Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Posttranslational modification of ion channels and related proteins is an important determinant of transcription, splicing, functional expression, localization, and macromolecular assembly. Ion channels and their modifications are important in virtually all physiologic processes and in the heart and vasculature in development, metabolism, pharmacology, and response to pathologic insults. This chapter focuses on the role of posttranslational modifications (PTMs) of cardiac Na channels and the effect of these processes on myocardial metabolism, remodeling with a particular focus on arrhythmia susceptibility. The reader is also referred to Chapter 1, Chapter 2, Chapter 3, Chapter 4, Chapter 5, Chapter 6, Chapter 7, Chapter 8, Chapter 9, Chapter 10, Chapter 11, Chapter 12, Chapter 13, Chapter 14, Chapter 15, Chapter 16, Chapter 17, Chapter 18 , and 21.
The voltage-dependent Na channel (Na V 1 family) is a multisubunit transmembrane glycoprotein that is highly regulated in health and disease (see Chapter 1, Chapter 2, Chapter 3, Chapter 4, Chapter 5, Chapter 6, Chapter 7, Chapter 8, Chapter 9, Chapter 10, Chapter 11, Chapter 12, Chapter 13, Chapter 14, Chapter 15, Chapter 16, Chapter 17, Chapter 18 , and 21). Voltage-dependent ion channels, such as the Na channel, facilitate the apparently incongruous rapid (>10 6 ions per second) yet highly selective flux of ions across the lipid bilayer down their respective electrochemical gradients. All ion channels exhibit two essential properties: gating and selective permeation. Gating is the opening and closing of the channel pore in response to a specific biologic stimulus, such as transmembrane voltage. Ion selectivity is in part determined by molecular sieving and, perhaps more importantly, by different energetic strategies for transiently binding the permeant ion in the pore. A number of signaling cascades active in normal physiologic and pathologic conditions associated with cardiac arrhythmias exquisitely regulate both processes.
Voltage-gated ion channel pore-forming (α)-subunits share common structural themes: a modular architecture, an ion-selective pore with highly conserved pore-lining residues for channels with similar selectivity, a common gating strategy using a charged membrane voltage sensor, and auxiliary subunits that regulate trafficking and function. Cryogenic electron microscopy (Cryo-EM) high-resolution structures of a range of mammalian Na V isoforms in varying conformations have recently been determined. The structures illuminate the atomic basis for essential channel functions and basis for inherited channelopathies. Na V 1.5 channels (encoded by SCN5A ) are among the most abundant ion channels in working cardiac muscle (∼10 6 copies per myocyte) and carry the major inward current (I Na ) for excitation. Expression of the Na V 1.5 channel pore-forming α-subunit is sufficient for current generation and determines the ion selectivity and conductance properties. The Na V 1 channel family transmembrane domain sequences are highly conserved from the eel electroplax to humans , ; however, there is significant divergence in key regulatory channel domains such as the cytoplasmic loops and carboxyl terminus in different Na V 1 isoforms. There are long and short variants of the linker between domains I and II; the long version of this linker contains multiple putative phosphorylation sites for several physiologically relevant kinases ( Fig. 39.1 ). Moreover, alternative splice variants of Na V 1.5 are associated with differences in functional expression in heritable and acquired cardiac disease.
