Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A 19-year-old woman presented to the emergency department with confusion, lethargy, and neck stiffness. Her dorm mates reported that she had experienced upper respiratory symptoms for 3 or 4 days before presentation. She had no significant past medical history. On physical examination, her findings were temperature, 98.6° F (37°C); pulse, 100 beats/min; respirations, 20/min; and blood pressure, 110/70 mm Hg. Although her neck was stiff, Kernig and Brudzinski signs were absent. The pharynx was slightly injected without exudate. Heart and lung examination results were normal. No rash was present. Neurologic examination revealed a slightly obtunded, sleepy woman with otherwise intact mental status, intact cranial nerves, no motor or sensory deficits, and normal reflexes.
The patient's white blood cell (WBC) count was 22,900/mm 3 , with a marked left shift, including 34% bands and 62% polymorphonuclear neutrophils (PMNs). Her platelet count was 120,000/mm 3 . Her electrolytes revealed a mild metabolic acidosis. Chest radiograph and brain computed tomography (CT) were normal.
Initial cerebrospinal fluid (CSF) examination was normal. However, she was admitted for observation. Very soon thereafter, she complained of increasingly severe headache and neck pain. On examination, she was found to be in a confused state and now had a fever of 39.1°C (102.4° F). Repeat lumbar puncture demonstrated a cloudy CSF with 670 WBCs (90% PMNs), a glucose level of 1 mg/dL, and a protein level of 220 mg/dL. Gram stain revealed rare gram-negative diplococci that on culture grew Neisseria meningitidis. Blood cultures also grew N. meningitidis. She was treated with penicillin G 24 million U/d IV and recovered completely. Before discharge she was given rifampin to eliminate nasopharyngeal carriage of N. meningitidis.
One of the most serious neurologic emergencies involves the evaluation and care of patients with bacterial meningitis. Usually, these individuals are previously healthy when they suddenly develop a severe headache, fever, and stiff neck. Despite more than 50 years of experience with antibiotic therapies, bacterial meningitis remains a very lethal disease. Expedient diagnosis is essential to prevent such an outcome. In the preceding vignette, typical for meningococcal meningitis, despite the history and findings suggestive of a meningeal infection, the initial CSF examination was normal. An emergency physician wisely admitted the patient for observation. When she experienced sudden deterioration, repeat CSF examination led to the diagnosis and appropriate therapy. Any delay in diagnosis and therapy of bacterial meningitis can be irretrievable, as death may occur unless appropriate antibiotic therapy is begun immediately.
