Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Vascular surgeons commonly treat patients with the manifestations of atherosclerosis. An improved understanding of atherosclerosis and advances in its treatment now provide scientifically based prevention and management strategies. Precise lesion classification, accurate imaging, a better understanding of atherogenesis, and increasingly effective medical treatment before and after vascular interventions all promise to result in improved long-term results. The pivotal role of lipids in the pathogenesis of atherosclerosis and the impact of lipid reduction on atherosclerotic plaque has long been known, as well as more recent information on the crucial roles of inflammatory and immune responses that affect arterial plaques. Novel risk factors, such as elevated blood levels of biomarkers, including inflammatory cytokines, metalloproteinases, and smooth muscle growth factors, including glucose and insulin for late progression, all contribute to our understanding of atherosclerosis and its progression. Advanced imaging can now also detect unstable plaques prone to rupture, thrombosis, and downstream embolization. Prospective randomized trials using drugs, micronutrients, and other interventions continue to provide therapeutic guidelines. Coronary thrombosis or stroke, the main causes of death in patients with peripheral arterial disease, have improved management strategies to increase overall survival and enhance long-term results of atherosclerosis management in other vascular beds.
Vascular surgeons must be familiar with the location and natural history of individual lesions and, in considering various interventions, distinguish primary prevention from secondary treatment. When active intervention is required, vascular surgeons must use strategies geared toward the specific pathology of lesions in particular arterial sites. For example, a stenotic lesion composed of smooth muscle and well-organized collagen, although capable of causing distal ischemia, is a much safer lesion than a plaque containing an unstable core of atheromatous debris beneath a tenuous cap. The smooth stenosis of an adductor hiatus plaque in the femoral artery, causing stable claudication, is clearly not as threatening as unstable symptomatic carotid or coronary plaques characterized by soft cores beneath friable caps. These lesions have differing vulnerabilities based on differing composition and morphology.
We now recognize that operative or endovascular treatment of segmental lesions does not prevent the progression of systemic atherosclerosis elsewhere and ensuring long-term results requires both medical treatment and lifestyle interventions.
Variations in patterns and rates of progression of atherosclerosis also have significant implications for the timing and choice of treatment. Considering the atherosclerotic process as a single disease leads to oversimplification; with considerable lesion diversity and clinical presentation, atherosclerosis also can be viewed as a polypathogenic process comprising a group of closely related vascular disorders. Multiple risk factors promoting atherosclerosis and its complications include dyslipidemia, smoking, diabetes, hypertension, and proinflammatory factors. The multiplicity of disease-promoting factors makes a single-disease–single-etiology view difficult to reconcile. This chapter discusses atherosclerosis as a single entity, while recognizing its heterogeneous pathologic characteristics and differing clinical presentations, which will be discussed in subsequent chapters. Despite the complexity and heterogeneity of the atherosclerotic process, new treatment strategies have resulted in increasingly successful treatment outcomes. More effective medical therapy and advances in endovascular and surgical approaches, based on a better understanding of the pathology and pathogenesis of atherosclerotic disease, have been fruitful in improving the survival and quality of life of patients with atherosclerotic disease in multiple vascular beds.
The term atheroma derives from the Greek athere , meaning “porridge” or “gruel” and sclerosis, meaning “induration” or “hardening.” A gruel-like color and consistency and induration or hardening exists to various degrees in different plaques, different disease stages, and different individuals. In 1755, von Haller first applied the term atheroma to a common type of plaque that, on sectioning, exuded a yellow, soft content from its core. Fig. 7.1 illustrates a typical fibrous plaque containing a central atheromatous core with a fibrous or fibromuscular cap, macrophage accumulation, and round cell adventitial infiltration. A previous generic definition of atherosclerotic plaque as “a variable combination of changes in the intima of arteries consisting of focal accumulation of lipids, complex carbohydrates, blood and blood products, fibrous tissue and calcium deposits” failed to encompass the full spectrum of atherosclerotic lesions. Advanced plaques invade the media, and at certain stages produce bulging or even enlarged arteries. Round cell infiltration, medial changes, and neovascularization characterize advanced atherosclerotic lesions. The atherosclerotic process ultimately involves the entire arterial wall. The process is complex and variable and, although not all atherosclerotic lesions evolve in the same way, certain progression patterns are more common than others.
