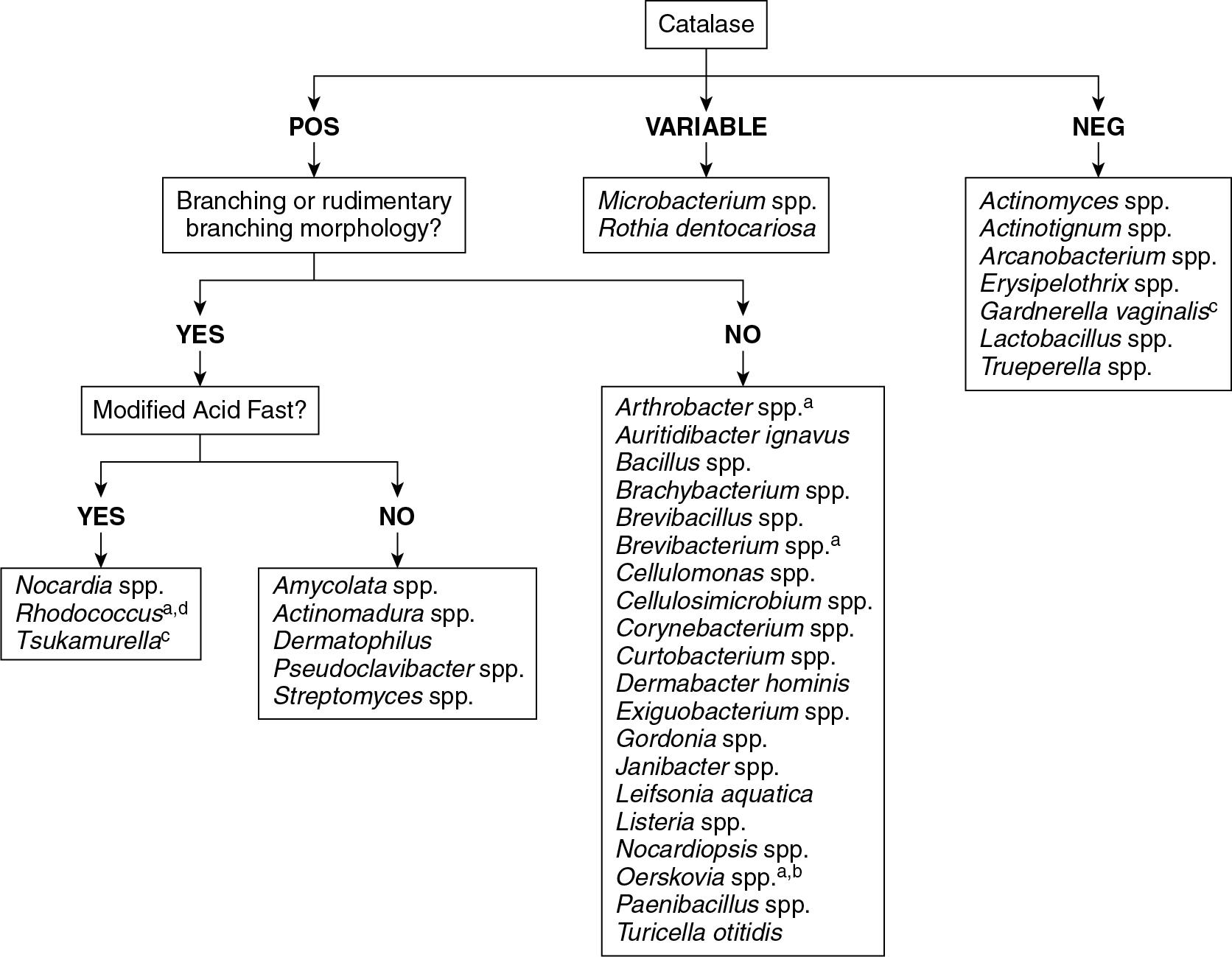
Bacillus spp. are a group of closely related organisms and there is little evidence evaluating the ability of MALDI-TOF MS to produce accurate identifications of these organisms. Of significance for any laboratory is the ability to recognize B. anthracis, which is a member of the B. cereus group. Most commonly used MALDI-TOF MS systems do not include B. anthracis in commercially available, US Food and Drug Administration (FDA)-cleared databases and therefore are unable to correctly identify this organism. For specialized databases that do include B. anthracis, MALDI-TOF MS can accurately identify this organism. It should be noted that routine identification of B. anthracis using MALDI-TOF MS is not recommended. Instead, Rule Out/Refer procedures from the Centers for Disease Control and Prevention (CDC) and the Association of Public Health Laboratories (APHL) should be followed.
Less information is available regarding the ability of MALDI-TOF MS to identify other gram-positive rods. Studies of isolates including Arcanobacterium haemolyticum, Trueperella bernardiae, Brevibacterium casei, Microbacterium spp., Arthrobacter spp., Rothia spp., Dermabacter hominis, Rhodococcus equi, and Listeria monocytogenes found that most of these isolates were correctly identified to the species level and none were incorrectly identified. ,
The ability of MALDI-TOF MS to accurately identify gram-negative bacilli and coccobacilli is well documented in the peer-reviewed literature. MALDI-TOF MS performance for Enterobacterales , glucose nonfermenting organisms, and fastidious gram-negative bacilli will be discussed.
Organisms within the Enterobacterales order are some of the most commonly encountered pathogens in the clinical microbiology laboratory ( Fig. 84.8 ). Traditional biochemical identification methods work relatively well for this group of organisms, and as a result, many laboratories may continue to use these methods in lieu of MALDI-TOF MS. In particular, many laboratories use “limited identification” strategies for Escherichia coli , as outlined in the CLSI document for the abbreviated identification of bacteria and yeast. According to this document, E. coli can be identified with indole (positive), oxidase (negative), lactose fermentation, and ß-hemolytic growth. Notably, approximately 5% of E. coli do not ferment lactose or are nonhemolytic. For nonhemolytic colonies, a negative pyrrolidonyl arylamidase (PYR) test can confirm the presence of E. coli . Nonhemolytic and lactose nonfermenters can be confirmed as E. coli with a positive rapid methylumbelliferyl-ß- d -glucuronidase (MUG) test.