Bacterial meningitis is defined as a microbial infection primarily involving the leptomeninges ( Fig. 44.1 ). Typically, bacteria seed the leptomeninges via the bloodstream or from a contiguous site of infection, such as sinusitis, otitis media, or mastoiditis. Rarely a defect in the normal anatomic barriers, as with a perforating cranial or spinal injury or congenital dural defect, leads to a predisposition to recurrent bacterial meningitis.
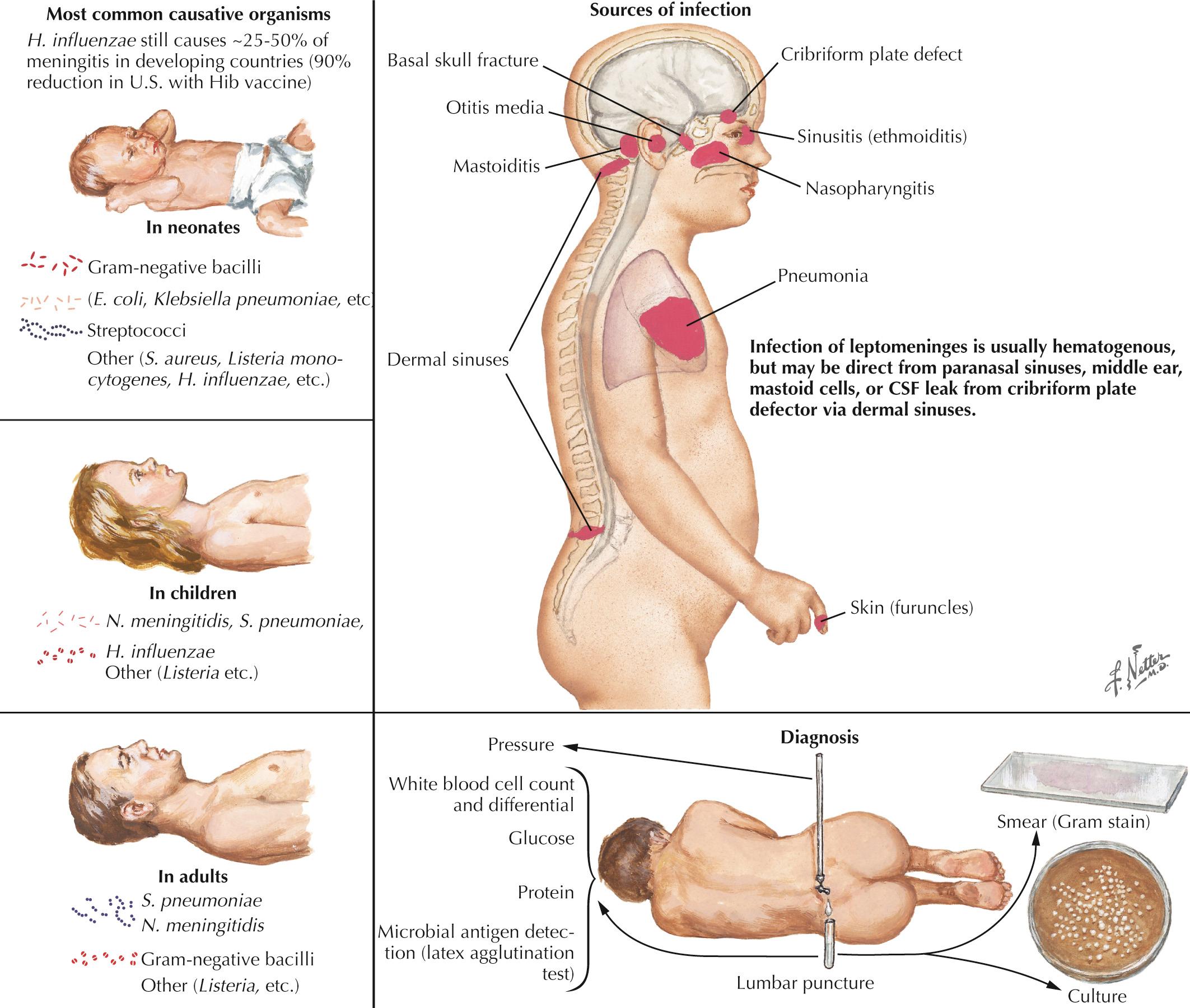
The responsible microorganisms often differ between children and adult patients. Those commonly responsible for meningitis in adults include Streptococcus pneumoniae, N. meningitidis, and Listeria monocytogenes. Haemophilus influenzae still causes 20% to 50% of meningitis in developing countries, but in the United States this rate has been reduced by 90% with the H. influenzae type b vaccine. In neonates, Escherichia coli and group B β-hemolytic streptococci cause most cases. L. monocytogenes particularly leads to meningitis in immunocompromised patients and rarely in the newborn. N. meningitidis infection often occurs with a primary sepsis and a characteristic petechial and/or purpuric rash or disseminated intravascular coagulopathy. Conditions predisposing to pneumococcal meningitis in adults include sickle cell disease as well as conditions predisposing to immune deficiencies, including alcoholism, cirrhosis, splenectomy, and HIV/AIDS. Basilar skull fracture may also pose a risk for invasive pneumococcal meningitis.
Gram-negative bacilli ( E. coli, Proteus, Pseudomonas, Serratia, Klebsiella, and Citrobacter ) are rarely found in community-acquired meningitis but more commonly occur in association with head or spinal trauma or after neurosurgery. These organisms must always be considered in immunocompromised hosts. Meningitis due to Staphylococcus aureus may follow penetrating trauma, neurosurgery, or bacteremia. Coagulase-negative staphylococci (Staphylococcus epidermidis) or S. aureus and other organisms are associated with infected ventricular shunts.
The onset of acute bacterial meningitis is rapid: hours to a day or so. Classic clinical findings include signs of an acute cerebral disorder, with lethargy, seizures, and agitation as well as specific signs of meningeal involvement manifested by severe neck stiffness, called meningismus, and fever that may not be immediately present. The patient rapidly becomes confused, sleepy, obtunded, and often comatose.
