Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
On a standard “adoption curve,” where interest in a new technology initially runs high, is followed by disappointment, then by maturation of the technology and finally a plateau of acceptance, cytology automation is firmly entrenched on the acceptance plateau. Over past editions of Comprehensive Cytopathology , that evolution has been well documented, initially with discussions of the research driving computerization and automated morphometry, followed by development of the requirements for clinical devices in the first and second editions, respectively. The third edition detailed newly developed clinical devices, their regulatory approvals and the first data related to their operating characteristics. Since that time, the “shine” of automation has been “tarnished and buffed” reflecting the entrance to a mature stage. Adoption has been widespread, but not without controversy. This chapter makes an attempt to paraphrase for the reader the sentinel points of this long process. It discusses the theoretical basis for, and principles behind, automation and its instrumentation. The controversies, conflicting data, and finally some observations from long-time users are presented. A glimpse at the future of this field closes the discussion.
The field of cytology automation has gone from a half-century of developmental work to clinical fruition in the form of primary screening instruments widely deployed in clinical cytology laboratories. Along the way, enormous amounts of time and energy have been spent on the development of specimen preparation methods and instrumentation, optical systems, computers, and algorithmic and other types of information-processing systems. This follows on from the original work necessary to the process, that being the morphometric measurements of cells, both normal and abnormal, that have provided the inputs necessary for these processes and devices. This technology has been commercialized at present only in the area of cervical cancer screening. This fact is a reflection of the substantial morphometric analysis done by the pioneers in the field, which could be then translated into computer-driven analyses. Morphometric data from other anatomic systems are slowly being developed at present and this bodes well for future efforts in other screening and diagnostic tasks. The future will undoubtedly see newly emerging technologies that leverage the study of these systems. In addition the rise of molecular methods may augment new imaging technology that could further enhance its accuracy and efficiency.
As one of the last high-volume, manually performed procedures in the laboratory, cervical cytology has always been a target for automation. About a half-century of time and energy has been devoted to this task, beginning with developmental work necessary to define what constituted morphologically, and more importantly morphometrically, the important qualitative and quantitative features of cells – benign, reactive, and neoplastic – obtained in a cervical cytology specimen.
In the days of Caspersson's quantitative analysis of nucleic acid and proteins by microspectrophotometry, it was estimated that gathering information about one cell required a week's work.
Detailed information on the relative or absolute number of measuring points per cell became available from the sophisticated machines of Caspersson, Hyden and Larsson, Deeley, Montgomery, Thornburg, and Tolles. However, despite this important and pioneering work, the potential for this detailed information remained largely unrecognized.
It was the widespread acceptance of the diagnostic capabilities of cytology that eventually generated demands for the automation of this highly time-consuming and tedious manual procedure. Both cytochemical and cytomorphologic criteria were used in the cytoanalyzer project of the US National Cancer Institute in an early attempt to develop an automated process. It was discontinued because of its inability to be able to resolve the complexity of routinely prepared conventional cytology specimens due to cell overlap and obscuring factors. In addition, the robustness of the software was inadequate for the task. However, this project allowed investigators to appreciate and develop approaches to the problems encountered in the early investigative process. Parts of the cytoanalyzer were used by Mendelsohn's group to build CYDAC (Cytophotometric Data Conversion), a system designed toward computer-assisted description and identification of intracellular cell patterns. In the 1960s, a number of cytochemical scanning methods and instruments of the integrating type were described. The most sophisticated optical and mechanical design entered into the Universal Recording Microspectrophotometer (UMSP-1) of Carl Zeiss, which incorporated an ingenious high-precision mechanical stage and achromat lenses corrected for the ultraviolet and visible range. Limitations precluded clinical applicability however, particularly the slow scanning mechanism.