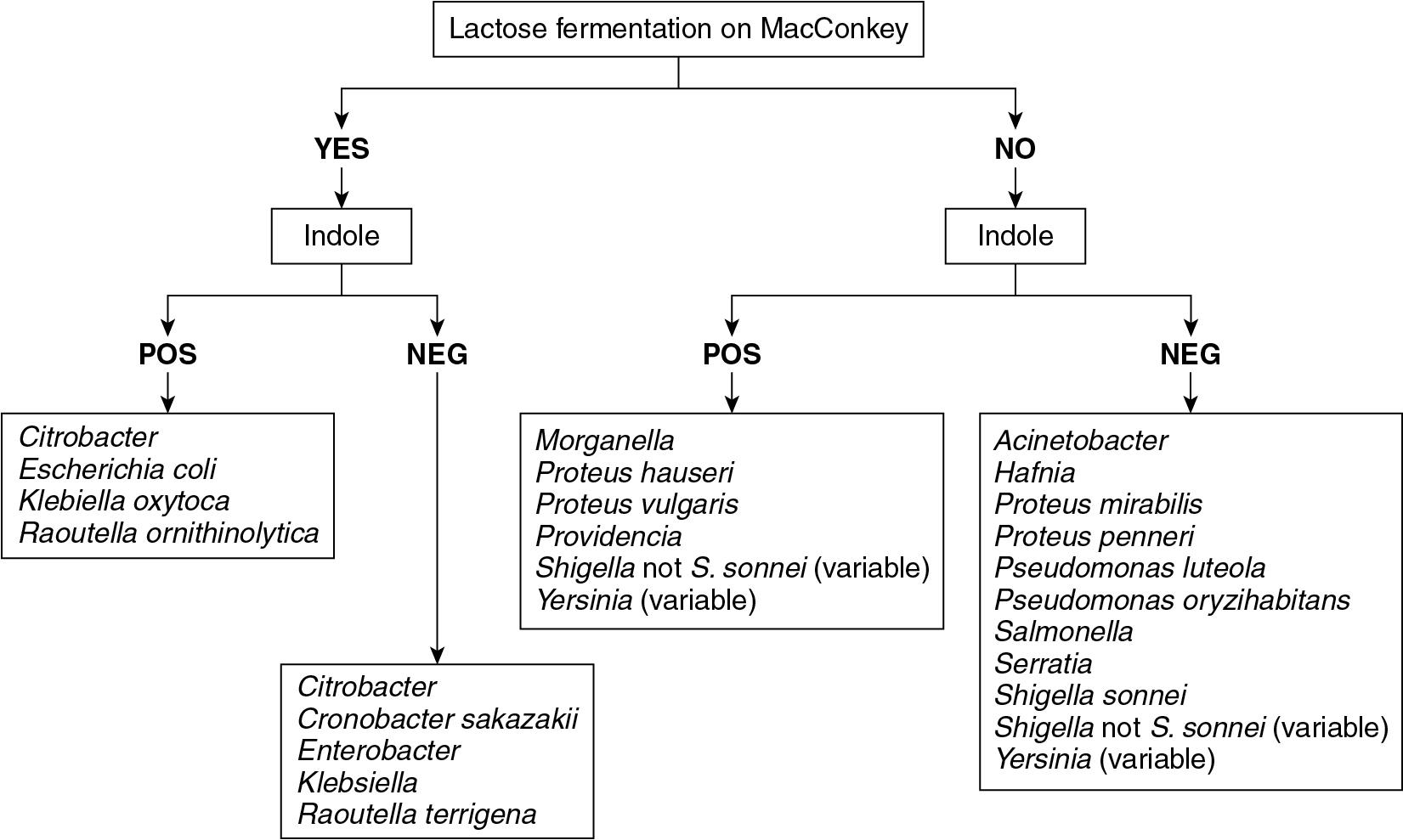
E. coli identification deserves special mention in this section because the inability of MALDI-TOF MS to distinguish E. coli from Shigella is probably its greatest weakness. In most systems, Shigella is not present in the database because its spectra are indistinguishable from those of E. coli. As a result, the default identification from MALDI-TOF MS is E. coli for either organism. It is particularly important to accurately identify Shigella spp. because there are significant public health implications associated with Shigella spp. infection. In addition, MALDI-TOF MS cannot provide Shigella spp. serotype information, thus laboratories may wish to retain the ability to serotype using conventional methods. Importantly, MALDI-TOF MS can reliably differentiate E. coli from non- Shigella spp. Enterobacterales . As a result, the method can be used confidently for the identification of isolates from specimens where the isolation of Shigella spp. would be a rare event. For other common clinical isolates of Enterobacterales , MALDI-TOF MS systems perform reliably, with misidentification rates of less than 1%. , Of note, MALDI-TOF MS identification of Yersinia pestis may pose a challenge, but the data are mixed in this regard. ,
One of the greatest benefits of MALDI-TOF MS is its ability to reliably identify glucose nonfermenting organisms ( Fig. 84.9 ). MALDI-TOF MS accurately identifies Pseudomonas aeruginosa , arguably the most clinically important glucose nonfermenting organism. Identification of isolates cultured from respiratory specimens of CF patients has improved markedly with the use of MALDI-TOF MS. Because MALDI-TOF MS identification is less affected by the growth conditions of an organism than traditional methods, as mentioned previously, it can reliably identify organisms with altered growth characteristics, such as those in CF patients. B. cepacia complex organisms are significant pathogens in CF patients, and accurate identification is vital to their proper management. An analysis of the accuracy of MALDI-TOF MS identification within the B. cepacia found that all systems were able to correctly identify members within the B. cepacia complex. , Importantly, MALDI-TOF MS can accurately identify other glucose nonfermenting, colistin-resistant organisms ( Pandorea and Ralstonia ), which have historically been difficult to confidently differentiate from B. cepacia complex. Of note, MALDI-TOF MS systems can have difficulty identifying Burkholderia mallei and B. pseudomallei. These are agents of bioterrorism that are not included in most FDA-cleared MALDI-TOF MS databases.