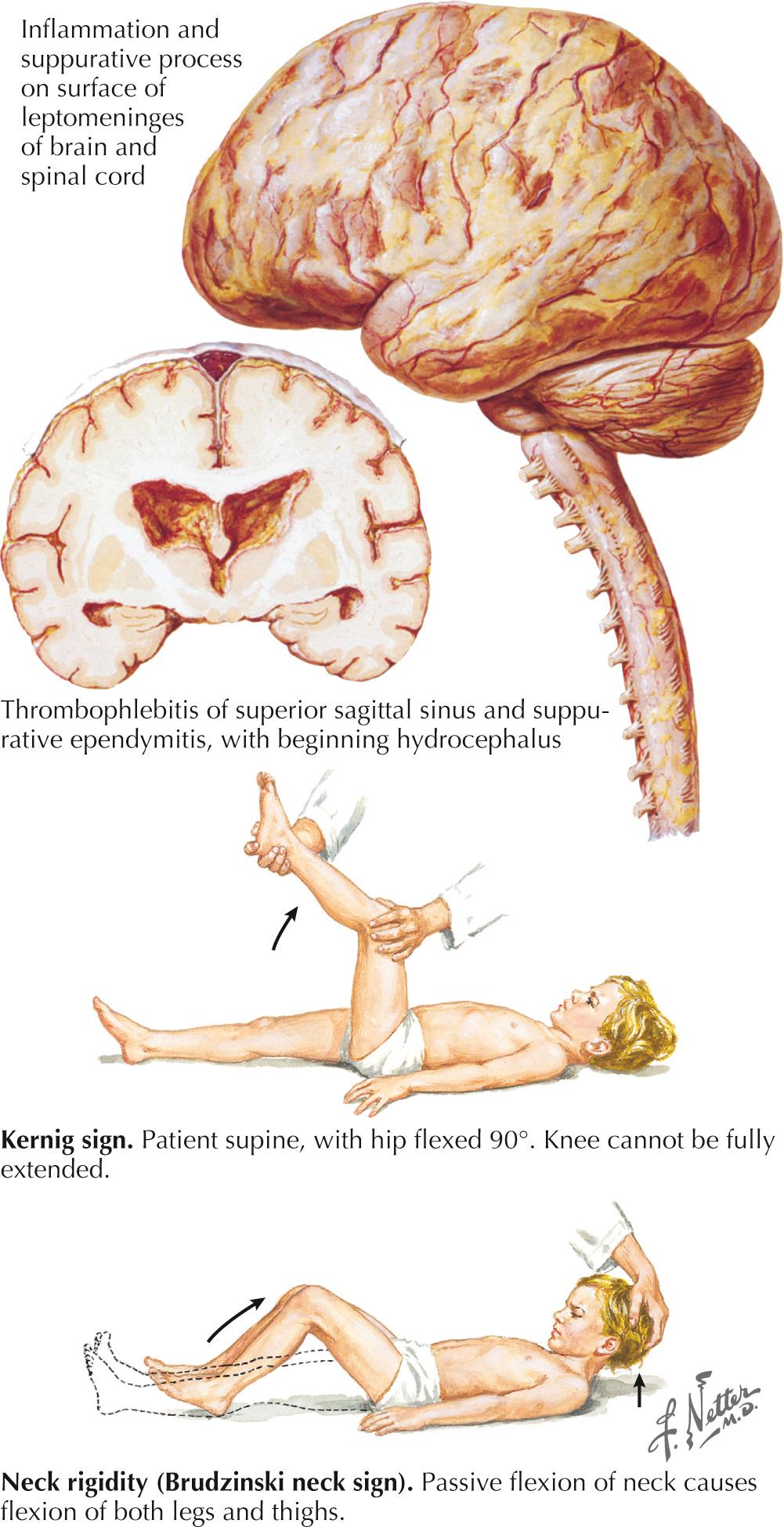
The examining physician must carefully search for signs of nuchal rigidity in any febrile patient who presents with a headache or any changes in the level of alertness. Two clinical maneuvers are very important for identifying the presence of inflamed meningeal coverings involving the lumbosacral nerve roots: examination for the Kernig and Brudzinski signs ( Fig. 44.2 ). The Kernig sign is elicited by flexing the patient's hip to a 90-degree angle and then attempting to passively straighten the leg at the knee; pain and tightness in the hamstring muscles prevent completion of this maneuver. This sign should be present bilaterally to support a diagnosis of meningitis. The Brudzinski sign is positive if the patient's hips and knees flex automatically when the examiner flexes the patient's neck while the patient is supine. Because host responsiveness to the infection varies, these signs of meningeal irritation are not invariably present, especially in debilitated and elderly patients and infants. When the clinical picture is typical of meningitis, it is also very important to exclude the concomitant presence of a focal parameningeal source such as a brain abscess. Further history, careful neurologic examination, and various imaging studies are essential ( Figs. 44.3 and 44.4 ). Frequently, concomitant dermatologic findings may be present. A maculopapular or petechial/purpuric rash usually indicates infection with N. meningitidis although an echovirus may mimic this. However, in viral meningitis, the CSF findings are significantly different, usually with predominant lymphocytosis, normal CSF sugar, and negative Gram stain. The dermatologic findings of N. meningitidis are usually secondary to an underlying vasculitis, concomitant coagulation defects, or a combination of the two. Meningococcal infection more commonly has a rash that affects the trunk and extremities, in contrast to the echovirus exanthema, which usually involves the face and neck early in the infection. Purpuric lesions may also rarely be found in a fulminant pneumococcal bacteremia with meningitis as well as staphylococcal endocarditis, the latter primarily involving the finger pads.