In 1968, Wied and collaborators introduced the taxonomic intracellular analytic system (TI-CAS), a device for cell identification based on computer evaluation of the intracellular patterns of image scan data. This effort stimulated the interest of others in Europe and Japan, leading to later systems such as the VCSA, Cerviscan, CYBEST, TUDAB, SAMBA, Diascanner, FAZY-TAN, LEYTAS, Magiscan, McGill System, and TULIPS.
Studies on the composition of cellular samples showed that the majority of abnormal cells in conventional Papanicolaou smears were in clusters. This understanding led to the conclusion that better cell presentation was needed to optimize the performance of scanning devices. Throughout the 1970s, the emphasis moved to development of techniques of cell sampling, preservation, disaggregation, and monolayering of cell preparations. Such specimens allowed for better visualization of cells for individual cell analysis, a process better suited to the computer hardware and software available. These processes have culminated in the present with successful introductions of automated cytology products into the marketplace. The 1980s saw the formation of companies such as Neopath, Cytyc, Neuromedical Systems, and Roche Image Analysis Systems, which brought forward and tested the products that we now routinely utilize in daily practice. Several organizations have sponsored and sustained those working in the field of automation for years. These include the Engineering Foundation of America, the Concerted Action on Automated and Analytical Cytometry of the European Economic Community, and Professor George L. Wied of the University of Chicago, who organized academic meetings and specific conferences on artificial intelligence and cytology automation. With the entrance of cytology automation into the “mainstream” of laboratory practice, the clinical cytology organizations have become far more active in the field. Regular symposia and educational sessions related to automation, including its practice and its controversies, are now routinely held at meetings of the American Society of Cytopathology, the Congresses of the European and International Academies, as well as at other local and national society venues.
Cervical cytology has always been a high-volume test, one of the last such tests to be performed in a completely manual fashion. Because manual interpretation of cells for the identification of abnormalities has always followed a morphologic criteria-based approach, it was only reasonable to hypothesize that such a process would be amenable to an “image analysis”-based computerization approach in which the computer software would be able to reliably “quantitate” widely recognized morphometric parameters such as nuclear size, nucleus to cytoplasmic ratios, chromatin texture, and distribution, and then sum (or otherwise analyze) such identified cellular changes into an interpretation, free of any human intervention. At first, such a hypothesis might seem to be very reasonable; however, this task was found to be far more complex due to a variety of factors. The conventional cervical cytology slide is very complicated. It may contain up to 500 000 cells of various types that may be displayed in an innumerable array of patterns and variations, with complex overlapping, obscuration by blood and inflammation, and a wide variety of presentations in which benign or reactive cells may mimic malignancy and malignant cells can mimic the benign. As such, early attempts at automation, geared toward machine final interpretation, were generally unsuccessful. Philosophic changes in approach were necessary if automation was to be successfully introduced as a mainstream process. Therefore, the concept of the device as an aid to human interpretation came to be regarded as a reasonable approach in distinction to methods seeking full device-only interpretation. This philosophic overlay is inherent in the three current approaches being utilized in automated screening: (1) the triage of slides by the device to those requiring manual review and those not requiring manual review (the Becton Dickinson FocalPoint Primary Screening System, FPPS); (2) the location-guided screening approach in which the device identifies cells or areas on the slide with high probability of abnormality for presentation to humans for final review and interpretation (Hologic ThinPrep Imaging System, Becton Dickinson FocalPoint GS); and (3) a combination of the first two methods (Becton Dickinson FocalPoint LGS).
Over this time, parallel efforts toward improving the cervical specimen were taking place. Clinical trials of liquid-based technology utilized new sampling devices such as endocervical brushes and “brooms” that provided a more cellular sample, particularly from the important transformation zone. Liquid-based cytology (LBC) specimens provided the uniformity and improved presentation which was lacking in conventionally prepared specimens. Cells in LBC specimens are generally better distributed on the slide, with improved cell-to-cell separation or “segmentation.” Such specimens are also less prone to cellular obscuration by blood and inflammation. Hence liquid-based specimens create a more optimized “platform” for automated screening, and the most modern clinical devices take advantage of this fact.