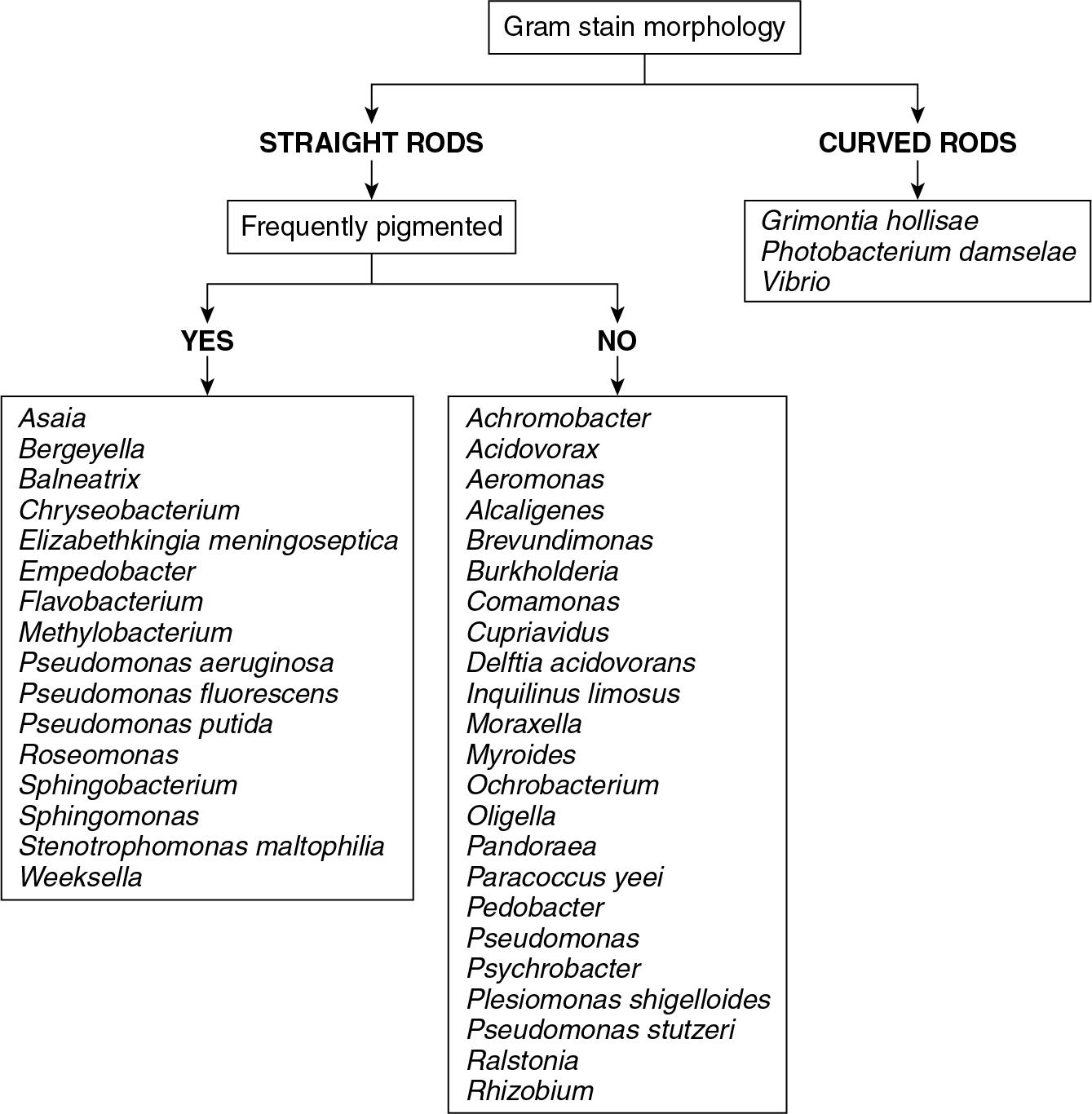
This general category of organisms includes significant diversity and a detailed discussion of MALDI-TOF MS performance for every fastidious gram-negative rod is outside the scope of this chapter. Rather, this discussion focuses on the more commonly encountered species ( Fig. 84.10 ).
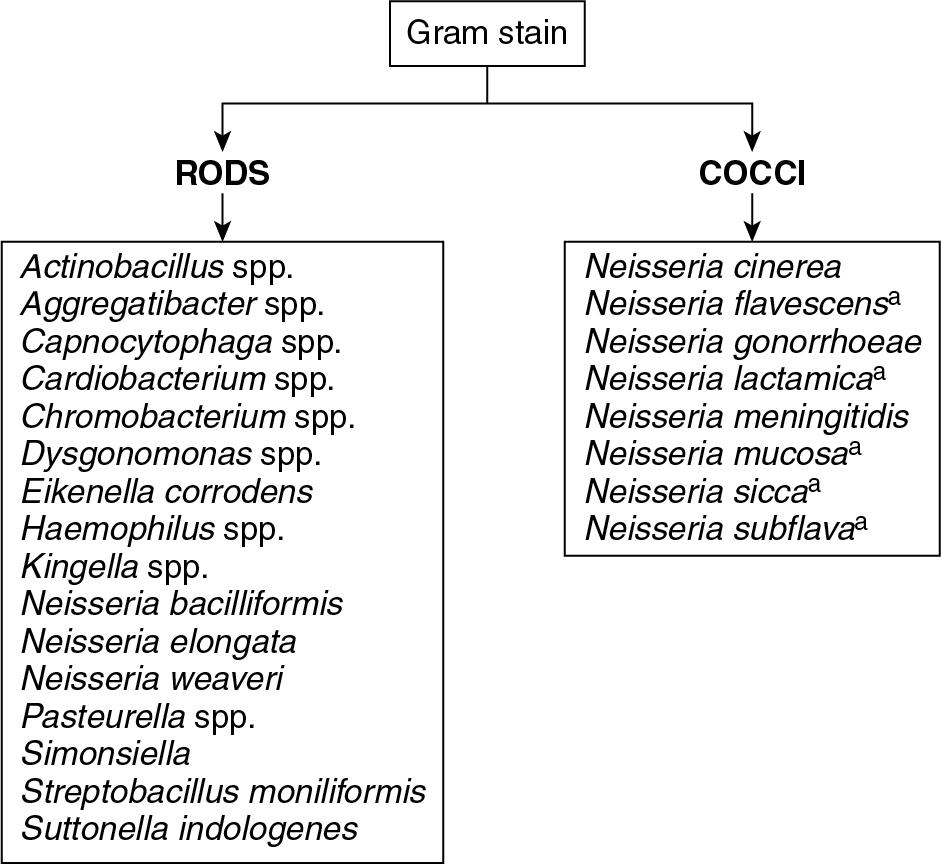
MALDI-TOF MS performance for the identification of fastidious gram-negative rods has excellent accuracy. Of the HACEK group organisms, a group of species that can cause subacute endocarditis (will be discussed in more detail later in the chapter), Haemophilus parainfluenzae, Aggregatibacter actinomycetemcomitans, Aggregatibacter aphrophilus (formerly Haemophilus aphrophilus ) , Aggregatibacter paraphrophilus (formerly Haemophilus paraphrophilus ) , and Eikenella corrodens were accurately identified to the species level. Limited data from other studies suggest that MALDI-TOF MS is able to accurately identify Kingella kingae, as well as Cardiobacterium hominis . ,
Practically speaking, genus-level identification is probably sufficient for many fastidious gram-negative rods. For example, it is probably not necessary to differentiate Dysgonomonas capnocytophagoides from D. mossii. Conversely, it could be clinically important to differentiate species within the Haemophilus genera or the Bordetella genera. Fortunately, MALDI-TOF MS appears readily able to identify most of these genera to the species level.