CSF examination is essential to the diagnosis of bacterial and other microbial forms of meningitis. When there are signs of focal neurologic involvement or increased intracranial pressure in patients with suspected meningitis, a lumbar puncture (LP) may be contraindicated. These include papilledema, coma, and focal neurologic findings such as dilated pupils, hemiparesis, and aphasia. Accompanying signs of increased intracranial pressure may include bradycardia, Cheyne-Stoke respirations, and even projectile vomiting. When a patient is immunocompromised or has an altered level of consciousness, new-onset seizures, or focal neurologic deficits, a computed tomography (CT) scan must be obtained prior to LP to exclude brain abscess or a parameningeal focus with significant mass effect.
As soon as the diagnosis of meningitis is considered, even before proceeding with a CT scan and LP, administration of empiric antibiotics covering the broad spectrum of gram-positive and gram-negative organisms is essential. One can later adjust the treatment once CSF culture examination results are available. If CT examination confirms that there is no mass lesion suggestive of a parameningeal focus with potential for herniation, one can then safely proceed with a CSF examination. If a parameningeal mass lesion is identified as a source of the meningitis, its treatment is paramount. The precise identification of the pathogenic bacterium, per se, will then become possible at the time of surgical decompression, and the spinal tap as such will not be required.
CSF analysis provides the only conclusive proof of bacterial infection of the subarachnoid space. It must include a Gram-stained smear to define the causative organism morphology. Gram stain correlates with the precise microbial etiology, as defined by the more specific bacteriologic culture, in approximately 80% of patients. This is a simple technique that allows for better selection of appropriate antibiotic therapy before definitive culture and sensitivity data are available. However, one does not need to await the results of Gram stain before immediate initiation of appropriate antibiotic therapy. Rapid detection of microbial antigens by immunochromatographic technique or latex agglutination tests can aid diagnosis when CSF Gram stain and cultures are not diagnostic. Newer polymerase chain reaction (PCR) technology has excellent sensitivity and specificity for the diagnosis of bacterial meningitis.
The initial CSF analysis needs to include measurement of the opening pressure, color (clear, turbid, or purulent), WBC count and differential, and levels of glucose and protein. Typically in bacterial meningitis, the CSF opening pressure is increased (>200 mm of water lying down and >35 mm water upright). The fluid is usually turbid or frankly purulent and contains predominantly (>80%) polymorphonuclear leukocytes. The CSF glucose level is very low, usually less than 40% of that found with measurement of concomitant serum glucose. A low glucose level (<40 mg/100 mL) is also found in some other types of microbial meningitides including L. monocytogenes, Mycobacterium tuberculosis, and Cryptococcus neoformans. Normal glucose levels are common in viral meningitis. Usually, CSF protein levels are increased, often greater than 100 mg/dL (reference range, <45 mg/dL). In patients with parameningeal foci such as a brain or epidural spinal abscess or with multiple septic emboli, CSF glucose may not be as low as with typical bacterial meningitis, even though in these instances, the CSF protein level is significantly increased.
Bacterial meningitis is an extremely severe, life-threatening infection. Any delay in its diagnosis by not initially assessing the patient or not beginning therapy at the first consideration of this critical diagnosis will increase morbidity and mortality. Antibiotic treatment must be initiated as soon as possible and later guided by CSF examination results. When CSF examination cannot be performed promptly, empiric therapy must be instituted immediately. Patients must receive high-dose IV antibiotics that can easily cross the blood-brain barrier. Empiric IV therapy with a third-generation cephalosporin such as ceftriaxone or cefotaxime plus vancomycin must commence pending results of the bacterial cultures. High-dose corticosteroids, administered before antibiotic therapy, are recommended for all children and should be seriously considered for adults with community-acquired meningitis.