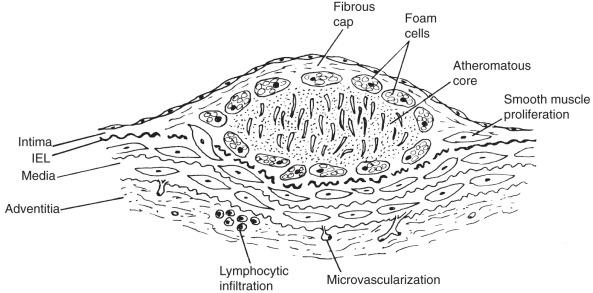
The development of the lipid atherosclerotic core and its relationship to an overlying cap relate directly to plaque complications. The lipid “core” develops early in atherosclerosis and accumulates in the deep aspects of early lesions, before actual fibrous plaque formation begins. Another important principle is recognition of the role of inflammation and immune reactions in the early and late stages of atherogenesis. The inflammatory cascade includes appearance of proinflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor (TNF)-α, and antiinflammatory cytokines such as IL-10 within arterial tissue and arising from the bloodstream. Lipid accumulation appears to attract inflammatory cells that produce cytokines locally, whereas cytokines or biomarkers derived from a variety of tissues appear systemically in atherosclerotic subjects. For example, in atherosclerotic patients with claudication, plasma levels of inflammatory cytokines TNF-α and IL-6 are elevated, whereas antiinflammatory IL-10 levels are reduced. Elevated levels of cytokines such as TNF-α and its receptors also affect the arterial wall. The atherosclerotic plaque contains leukocytes, of which approximately 80% are monocytes or monocyte-derived macrophages. Lymphocytes, predominantly memory T cells, constitute 5% to 20% of this cell population. Inflammation, size, and composition of the lipid core determine plaque instability or “vulnerability,” which may result in sudden expansion, rupture, release of distal emboli, and vascular occlusion.
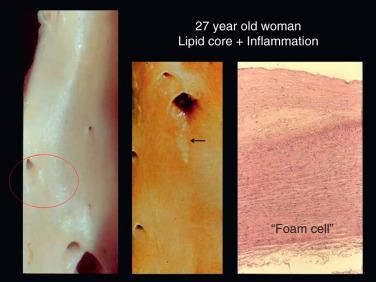
The first stages of atherosclerosis, fatty streaks (minimally raised yellow lesions), develop in vulnerable segments of the arterial tree. These lesions contain lipids deposited intracellularly in macrophages and smooth muscle cells. Stary and colleagues defined initial fatty streaks and intermediate lesions of atherosclerosis as follows: type I lesions in children are early microscopic lesions, consisting of an increase in intimal macrophages and the appearance of foam cells. Type II fatty streak lesions are grossly visible ( Fig. 7.2 ); in contrast to type I lesions, type II lesions stain with Sudan III or IV. Foam cells and lipid droplets appear in intimal smooth muscle cells and heterogeneous droplets of extracellular lipids characterize type II fatty streaks. Type III lesions are intermediate lesions, considered to be the bridge between the fatty streak ( Fig. 7.3 ) and the prototypical type IV atheromatous fibrous plaque (see Fig. 7.1 ). Type III lesions occur in plaque-prone locations in the arterial tree at sites exposed to forces (particularly low-shear stress), promoting increased low-density lipoprotein (LDL) influx.