MALDI-TOF MS does well identifying many species of Campylobacter , the most common curved gram-negative rods encountered in the clinical microbiology laboratory. Multiple studies have shown the ability of MALDI-TOF MS to accurately differentiate commonly encountered Campylobacter spp., including C. jejuni , and C. coli . , Others have shown that MALDI-TOF MS can discriminate between Vibrio cholerae and aeromonads, which can sometimes mimic V. cholerae in stool culture.
There is comparatively little peer-reviewed data evaluating the performance of the MALDI-TOF MS and its ability to accurately identify gram-negative diplococci ( Neisseria spp. and Moraxella catarrhalis ) (see Fig. 84.10 ). M. catarrhalis is readily identified with MALDI-TOF MS, but some studies have found MALDI-TOF MS struggles with differentiation of pathogenic Neisseria meningitidis from nonpathogenic Neisseria spp., such as Neisseria polysaccharea. , In addition, MALDI-TOF MS may not accurately differentiate among nonpathogenic Neisseria spp. such as Neisseria subflava, Neisseria sicca, and Neisseria cinerea.
In most circumstances, it is not clinically relevant to differentiate among nonpathogenic Neisseria spp. However, it is extremely important that laboratories be able to accurately identify N. gonorrhoeae and N. meningitidis , not only so that laboratories can properly guide patient care, but also, in the case of N. meningitidis, so that laboratorians can protect themselves from exposure to a potentially life-threatening infection. Although laboratory-acquired N. meningitidis infection is rare, it results in high morbidity and approximately 50% mortality. Given the inability of MALDI-TOF MS to reliably identify some Neisseria spp. isolates, it is critical that laboratories maintain back-up methods for confirmatory identification. These back-up methods include 16S rDNA sequencing (not widely available), biochemical reactions, such as gamma-glutamyltransferase activity, and production of acid from sucrose, or from glucose and maltose on cysteine-rich media.
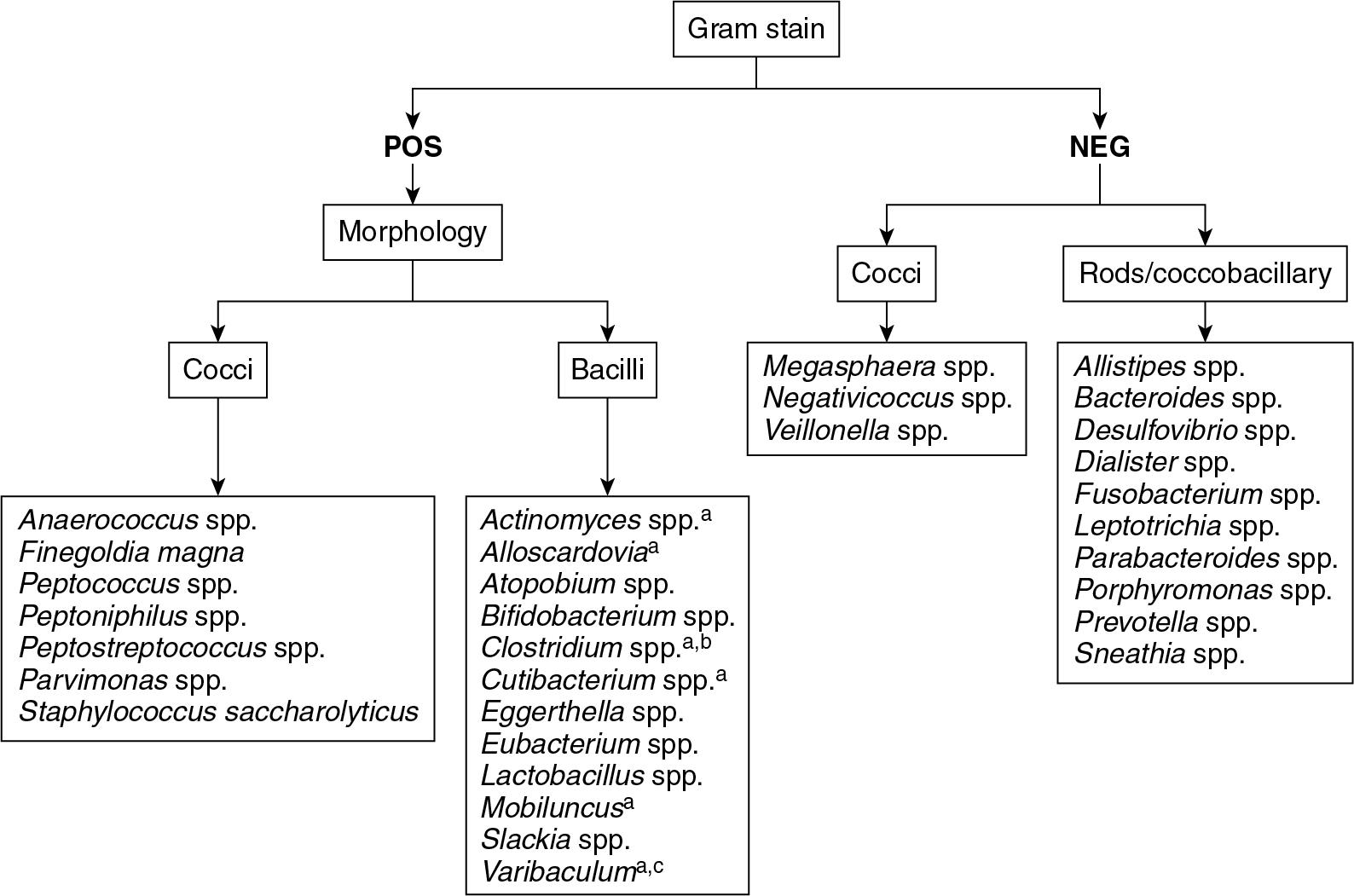
Anaerobic infections are frequently polymicrobic, as they result from either endogenous dissemination of commensal microbiota or from traumatic penetration, which introduces environmental anaerobes to ordinarily sterile body sites. Before implementation of MALDI-TOF MS, laboratory methods failed to reliably identify many anaerobic organisms. However, MALDI-TOF MS can rapidly and accurately identify many important anaerobic organisms including Clostridium spp., Bacteroides spp., Cutibacterium (formerly Propionibacterium ) spp., Peptostreptococcus spp. and related organisms, Fusobacterium spp., Actinomyces spp., and Lactobacillus spp. , Similar to other bacterial categories, MALDI-TOF MS may not identify all anaerobes, but it rarely generates erroneous results.
MALDI-TOF MS has become so effective in identifying anaerobic organisms that many laboratories have altered their traditional protocols such that routine aero-tolerance testing (comparative growth studies to determine whether an organism is an obligate anaerobe) is no longer routinely performed. Before MALDI-TOF MS, laboratories would subculture suspected anaerobes to both aerobic and anaerobic conditions and compare the rates of growth. Strict anaerobes would be confirmed by growing under anaerobic but not aerobic conditions. This procedure delays results because it requires at a minimum overnight incubation. Despite the ease and accuracy of MALDI-TOF MS identification of anaerobes, it is important that laboratories maintain a working knowledge of some basic organism characteristics such that MALDI-TOF MS results can be questioned and verified. Important identifying characteristics include the Gram stain, morphology, colony pigment, antibiotic resistance profiles, and fluorescence. See Fig. 84.11 for an outline of clinically important anaerobes and their defining characteristics.