Another important piece of the automation puzzle is the role of the regulatory agencies, most notably the US Food and Drug Administration (FDA), which has to pass on the safety and effectiveness of such devices prior to commercial release. It was clear that the initial utility of automated screening devices destined for commercial release would be tested and first released in a quality control (QC) use mode. Automated screeners would be utilized to “check” previously manually screened slides looking for false-negative cases. During such activities, the overall performance of the devices could be tested. In addition, the formulation of the types of trials necessary to adequately investigate and document these performance or operating characteristics without compromising patient care could also be developed. Professional societies published documents proposing performance measures, and the FDA and its advisory panels judged the data presented by the manufacturers.
Following acceptable QC performance, the introduction and vetting of primary screening devices could be initiated.
There are two ultimate goals of automation: (1) to improve the accuracy of the interpretation in terms of both false-negative and false-positive performance, and (2) to improve productivity of the workforce. A false-negative specimen is defined as one in which an interpretation is rendered of no disease being present when indeed such is not the case. False-negative specimens are the most concerning, as they can lead to delayed detection and treatment with its inherent morbidity and mortality. However, false-positive results, defined as a positive result when no disease is present, are also important to avoid, as they can lead to overtreatment, which can be associated with morbidities such as hampered fertility and patient anxiety. In addition, false-positive results lead to inefficiencies in the system associated with greater than necessary time, workload, and expense. Cervical cytology automation – the combination of LBC and computerized automated screening – can theoretically enhance the accuracy of the Papanicolaou test in terms of both false-negative and false-positive parameters.
LBC has the potential to enhance accuracy in the following ways:
Complete (or nearly so) capture of all cellular material sampled from the patient. Compared with the making of a conventional slide, in which, on average, only about 35% of sampled material is transferred to the glass slide for review, liquid-based technology captures most of the cells sampled. This diminishes the likelihood of false-negative tests due to “transfer” error in which positive cells are left on the sampling device following the making of a conventional smear ( Fig. 35-1 ).
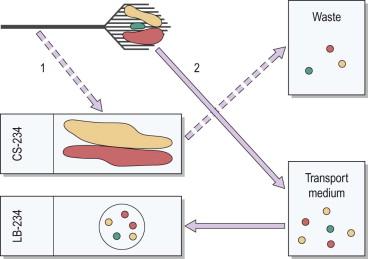
Specimen randomization. Liquid-based slides contain a subsample of cells that have been randomized during the preparation process. Randomization allows for a more representative cell pattern being present on the slide, containing all the types of cells sampled from the patient. This compares with the non-randomized subsample routinely transferred in the making of a conventional smear. Randomization is important in reducing false-negative tests, particularly in the circumstance in which only a few abnormal cells are sampled or in circumstances in which abnormal cells may be geographically isolated to more poorly transferred areas of the sampling device ( Fig. 35-1 ).
Improved preservation of the cellular sample. Cells sampled and then immediately immersed in liquid fixative transport media show, on average, better fixation, and thus better visual preservation, than will smeared cellular material on a conventional slide. Artifacts such as air-drying and other cellular degeneration changes are virtually eliminated in liquid-collected specimens. Better preservation allows for more accurate assessments of nuclear size, nucleus to cytoplasmic ratio, and chromatin structure. This improved cellular appearance increases accuracy (both false negative and positive) via improved ability to correctly identify, recognize, and classify cellular changes.
Improved individual cell visualization. Because overall cellularity on the slide surface is controlled in the liquid-based processes, and because of the ability to equally distribute cells in a uniform fashion across the slide surface, liquid-based slides allow for improved visualization of cells due to lack of overlapping or 3-dimensional aggregation. Again, this increases accuracy of cell classification (both false negative and positive) via improved ability to correctly identify, recognize, and classify morphologic changes.