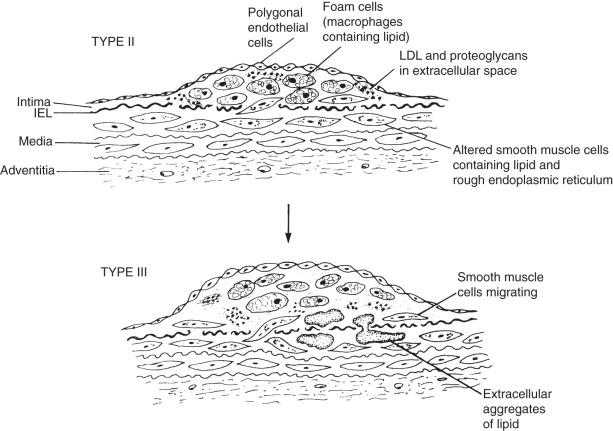
The fatty streak type II lipids are chemically similar to those of plasma. Plasma lipids may enter the arterial wall in several ways. As described in a review of pathogenesis, LDL accumulation can occur because of (1) alterations in the permeability of the intima; (2) increases in the intimal interstitial space; (3) poor metabolism of LDL by vascular cells; (4) impeded transport of LDL from the intima to the media; (5) increased plasma LDL concentrations; or (6) specific binding of LDL to connective tissue components, particularly proteoglycans in the arterial intima. Experimental studies show that LDL cholesterol accumulates in the intima even before lesions develop and in the presence of intact endothelium. These observations support the classic descriptions of lesion formation by Aschoff in the early 20th century and Virchow in the mid-19th century.
A second event in early atherogenesis, as shown in animal experiments, is binding of monocytes to the endothelial lining, with their subsequent diapedesis into the subintimal layer, to become tissue macrophages. Fatty streaks are populated mainly by monocyte-derived macrophages. These lipid-engorged scavenger cells mainly become the foam cells characterizing fatty streaks and more advanced lesions. LDL, altered by oxidation or acetylation, is taken up by the macrophages to form foam cells. Oxidized LDL itself is a powerful chemoattractant for monocytes. Another component of this theory suggests that the endothelium modifies LDL to promote foam cell formation. In either case, oxidative reactions are seen as enhancing atheroma development.
The initial interactions of plasma LDL with the arterial wall and macrophages form the basis for the earliest lesion formation. LDL traverses the endothelium mostly through receptor-independent transport, possibly also through cell breaks. Endothelial cells, smooth muscle cells, and macrophages are all capable of promoting oxidation of LDL. The oxidized LDL, in turn, further attracts monocytes into the intima to promote their transformation into macrophages. Macrophages produce cytokines, thus initiating the inflammatory cascade. Oxidized LDL also induces genes and their products, which are not ordinarily expressed in normal vascular tissue. A notable example is tissue factor, the cellular initiator of the coagulation cascade, abundantly expressed by atheroma monocytes and foam cells. Expression of tissue factor requires the presence of bacterial lipopolysaccharide, suggesting that hypercoagulability in atherosclerosis is enhanced by infectious factors.
Large numbers of macrophages and T lymphocytes in the plaques suggest cellular immune responses; oxidized lipoproteins, heat shock proteins, and microorganisms are all possible antigens. A study analyzing endarterectomy specimens by immunohistochemistry and reverse transcription polymerase chain reaction showed proinflammatory T cell cytokines, IL-2, and interferon-7 in a large proportion of plaques, indicating that a helper T cell 1–type cellular immune response likely occurs in the atherosclerotic plaque.
Animal studies reveal that endothelial cells tend to be oriented parallel to the direction of flow; these cells show increased stigmata or stomata, increased proliferation, and a decrease in microfilament bundles. In humans and animals, endothelial cells become polyhedral or rounded; in humans, there is also increased formation of multinucleated cells and cilia. Animal studies reveal increased proliferation and cell death, with retraction and exposure of subendothelial foam cells. The endothelium becomes more permeable to macromolecules in experimental models; in humans, it exhibits increased tissue factor expression and mural thrombus formation. Leukocyte adherence increases with the expression of a monocyte adhesion molecule (vascular cell adhesion molecule 1 [VCAM-1]). Endothelium-derived relaxing factor and prostacyclin release are decreased and vasoconstriction is enhanced.
The smooth muscle shows increased proliferation, with increased rough endoplasmic reticulum, phenotypic changes, and increased production of altered intracellular and extracellular matrices. In humans, these matrices include increased expression of type I and type III collagen, dermatan sulfate, proteoglycan, and stromelysin. Smooth muscle cells also produce cytokines, including macrophage colony-stimulating factor, TNF, and monocyte chemoattractant protein-1. Myocytes accumulate native and modified lipoproteins by both native receptor pathways and nonspecific phagocytosis. These cells also express increased lipoprotein lipase activity; experimentally, they display a scavenger receptor similar to that of foam cells.