Aerobic actinomycetes are made up of a broad group of gram-positive bacillary organisms that are often characterized by the presence of rudimentary branching. A positive modified acid-fast stain is also considered a key indicator among many genera of this group. Although over 40 genera have been classified as part of the aerobic actinomycete group, only a small minority are associated with human infections— Nocardia , Rhodococcus , Gordonia , Tsukamurella , Actinomadura , Streptomyces , and Tropheryma . Other members of this group such as Amycolata, Amycolatopsis, Pseudonocardia, Dermatophilus, Dietzia, and Nocardiopsis , although occasionally isolated from patient specimens, are saprophytes and should almost always be considered contaminants or colonizers.
Aerobic actinomycetes take up to two weeks to culture. Hence, infections due to these organisms are often overlooked as they are frequently overgrown by other microbiota associated with the site of infection and/or they require extended incubation time to grow the organism. Historically the identification of aerobic actinomycetes required evaluating both microscopic and macroscopic morphologies, in addition to testing for the metabolism of glucose, arylsulfatase production, growth in lysozyme, and determining the absence or presence of mycolic acid, a cell wall component. These methods are laborious and time consuming and are only able provide a presumptive or genus-level identification in most cases. ,
The use of MALDI-TOF MS for identification of actinomycetes has both shortened the time to identification and allowed for species-level identification that would have necessitated the need for DNA sequencing in the past. , However, unlike most bacteria identified using MALDI-TOF MS, aerobic actinomycetes may require additional steps prior to spotting to the target slide. These inactivation steps entail the use of glass beads with ethanol to disrupt the cell wall, and formic acid and acetonitrile to complete the extraction of the proteins. Both the MALDI Biotyper (Bruker) and the VITEK MS (bioMérieux) can identify most pathogenic aerobic actinomycetes (i.e., Nocardia , Rhodococcus , Gordonia , Tsukamurella , Actinomadura , and Streptomyces ), with the only limitation being the robustness of the MALDI-TOF MS database. , ,
Provides rapid identification of bacteria from as few as one isolated colony
Produces more accurate results, with higher resolution, than biochemical growth-based methods
Reduces need for organism identification by sequencing
Low cost per test
Systems unable to adequately differentiate key organisms (e.g., Shigella/ Escherichia coli, Streptococcus pneumoniae/Streptococcus mitis/oralis
Expensive initial investment in instrumentation (but cost-effective long term)
Bacterial identification based on conventional microbiologic phenotypic tests and MALDI-TOF MS does not always allow for accurate or unequivocal identification of bacteria. Historically, a major problem for clinical microbiologists was how to identify bacterial isolates that exhibited biochemical and/or other phenotypic characteristics that did not fit the pattern of any known genus and species or that was extremely slow to grow and thereby was challenging to identify by phenotypic characteristics. In the last decade or so, gene sequencing, especially small-subunit (16S) rDNA sequencing, has become a widely accepted tool for identifying bacterial isolates, in large part as a result of wider availability of polymerase chain reaction (PCR) and refinements in DNA sequencing. With the development and introduction of standard DNA extraction and amplification protocols, easy-to-use, commercially available reagents, and automated sequencers, this molecular approach to identification has been incorporated into the routine practice of reference laboratories and some hospital-based diagnostic laboratories. The impact of sequencing on infectious diseases cannot be underestimated; this approach has not only increased the ability of the laboratory to accurately identify clinical isolates that were poorly described and rarely isolated or were non–wild-type strains, but has now become routinely used for identifying mycobacteria, so that novel pathogens can be recognized. With identification and detection of novel pathogens, sequencing has also provided insight into the agent causing an infectious disease, including its pathologic associations, and has provided options for possible effective antimicrobial therapy.