When culture and sensitivity data are available, a specific antimicrobial therapy can be determined. Penicillin G is recommended for documented meningococcal meningitis.
Antimicrobial therapy for meningitis caused by S. pneumoniae must be based on antibiotic sensitivity test results. If the strain is susceptible to penicillin, penicillin or ceftriaxone is recommended. Ceftriaxone or cefotaxime is recommended when the strain is not susceptible to penicillin and is susceptible to cephalosporins. If the strain is susceptible to neither cephalosporins nor penicillin, vancomycin must be added to a third-generation cephalosporin (cefotaxime or ceftriaxone). In patients older than 50 years of age, pregnant women, immunocompromised hosts, or alcoholics, empiric therapy with ampicillin must be added to vancomycin and a third-generation cephalosporin to provide coverage for L. monocytogenes.
Among patients with bacterial meningitis, approximately 15% experience acute and chronic complications, including various cranial nerve dysfunctions, particularly those affecting extraocular function (cranial nerves [CNs] III, IV, and VI), CN VII, and sometimes CN VIII, although this is less common today, with the antibiotics lacking specific ototoxicity or vestibular toxicity. However, permanent sensorineural hearing loss occurs occasionally, most commonly with pediatric meningococcal infections. Assorted cranial neuropathies are generally secondary to the exudate that is common with the more purulent forms of bacterial and tuberculous meningitis.
Focal or generalized seizures, various focal cerebral signs, coma, and acute cerebral edema occasionally occur. Findings mimicking a stroke—such as a hemiparesis, aphasia, and hemianopsia—are relatively infrequent; persistence of such findings suggests secondary cerebral arteritis, cerebral venous thrombosis, or rarely a mass lesion, especially an abscess. Even with astute and early diagnosis, mortality rates are still at least 10% for meningococcal and 30% for pneumococcal meningitis, although the latter has very significantly decreased in frequency with the recent introduction of a pneumococcal immunization. Whenever any diagnostic delay occurs leading to less than immediate treatment, mortality and morbidity are significantly higher.