Macrophages proliferate and express monocyte chemoattractant protein-1, macrophage colony-stimulating factor, TNF, IL-1 and other interleukins, and platelet-derived growth factor (PDGF), along with cluster of differentiation (CD) immune antigens and, as previously described, tissue factor. Plaque macrophages contain increased free and esterified cholesterol, increased acetyl coenzyme A, cholesterol acyltransferase, and acid cholesterol ester hydrolase. Neutral cholesterol ester hydrolase is decreased. These altered cells also express the scavenger receptor 15-lipoxygenase and exhibit increased lipoprotein oxidation products in humans and in animal models. These extensive changes indicate the complexity of the morphologic, functional, biochemical, and genetic expressions of the arterial wall in early atherosclerosis. The reader is referred to an original report for comprehensive details, with references for the cellular alterations given.
Intimal gelatinous lesions may sometimes be considered as precursors of atheromas. Haust described these lesions in 1971, first noted in 1856 by Virchow as potential progenitors of advanced atherosclerosis. Smith later described their identification and composition. Gelatinous plaques are translucent and neutral in color, with central grayish opaque areas. Most plaques are characterized by finely dispersed, perifibrous lipid along with collagen strands around the lesions. Grossly, gelatinous lesions feel soft. With gentle lateral pressure, these plaques “wobble.” The gelatinous material separates easily from the underlying arterial wall without entering a conventional endarterectomy plane. Gelatinous plaques appear in the aorta as extensive areas of flat, translucent thickenings, particularly in its lower abdominal segment. These lesions have a low lipid and high fluid content. In some plaques, numerous smooth muscle cells are present and the lesions contain substantial amounts of cross-linked fibrin.
Fig. 7.1 typifies a common prototypical atherosclerotic lesion: fibrous or type IV plaque. These lesions are composed of large numbers of smooth muscle cells and connective tissue, which form a fibrous cap over an inner yellow (atheromatous) core. This soft core contains cholesterol esters, mainly cholesteryl oleate, likely derived from disrupted foam cells. A second type of particle contains both free cholesterol and cholesterol linoleates. The early core is associated with vesicular lipids that are rich in free cholesterol. These particles likely are derived directly from LDL, possibly by modification of LDL by specific lipolytic enzymes capable of hydrolyzing LDL cholesterol esters. Lipoprotein aggregation and fusion are thought to be the chief pathway of cholesterol ester accumulation. Fibrous plaques contain large numbers of smooth muscle cells, connective tissue cells, and macrophages. Over 2 decades ago, the importance of the composition and integrity of the atheromatous cap were emphasized, as this structure stabilizes the atheroma to prevent intraluminal rupture of its soft core.
Fibrous plaques appear later than fatty streaks and often in similar locations. Fibrous plaques generally evolve from fatty streaks or from areas of fatty streak involvement. Gelatinous plaques, injured arterial areas, or thrombi less commonly may lead to fibrous plaque formation. Mural thrombi sometimes are converted into atheromas, as demonstrated by experimental intraarterial catheter implantation.
Fibrous plaques protrude into the arterial lumen in fixed cut sections; however, when arteries are fixed at arterial pressure, they produce an external bulge. For example, coronary plaques in vivo must occupy at least 40% of the arterial wall before angiographic detection is possible. Within certain limits, atheromatous luminal intrusion is compensated by arterial enlargement. Compensatory remodeling of coronary arteries in subhuman primates and humans has been stressed as a protective response. However, with lesion progression, ulceration, rupture, or overlying thrombosis, the arterial lumen may become suddenly compromised, a sequence characterizing coronary atheromas. A unique adaptive response involving dilation, with atheromatous involvement of the entire arterial wall and participation of inflammatory cells, immunologically active T lymphocytes, and elastolysis may predispose to aneurysm formation.