Since the development of PCR and DNA sequencing, comparisons of gene sequences between bacterial species has shown that 16S rDNA contains regions that are highly conserved and regions that are variable, such that they can be used to discriminate among many bacterial species. An organism’s sequence has been described as a “molecular clock” that can estimate relationships among bacteria (phylogeny), thereby playing a significant role in reclassifying bacteria into completely new species or genera. In addition, more recently 16S rDNA gene sequencing has become an important tool for organism identification.
Although many different gene targets can be used for bacterial identification, 16S rDNA (which encodes for a part of the 30S ribosome) remains the primary target. This gene, which is about 1550 base pairs, has several important advantages over other targets.
In addition to highly conserved regions that serve as primer binding sites, 16S rDNA sequences contain hypervariable regions that provide species-specific signature sequences useful for identification of bacteria. Of note, partial 16S rDNA sequencing (approximately 500 bp) has emerged as an identification method that is accurate and fast for a wide variety of aerobic and anaerobic bacteria with successful implementation in the clinical laboratory.
A major limitation of 16S rDNA sequencing is its inability to discriminate among some highly related bacterial taxa. For example, Bacillus cereus and Bacillus anthracis have identical 16S rDNA sequences, and therefore alternative targets must be selected. Similarly, there are a number of bacterial species, such as Streptococcus pneumoniae, Streptococcus mitis, and Streptococcus oralis, and some rapidly growing mycobacterial species that have similar 16S rDNA sequences, and as such, 16S rDNA sequence-based identification systems are unable to differentiate between them. Therefore a number of alternative gene targets are used to better separate certain species (e.g., rpoB , tuf, gyrA or gyrB , and sodA genes), as well as heat shock proteins. , Primers used for bacterial sequencing are selected to target regions of the bacterial genome that are universal and conserved for a wide spectrum of bacteria.
This sequencing method, used by many laboratories, is a variation of the Sanger chain-termination technique developed two decades ago. The method is reviewed in detail in Chapter 64 . In brief, DNA extracted from the bacterial isolate serves as a template to which specific, short, complementary oligonucleotides (i.e., universal primers complementary to conserved regions of the 16S rDNA) anneal and initiate DNA synthesis. Newly created fragments are complementary to the template DNA. In the following cycle-sequencing reaction, the purified PCR products serve as the template, and forward or reverse primers are added to a mixture containing deoxyribonucleoside triphosphates (dNTPs) and four different, fluorescently labeled, dideoxyribonucleoside triphosphates (ddNTPs; dye terminators), Taq polymerase, and buffer. During this cycling reaction, DNA is denatured, the forward or reverse primer anneals and is extended such that sufficient fluorescent-labeled DNA is generated for subsequent sequencing, and during the extension step, chain termination occurs. In the reaction mix, dNTPs and ddNTPs are at concentrations such that some fluorescently labeled ddNTP will be incorporated instead of a dNTP at each nucleoside position in newly generated fragments. This results in terminated products of varying lengths. During capillary electrophoresis, these fragments separate by length, and because the terminating nucleoside is known (fluorescently labeled ddNTP), the sequencing machine will detect changes in fluorescence and use these changes to generate the nucleic acid sequence. The two strands of DNA are sequenced separately, generating forward and reverse complementary sequences thereby allowing for resolution of any base ambiguities or other errors. A generated DNA sequence (i.e., consensus sequence) is assembled by aligning the forward and reverse sequences, and then compared to a database using analysis software. Following query of the database, identification of the unknown bacteria can be determined. See At a Glance feature for an overview of sequencing. The utility of 16S rDNA sequencing as a tool in microbial identification depends on two key elements: correctly identifying or “labeling” each sequence and deposition of complete, unambiguous nucleotide sequences into public or private databases. There are a number of well-known databases of 16S rDNA sequences, many of which contain high-quality, current, and optimally curated sequence data. For example, GenBank, which contains the largest database of nucleotide sequences, contains over 90,000 deposited 16S rDNA sequences.
Typically, nucleotide sequences are reported in terms of percent identity . This phrase refers to the number of identical nucleotide bases shared by the query and reference sequences divided by the number of nucleotide bases sequenced. Interpretive criteria were published by the Clinical and Laboratory Standards Institute (CLSI) in 2008 and updated in 2018. An additional CLSI document addresses diagnostic sequencing using capillary-based sequencers and other topics such as isolation and extraction of nucleic acid; template preparation; sequence generation, alignment, and assembly; validation and verification; ongoing quality assurance; and reporting of results.
Finally, there are primer candidates for broad-range amplification involving the V1 to V3 area of the 16S rDNA that do not have significant cross-reactivity with human DNA. By using these primers and other advances, such as dual priming oligonucleotides, the detection of bacterial 16S rDNA sequences directly from human clinical material is now possible. , ,
Although it is clear that 16S rDNA sequencing has a significant role in the identification of bacteria in the clinical laboratory, it is not foolproof or applicable in every situation. Misidentification of bacteria by 16S rDNA sequencing has been reported for organisms such as S. pneumoniae, E. coli, and B. anthracis . Therefore it is important to correlate sequence-based findings with the phenotypic traits of the organism being characterized. In addition, various software packages can give different results. Finally, because unusual taxa are detected and reported by gene sequencing, it is important for the laboratory to provide additional information to help clinicians in placing the new species in a familiar context.