Chemoprophylaxis is particularly recommended for persons in close contact with patients who have acquired meningococcal meningitis, especially in confined settings such as college dormitories and army barracks. Rifampin is preferred; ciprofloxacin is also effective.
Vaccines are available for three common organisms. H. influenzae type b protein-polysaccharide vaccine is highly effective in preventing meningitis in newborns and young infants. N. meningitidis (meningococcus) serogroups A, C, Y, and W135 polysaccharide vaccine is recommended for high-risk adults and contacts of persons with meningococcal disease. An additional monovalent serogroup B vaccine is recommended for individuals with complement deficiency, functional or acquired asplenia, and those exposed to N. meningitidis in an outbreak or laboratory setting. For S. pneumoniae (pneumococcus) infection, 13 valent protein conjugate vaccine is recommended for all children. Additionally, protein polysaccharide vaccine is given for those children who are at high risk for acquiring pneumococcal infections. Currently, 23 valent pneumococcal polysaccharide and 13 valent protein conjugate vaccines are recommended for adults based on age and risk.
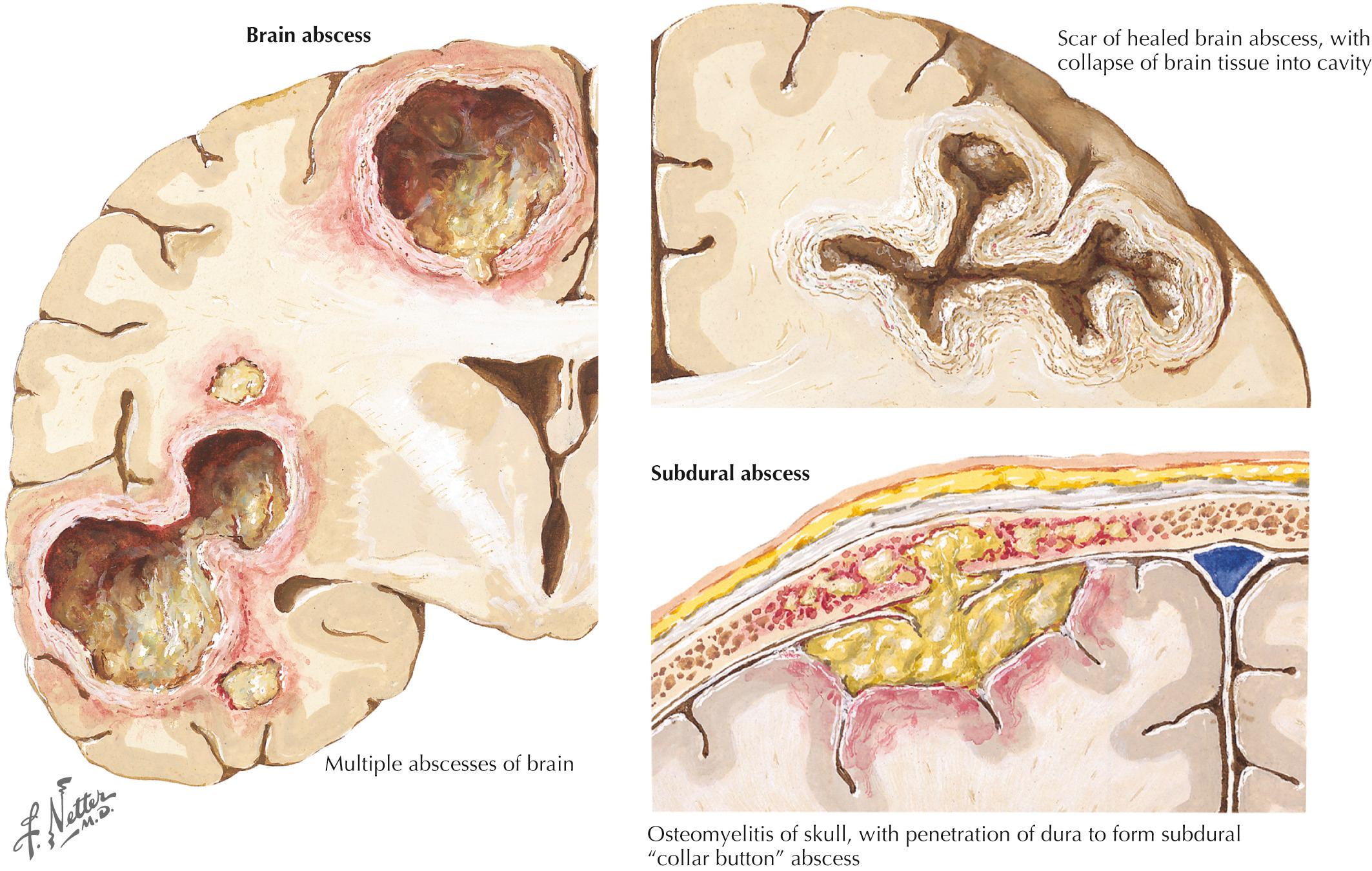
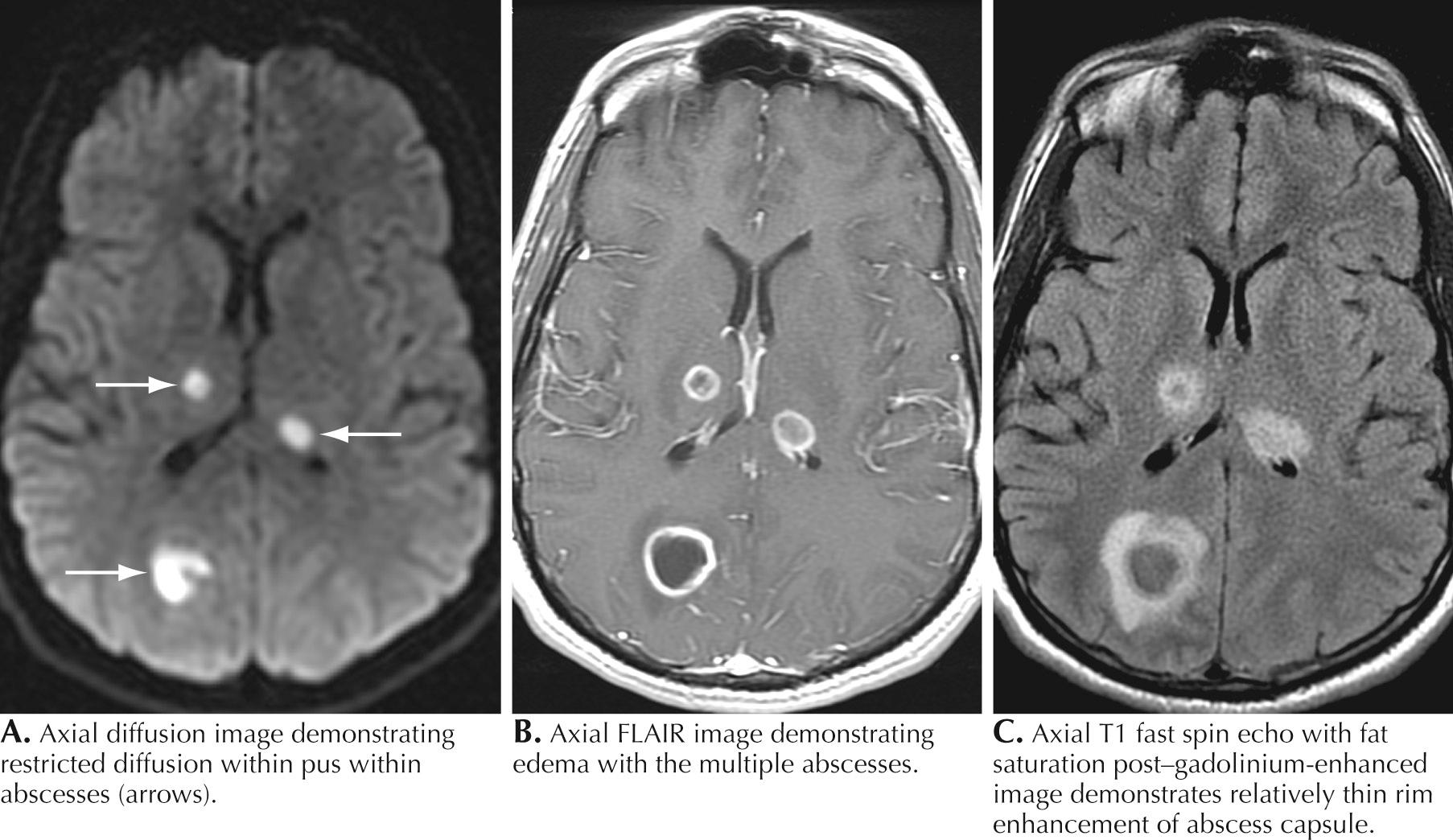
A 76-year-old man presented with sinus headaches and underwent surgical drainage of the frontal sinuses. Postoperatively, he had headaches, seizures, and mild right-sided weakness, diagnosed as a mild cerebrovascular accident (CVA). He was discharged home, where he had some difficulty walking and his speech was “not quite normal.” He gradually worsened and, 6 weeks after his surgery, could not hold anything in his right hand and became aphasic. He complained of chills but had no fever. On examination, he was awake and alert but globally aphasic, the cranial nerves were