Decrease in obscuring factors. The liquid-based processes generally improve cellular visualization via decreases in physiologic obscuring factors such as blood or inflammation. The cells in conventional smears may be obscured, leading to unsatisfactory or limited specimens when such factors are present. Liquid-based samples are largely immune (with caveats discussed below) to such encumbrances, leading to accuracy improvements directly tied to “visual accessibility” of the cells.
Consistent location and limited size viewing area. Because liquid-based methods place cellular material in a limited area of the glass slide, always in the same place, screening (following training and initial experience) is more efficient and is associated with less viewer fatigue, leading to fewer “habituation,” or inattention, lapses. Such slides do not require “hunting down” cellular material at any location on the slide, including outside the coverslip area and adjacent to (or even under) the slide label.
Complete cellular capture
Specimen randomization
Improved cellular preservation
Improved individual cell visualization (improved segmentation)
Decrease in obscuring factors (blood and inflammation)
Consistent cell location and viewing size area.
All these features allow for standardization of the Papanicolaou slide with uniformity of specimen and cell appearance from case to case. Overall, this uniformity leads to population-wide specimen improvement. The process makes all preparations “optimal,” eliminating the “highs and lows” inherent in the highly variable conventional preparation method.
Computerized automated screening has the potential to enhance accuracy in the following ways:
The devices scan the entire cellular content of the slide without attention lapses or missed fields of view (FOVs). Computers have the advantage of being “tireless” and will scan all that they are programmed to review. Unfortunately, even the best of human reviewers will be inattentive for or miss some portion of a slide.
The devices apply a standard set of analyses in a uniform fashion to all cells or FOVs on the slide. Theoretically, computerized cell or FOV measurements should lack the variability, or non-objectivity, that is inherent in human analyses and which is subject to a wide variety of biases. Although not widely appreciated, human ability to “measure” an object, such as a nuclear size, is affected by the cell's local environment, and biases such as preconceptions about the case diagnosis (e.g., if an observer already “thinks” that a case represents a dysplasia, perception of size is likely to fall within the bounds of known “dysplasia criteria” regardless of the actual measured value ).
In the automated approach, in which slides are triaged into cases requiring review and those not requiring review, based on overall probability of abnormality on the slide, categorization as a low-probability “no review” slide improves the specificity of abnormal detection. Put another way, if humans were allowed to screen this population of slides having very low probability of abnormality, a “finding” would more likely be a false, rather than a true, positive finding.
In the location-guided screening approach, in which a limited number of “highest probability” areas of the slide are viewed by the human screener, the increased “signal to noise” ratio (meaning more abnormal cells are presented per normal cell) improves screener vigilance and hence sensitivity to the abnormal cells present. This concept has been clearly shown in the literature about other types of screening procedures, such as visual screening for prohibited objects at airport checkpoints. If the prohibited objects are more prevalent in the total population of objects viewed, the sensitivity of detection increases. This phenomenon has been recently demonstrated in an experiment using cervical cytology images in blocks of high and low abnormality prevalence. Detection sensitivity was proportional to prevalence. A decreased false-negative rate might be expected with the use of this focused screening approach. In addition, in a similar fashion to the previous concept, when screeners do not view the “low-probability” areas of the slide, the likelihood of false-positive results should be diminished. This latter point has also been demonstrated with human papillomavirus (HPV) testing showing a lower positive rate in specimens interpreted as negative after being reviewed in a guided screening device, when compared to manually screened negative specimens.
The device scans the entire slide without attention lapses or missed FOVs
The device applies a standard set of analyses in a uniform fashion to all cells and FOVs
In slide-ranking devices, slides triaged to no manual review increase specificity by decreasing false-positive tests
In slide-ranking devices, increased screener vigilance in high-probability cases increases sensitivity by decreasing false-negative cases.
The combination of LBC preparation techniques with computerized automated screening has the potential to have a synergistic effect on accuracy. Better visualization of cells inherent in the liquid-based technique should also allow for more accurate and efficient analyses by computer devices in a similar fashion to human observers. This hypothesis will be put to the test when data from the clinical trials and independent studies using these systems are presented below.