One of the downstream effects of the implementation of molecular methods such as MALDI-TOF MS and 16S rDNA sequencing for the identification of bacteria is the ability to identify/discriminate organisms that previously could not be readily identified using traditional biochemical methods. Numerous publications have highlighted these organisms. Some pertinent examples include the identification of animal-associated staphylococci such as Staphylococcus intermedius group and Staphylococcus schleiferi and pathogenic Corynebacterium spp. that have important clinical associations, including C. kroppenstedtii and C. macginleyi to name a few.
Despite this increased ability to discriminate potentially clinically relevant species using MALDI-TOF MS, education of medical staff is necessary to ensure understanding of this “new” information. For example, clinical staff were historically trained to ignore Corynebacterium spp. as potential contaminants. In the case of Corynebacterium spp. with important clinical associations such as those listed above, these identifications should not be ignored.
16S rDNA contains both highly conserved and hypervariable regions, permitting specific identification of many bacterial species.
Universal primers amplify all bacteria while the internal sequence is used for bacterial identification.
Misidentification of bacteria by 16S rDNA sequencing has been reported for Streptococcus pneumoniae, Escherichia coli, and Bacillus anthracis due to similarity of the 16S rDNA sequence with related species.
Historically the use of nucleic acid amplification tests (NAATs) has been associated with basic science research rather than the clinical laboratory setting, as the development of these “high-complexity” tests required a skilled work force that understood molecular methods and a laboratory space that could assure that the multi-step process of testing would limit contamination. However, advances in molecular methods, such as transcription mediated amplification (TMA), real-time polymerase chain reaction (RT-PCR), and quantitative PCR (qPCR), has allowed for the commercialized development of sample-to-result instruments, thereby eliminating the need for highly skilled technologists and a dedicated space for some molecular testing.
Most of the nucleic acid-based tests used in the detection of bacteria are qualitative assays. Typically, these qualitative tests are used to identify pathogens from a sterile source, such as blood or CSF. On nonsterile sources, these qualitative tests focus on definitive pathogens, such as Chlamydia trachomatis or Neisseria gonorrhoeae , which are not part of normal microbiota and where a positive result would be clinically actionable. The use of NAATs has also proven useful in identifying organisms that require extended incubation times (e.g., Mycoplasma pneumoniae ), selective media (e.g., Bordetella pertussis/parapertussis ), or cell lines to grow (e.g., C. trachomatis ). The use of molecular assays has also had practical effects in the appropriate management of antimicrobial therapy. Examples of patient management being influenced by molecular testing include patients being screened for methicillin-resistant Staphylococcus aureus (MRSA) colonization to determine if empiric vancomycin therapy should be discontinued in patients presenting with a lower respiratory tract infection or if decolonization measures should be instituted prior to surgery. Furthermore, these molecular-based tests have practical implications from an infection prevention perspective as well, as patients colonized or infected with MRSA, vancomycin-resistant Enterococcus (VRE), or carbapenem-resistant Enterobacterales (CRE) can be rapidly placed on appropriate precaution measures, thus limiting the subsequent transmission of these organisms.
Although NAATs have many positive aspects, one of the key constraints to molecular-based testing, compared to culture, is its inability to detect the organism that is causing the infection unless the correlative primer set is present in the assay. Alternatively, from a clinician’s perspective it can be just as difficult to determine the etiologic agent based simply on the signs and symptoms of the patient, which leads to the ordering of multiple culture types (i.e., aerobic/anaerobic bacterial cultures, fungal cultures, and/or AFB cultures) to assure that the pathogen of interest can be identified. This dilemma has been partially resolved with the advent of multiplex syndromic panels, where the selection of targets is based on pathogens most commonly associated with a specific infectious disease syndrome, such a bacteremia, meningitis/encephalitis, or gastroenteritis. The implementation of these syndromic panels has allowed for faster turnaround times, greater sensitivity, and significantly better patient outcomes as patients can now be actively managed in real time. For additional details on molecular-based testing and its role in the clinical microbiology setting, refer to Chapter 67 .
In recent years, direct specimen testing for pathogen identification has become more commonplace in clinical laboratories. These technologies include, but are not limited to, broad-range 16S rDNA PCR and sequencing, magnetic resonance testing, and other near-direct specimen testing methods, such as direct MALDI-TOF MS identification of positive blood cultures, and rapid culture-based systems for pathogen identification and susceptibility testing from positive blood cultures.
Broad-range sequencing is a method of pathogen identification whereby a patient specimen is submitted for 16S rDNA PCR (or other targets, such as hsp65 and rpoB for mycobacteria) and sequencing of the PCR product. There are a number of situations where this technology could add tremendous clinical value. However, this testing should be used with discretion, as it is often costly and, in some instances, unnecessary.
One of the advantages of broad range 16S rDNA sequencing is the ability to identify bacteria present in normally sterile specimens, like tissue and CSF. These identifications are unbiased with respect to viability and cultivability of the organism. Indeed, this aspect of this testing is useful, especially when specimens are inadvertently placed into formalin prior to microbiological culture, as formalin-fixed paraffin-embedded tissue is generally acceptable for this testing, especially if potential pathogens are noted in the pathology report. Challenges associated with broad-range sequencing include contamination, long turnaround times, and the inability to obtain antimicrobial susceptibility testing. An in-depth review of broad-range sequencing is beyond the scope of this chapter. ,
Magnetic resonance technology has come into use in some clinical microbiology laboratories in the past decade. T2 Biosystems (Lexington, MA) currently has two FDA-cleared panels on their T2Dx system for direct detection of pathogens from whole blood specimens, for both candidemia and bacteremia. This technology leverages the ability of magnetic resonance to track changes in water molecule interactions. Recent studies of this technology show very good test performance characteristics for the targets on the test panel (90% for both sensitivity and specificity), but low overall performance for all bacteremia (43%). , However, many questions about the utility of this test remain, particularly in regard to how the results are used for patient management and antimicrobial stewardship.