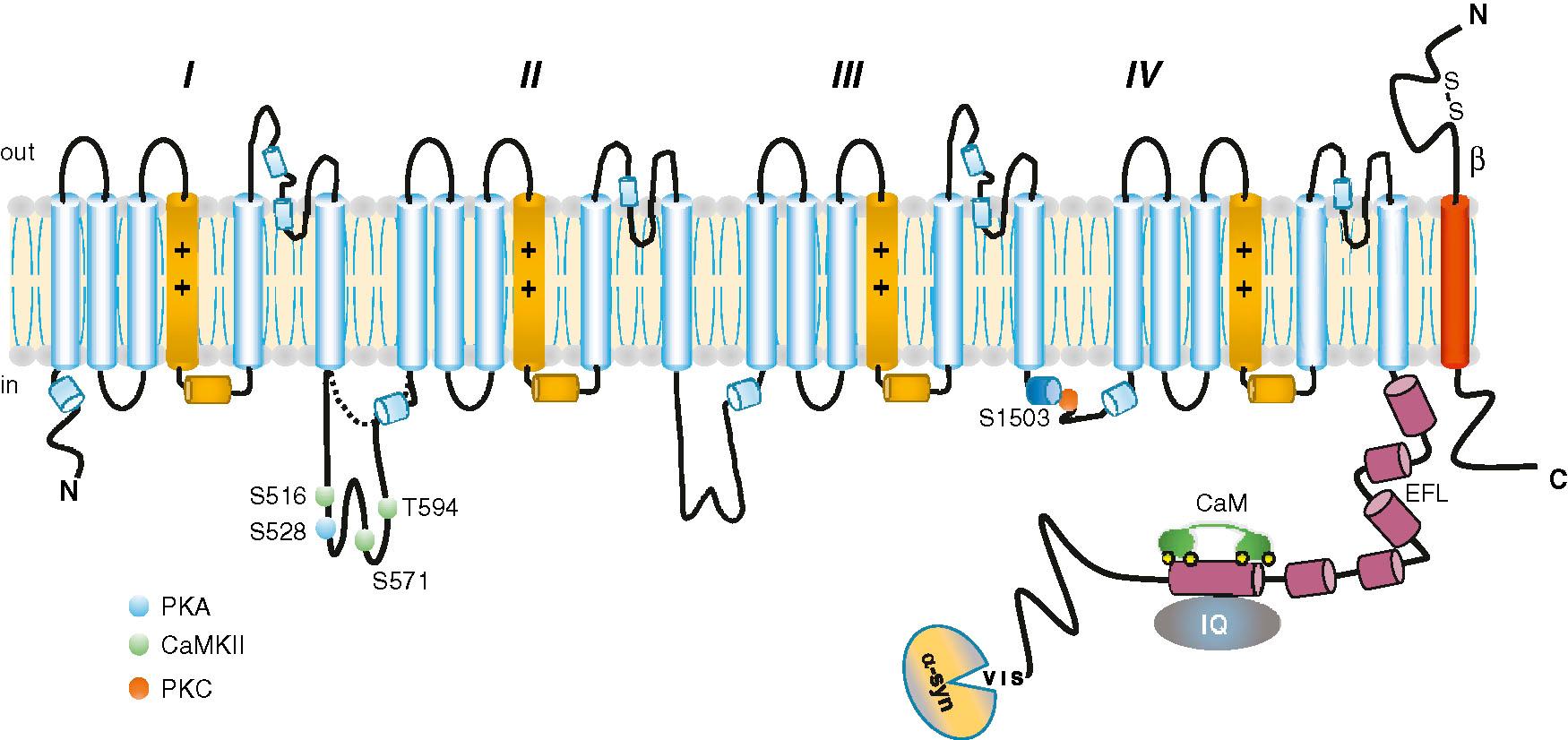
Four genes encode five different β-subunit proteins (Na V β1, 1B, 2, 3, and 4 and SCN1B-SCN4B ), each with a single membrane-spanning domain (type 1 topology) and a large extracellular V-like immunoglobulin fold often found in cell adhesion molecules (CAMs). The only exception is the Na V β1B splice variant that is secreted and lacks the small carboxyl-terminal cytoplasmic domains characteristic of the other isoforms. β-Subunits are present in a wide range of tissues, and a general function is to promote the expression, macromolecular assembly, and specific subcellular localization of Na V β-subunits. The structure of Na V β-subunits is similar to that of classes of CAMs, and β-subunits promote adhesion and can do so even in the absence of Na V 1 α-subunits (thus could influence conduction). The distinct kinetic, voltage-dependent and pharmacologic properties of Na V channels are dependent on the specific α-β-subunit combination and cell expression systems.
The role of β-subunits in function and regulation of Na V 1.5 channels in the heart is uncertain. However, variants in a number of β-subunits have been implicated in the production of cardiac arrhythmias, sudden infant death syndrome (SIDS), and sudden unexplained death in epilepsy (SUDEP). Mutations in SCN1B, SCN2B , and SCN3B have been linked to Brugada syndrome (BrS), SCN3B in SIDS and idiopathic ventricular fibrillation, and SCN4B in long QT syndrome (LQTS) and SIDS. Variants in SCN1B, SCN2B, SCN3B, and SCN4B have been associated with atrial fibrillation. The presence of an arrhythmic phenotype in scn1b and scn3b null mice is consistent with a functional effect of these subunits in the heart, although the interpretation in scn1b−/− mice is complicated by the presence of a complex neurologic and developmental phenotype.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here