Potential productivity improvements with automated cytology methods and devices are as follows:
LBC specimens can be screened by humans and by automated devices at a faster speed than conventional cytology specimens. Studies have shown that productivity gains of about 20% for manual screening can be expected once personnel are trained and have gained experience. Smaller screening areas and uniformity of cellular presentation is the likely explanation.
Devices that triage slides into categories requiring human review and those that do not benefit from fewer slides entering the human review process. At present, this feature can exclude up to 25% of slides based on FDA regulatory operating parameters, but it is as many as 50% in devices approved for use outside the USA. Data from the UK MAVARIC trial showed productivity gains with this feature.
Location-guided screening devices requiring only limited reviews of machine-selected fields substantially reduce the average time spent screening a slide. At present, in the currently approved Hologic device, this productivity gain is reported to range from 27% to 100%. Data from the BD FocalPoint device shows more modest improvements of 15–20%. In the UK MAVARIC study the time taken to read both ThinPrep and SurePath slides using their respective guided-screening automated devices was three- to four-fold shorter than for manual screening.
Suffice it to say that automation provides theoretic and, in many cases, achieved improvements in accuracy, both sensitivity and specificity, as well as productivity gains. Such achievements are important in an era of declining resources, in both reimbursement and technologist labor, and the increased threat of costly litigation for inaccurate results. With the prevalence of high-grade lesions predicted to decline in the population due to HPV vaccination, the potential “prevalence-boosting” capabilities of both “no review” and guided screening devices will become more important in order to maintain detection sensitivity.
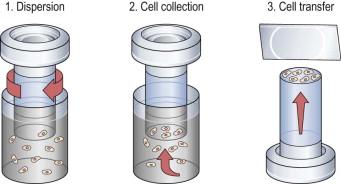
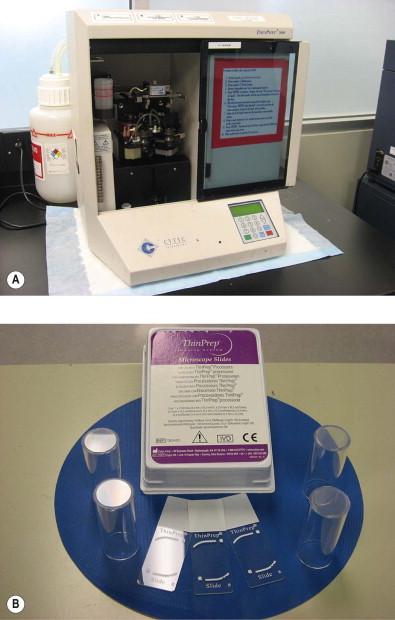
The ThinPrep Pap Test (Hologic, Bedford, MA) was the first FDA-approved cervical cytology method, receiving clearance in 1996. Collection of a ThinPrep specimen requires the use of either a broom-type device or the combination of an endocervical brush and spatula. Cells are washed off the sampling device(s) into a transport vial (PreservCyt, Hologic) containing a proprietary preservative mixture that is methanol based. The vial is received in the laboratory, where the cell suspension undergoes homogenization, and therefore randomization, by a vortexing cell dispersion method. Cells are transferred by suction to a filter ( Fig. 35-2 ). Cellularity of the specimen is controlled by monitoring of a pressure gradient across the filter. When this pressure gradient reaches a defined level, the suction stops and the cells are transferred to a glass slide by blotting and the application of gentle pressure across the filter in the opposite direction. The final result is a circle of deposited cells measuring 20 mm in diameter ( Fig. 35-3 ). There are two models of the ThinPrep processing device, both of which operate in a fundamentally similar fashion as described above; the T2000 model ( Fig. 35-4 ) is a single specimen load device, and the T3000 model ( Fig. 35-5 ) is an automated multiload device designed for high-volume laboratories.

Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here