In addition to the direct specimen testing assays, described above, there are a number of near-direct methods available for identification and susceptibility testing from positive blood culture broth, including rapid culture-based and molecular FDA-cleared assays, in addition to laboratory-developed tests that identify and test bacteria purified from positive blood culture broth by MALDI-TOF MS and/or various antimicrobial susceptibility systems. These methods, especially those that provide direct susceptibility testing from blood culture broth, can provide clinically actionable information in a much shorter time frame than typical culture and susceptibility testing.
To mitigate false-positive results when using highly sensitive, molecular methods, contamination should be proactively monitored. Contamination can be assessed by monitoring the positivity rate of the assay and periodic environmental monitoring (swipe) tests. An unexplained and sudden increase in the positivity rate may be an indication of contamination. Therefore swipe tests that look for potentially contaminating nucleic acid amplicons should be performed periodically on instruments, pipettes, reagents, consumables, and each workstation. A positive swipe test allows one to determine the specific area of contamination, as well as determine if the area has been appropriately cleaned when follow up testing is negative. Even if there is not an appreciable change in the positivity rate, swipe tests should be performed at regular intervals as part of good laboratory practice.
Urinary tract infection (UTI) is one of the most common infectious presentations, resulting in over 4 million ambulatory care visits in the United States each year. UTI can occur in two general anatomic locations within the urinary tract. Cystitis and urethritis are infections in the lower urinary tract, which includes the bladder and urethra, respectively. These infections are particularly common in women because they have a shorter urethra, which allows anogenital microbiota easier access to the bladder. In contrast, men have a longer urethra and UTI is rare in men less than 60 years of age. Upper UTI (i.e., pyelonephritis) is infection of the ureters and renal parenchyma.
Infections of the genital tract can occur in both men and women, and it is important to understand the anatomy and epidemiology of each when processing and interpreting cultures. Genital tract infections can result from several different exposures including sexual contact, altered commensal microbiota, or migration of organisms to normally sterile spaces.
UTIs are not only subdivided by their location in the upper or lower urinary tract but also by the way in which they are acquired (community-acquired or hospital-acquired) and whether the patient has urinary tract abnormalities and/or other risk factors (i.e., complicated vs. uncomplicated). Important risk factors for complicated UTI include chronic catheter placement, which can lead to the development of catheter-associated UTI, frequent self-catheterization (such as those with neurogenic bladders), and those with complex urogenital abnormalities/complications requiring the placement of a nephrostomy or ureterostomy tube.
Sexually transmitted infections (STIs) are some of the most common human infections, with nearly half a billion cases worldwide annually. Infections of the genital tract that result from nonsexual exposures include those introduced by instrumentation or foreign bodies. Regardless of the route of exposure, these infections can be either symptomatic or asymptomatic. Asymptomatic infections are a significant problem because they often go untreated, leading to further spread or complications such as pelvic inflammatory disease (PID) and infertility.
STIs can present with urethral discharge and/or lesions on the skin and mucous membranes. Vaginitis is an inflammation of the vaginal mucosa and presents with symptoms of itching, abnormal discharge, and malodor. Bacterial vaginosis (BV) is a distinct disease caused by dysbiosis of the vaginal microbiota resulting from proliferation of Gardnerella vaginalis and other aerobic and anaerobic organisms and the loss of commensal lactobacilli. BV is associated with vaginal irritation and a foul-smelling odor. Cervicitis (inflammation of the cervix) is common in women with N. gonorrhoeae and/or C. trachomatis infection and can lead to purulent discharge from the endocervix.
PID results from the inflammation that occurs when organisms reach the endometrium and fallopian tubes. These infections can present with vague constitutional symptoms such as fever, weight loss, and headache but also commonly include abdominal pain, dysuria, and vaginal discharge. PID is a serious infection and can lead to infertility, if untreated.
Several urine specimens can be collected for the diagnosis of UTI. The most convenient specimen is the clean-catch urine or mid-stream urine, which can be self-collected by first washing out the initial stream of urine and collecting the so-called mid-stream urine. This process helps to void the contaminating microbiota that is present in the urethra. In catheterized patients, urine can be collected directly through the catheter. This is a higher quality specimen as it represents urine collected directly from the bladder. However, if the patient has an indwelling catheter, growth in urine culture may represent colonization of the catheter. As such, indwelling catheters should be changed prior to collection of urine for culture. Undoubtedly, the highest quality specimen is obtained via suprapubic aspiration (SPA) of urine. This is a sterile procedure in which a needle is inserted directly through the abdominal wall into the bladder, and urine is withdrawn without risk of contamination. Despite the high quality of this specimen, it is rarely obtained due to the technical expertise required for collection and the invasiveness of the procedure. In lieu of SPA, most providers opt to collect urine via straight catheterization, sometimes known as an “in and out” catheterization. This is a commonly employed approach in children who are unable to provide midstream urine. Although bag urine specimens can be useful for performing urinalysis in young children, this specimen type is of very low quality for culture because of the high likelihood of generating a false-positive result.
It is essential that urine specimens be transported in a timely manner to the laboratory so they can be processed before bacterial overgrowth can occur and obscure semi-quantitative urine culture results. If timely transport is not possible, urine specimens can be refrigerated to suppress growth or urine may be placed in a preservative tube containing boric acid. Although boric acid preservation is a commonly used approach, there is a paucity of evidence that supports its efficacy.
In some cases, the collection of urine or other genitourinary specimens (i.e., vaginal, endocervical, or urethral specimens) require specific collection and transport devices/media. Examples include manufacturer-specific NAAT transport devices or specific transport media needed to maintain the viability of fastidious pathogens, such as N. gonorrhoeae and C. trachomatis . Due to the fastidious nature of these organisms, rapid transport to the laboratory for processing is encouraged for culture setup. Special collection devices for preserving the viability of gonococci for culture exist and include Stuarts or Amies charcoal transport media. In non-genital tract specimens, cultivation of Chlamydia requires that the specimen be collected in special transport media that includes antibiotics to inhibit the overgrowth of resident microbiota. In contrast to gonococci, refrigeration may preserve viability of Chlamydia in clinical specimens, but these specimens should not be held longer than 24 hours before processing. If it is not possible to process specimens within 24 hours of collection, samples should be frozen. The specimen of choice for BV is vaginal discharge resulting from sloughed off epithelial cells.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here