intact, gaze was conjugate, and there was a right hemiparesis. His WBC count was 9700/mm 3 with a normal differential. Brain CT demonstrated a hypointense structural lesion with midline shift in the left frontal lobe. Brain magnetic resonance imaging (MRI) demonstrated a multiloculated lesion with marked ring enhancement and surrounding edema extending through the frontal lobe and posteriorly toward the left parietal lobe (see Fig. 44.3 ). There was 1.5-mm midline shift. The lesion was aspirated using a stereotactic technique; Gram staining revealed many polymorphonuclear leukocytes (PMNs), many gram-positive cocci, and rare gram-negative rods. Culture grew Proteus mirabilis and Bacillus species. He was treated for 4 months with ceftriaxone and metronidazole with full resolution of speech and recovery of ambulation with the assistance of a walker.
Comment: Although parameningeal infections are relatively uncommon disorders, these lesions must always be considered in the differential diagnosis of any acute cerebral or spinal lesion (see Fig. 44.3 ). These processes may not be suspected and thus go unrecognized until it is too late to prevent permanent neurologic deficits. CT and MRI scanning are useful tools to exclude such predisposing lesions. Although these abscesses are easily considered within the setting of an overt infection, a precise microbial source is not always defined by the character of the clinical presentation. It is essential always to consider whether any acute spinal or cerebral lesion possibly has an infectious basis. This is particularly important in the setting of a chronic illness such as diabetes mellitus, which often predisposes individuals to spinal epidural abscesses. The highest diagnostic and therapeutic priority is required in these settings. When they are identified, such processes are among the most urgent neurologic emergencies. These require immediate diagnostic and therapeutic attention. Even when appropriate diagnostic and therapeutic focus occurs, the patient's outcome may still be guarded, as in the preceding vignette.
A 26-year-old woman had a sudden onset of numbness in her right hand and face. This cleared within a few minutes, only to recur on two more occasions within the next 48 hours. She then suddenly became aphasic, with numbness and weakness of her right hand and face. Neurologic examination demonstrated a fluent aphasia with a right central facial and hand weakness. Her muscle stretch reflexes were enhanced in her right arm, associated with a right Babinski sign. She had a grade III/IV systolic murmur at the apex of her heart. Brain imaging demonstrated a left parietal temporal mass suggestive of a cerebritis or an abscess. Empiric antibiotics were begun. Within 36 hours, blood cultures demonstrated a gram-positive diplococcus. The antibiotics were adjusted appropriately. Within 48 hours, her condition had stabilized. Repeat imaging studies 1 week later showed improvement. Surgical decompression was considered early on; however, the antimicrobial therapy was sufficient. She gradually improved, having an almost complete recovery. Subsequently, when her speech improved, she recalled having had a dental hygiene appointment a few weeks before the onset of her illness. Previously, she had not been aware of having a mitral valve lesion. She was instructed that in the future, she would need antimicrobial therapy prior to any dental or other medically invasive procedure.
Brain abscess, which may be indolent or fulminant, results from direct extension of a contiguous focus, such as middle ear or sinus infections, congenital heart disease with a right-to-left shunt, or very rarely a pulmonary arteriovenous malformation having similar shunt mechanisms. Hematogenous spread may occur from distant infection sites in the head and neck, heart (infectious endocarditis), lung, or abdomen, or from the direct introduction of bacteria after a penetrating head injury. Brain abscess can occur after surgical procedures, as in the vignette on [CR] . The cardinal symptom of brain abscess is relentless and progressive headache, usually followed by focal neurologic manifestations. Only two-thirds of patients have fever. Papilledema and other signs of increased intracranial pressure may occasionally develop; however, the availability of imaging studies makes it more likely that the abscess will be identified prior to its obtaining significant enough mass to create increased intracranial pressure.
Most cases of brain abscess are polymicrobial. Etiologic agents often include aerobic bacteria such as streptococci, Enterobacteriaceae, and staphylococci. Streptococcus anginosus group normally resides in the oral cavity, appendix, and female genital tract and has a proclivity for abscess formation. Anaerobic microorganisms such as Bacteroides and Prevotella species, are present in up to 40% of cases. Fungi are uncommon but increasingly recognized among immunosuppressed patients.
MRI is most helpful for making the initial diagnosis (see Fig. 44.3 ). The characteristic appearance is a focal cerebral lesion with a hypodense center and a peripheral uniform ring enhancement subsequent to the injection of contrast material. Sometimes, there is a concomitant area of surrounding edema. In these circumstances, if at all possible, lumbar puncture should be avoided to prevent abscess herniation or rupture into the ventricular system.
Therapeutically, the abscess may be directly aspirated. Empiric medical therapy is started with a third- or fourth-generation cephalosporin or penicillin plus metronidazole, depending on the setting. Surgery may not be necessary if follow-up CT demonstrates decreased abscess size. Brain edema associated with acute brain abscess necessitates the use of corticosteroids and mannitol as well as anticonvulsants to prevent seizures.
This is another form of life-threatening neurologic infection. It is typically characterized by a purulent collection within the potential space between the dura mater and arachnoid membrane (see Fig. 44.3 ). An active paranasal sinusitis, particularly originating within the frontal sinuses or mastoid air cells, usually precedes extension of the infection into the subdural space. Occasionally, it is directly introduced through operative or traumatic wound sites.
Streptococci usually comprise 50% of cases; S. aureus, gram-negative bacteria, and anaerobic bacteria such as microaerophilic streptococci, Bacteroides species, and Clostridium perfringens are sometimes identified. Occasionally polymicrobial infections occur.
Localized swelling, erythema, headache, or tenderness of the site overlying the primary infection may occur. As the illness progresses, the headache becomes generalized and severe, with high fever, vomiting, and development of nuchal rigidity. Seizures, hemiparesis, visual field defects, and papilledema sometimes occur.
CSF contains 10 to 1000 WBCs; protein level is increased, and glucose level is usually normal, in contrast to bacterial meningitis; this is a particularly important clue if imaging studies have not been obtained previously. CT or MRI demonstrates a low-absorption extracerebral mass. A thin, moderately dense margin may be visualized with the contrast medium (see Fig. 44.3 ).
Treatment includes a combination of prompt surgical drainage and intensive antimicrobial therapy. The initial antibiotic choice requires intravenous third- or fourth-generation cephalosporin for aerobic bacteria and metronidazole for anaerobic bacteria. Prophylactic use of anticonvulsants and corticosteroids may also be required.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here