During the early stages of evolution from fatty streak to fibrous plaque, cholesterol esters appear in ordered arrays of intracellular lipid crystals. In intermediate type III and fibrous plaques, the lipids assume isotropic forms and occur extracellularly. Cholesterol esters and oxysterols are highly irritating, causing severe inflammatory reactions in the connective tissue ; they probably behave similarly within the arterial wall to promote inflammation, fibrosis, and lymphocytic infiltration. Advancing neovascularization from the adventitia characterizes intermediate fibrofatty and fibrous plaque lesions. Atherosclerotic lesions contain immunoglobulin (Ig) G in large quantities, as well as other immunoglobulins and complement components. The IgG recognizes epitopes characteristic of oxidized LDL, indicating that immunologic processes characterize more advanced atherosclerotic plaques. This process is associated with systemic effects; for example, patients with carotid atherosclerosis have higher antibody ratios of oxidized LDL and IgM than do comparable non-atherosclerotic controls.
Experiments in complement-deficient rabbits suggest that the chronic inflammation of atherosclerosis is driven mainly by activation of the complement and monocyte-macrophage systems. In this sequence, enzymatic degradation, not oxidation, is considered to be the central predisposing process.
Fibrous plaques become complicated by calcification, ulceration, intraplaque hemorrhage, or necrosis. These later developments cause the clinical complications of myocardial infarction, stroke, and arterial occlusion causing gangrene. Aneurysm formation also may represent unique interactions with the atherosclerotic process. Alternatively, aneurysms have been viewed as nonspecific, inflammatory, degenerative, or purely mechanical arterial responses. Patients harboring aortic aneurysms have a higher prevalence of risk factors for atherosclerosis and concurrent atherosclerotic involvement of other arteries, suggesting a unique response to atherosclerosis involving a specific arterial segment in certain individuals.
As with early plaque evolution, advanced atherosclerotic lesions have been described and classified. The type IV lesion has extracellular lipid as the precursor of the core. Lesions that contain a thick layer of fibrous connective tissue are characterized as type V lesions, whereas those with fissures, hematoma, or thrombus are characterized as type VI lesions. Type V lesions have been further described as largely calcified (type Vc) or consisting mainly of connective tissue with little or no lipid or calcium (type Vb). This definition of advanced disease includes atherosclerotic aneurysms, although aneurysm formation may follow other distinct sequences.
Virchow believed that the cellular changes characterizing atherosclerosis were simply reactive responses to lipid infiltration. Later, Aschoff remarked, “From plasma of low cholesterin content no deposition of lipids will occur even though mechanical conditions are favorable.” As can be seen from fatty streak to fibrous plaque evolution, lipids, particularly LDL cholesterol, have a pivotal role in lesion morphology, composition, and evolution. Early experiments by Anitschkow with cholesterol-fed rabbits appeared to validate a simple “lipid filtration hypothesis.” However, the atherosclerotic process was soon appreciated to be much more complex. Atherosclerosis develops in various species in proportion to the ease with which an experimental regimen displaces the normal lipid pattern toward hypercholesterolemia, particularly hyperbetalipoproteinemia. At the same time, arterial susceptibility and inflammatory responses vary among locations, species, and individuals. Enhanced inflammatory responses, genetically determined by toll-like receptors, influence atherogenesis.
Canine and subhuman primate (rhesus and cynomolgus monkey) models develop atherosclerosis in response to dietary manipulation and demonstrate plaque regression in response to serum cholesterol lowering. However, lesion production in susceptible species is not a result of simple dietary cholesterol overload. Any diet that causes hypercholesterolemia induces atherosclerosis. The presence of excess cholesterol is not necessary in atherogenic diets. In developmental subhuman primate feeding experiments, reduction of cholesterol content to 0.5% combined with sugar and eggs produced rapidly progressive plaques, whereas high cholesterol addition (up to 7% by weight) did not. In rabbits, a variety of purified cholesterol-free diets with various amino acid compositions induces hypercholesterolemia and atherosclerosis. Epidemiologic observations provide important circumstantial evidence linking hyperlipidemia to atherosclerosis. Genetically determined hyperlipidemias provide compelling evidence that elevated LDL cholesterol is a prime etiologic factor in atherosclerosis, despite objections that highly cellular lipid-laden atheromas in these individuals might be atypical lesions.
This important observation earned Brown and Goldstein a Nobel Prize. Serum cholesterol levels are markedly elevated early in life; individuals with the homozygous condition die prematurely from atherosclerosis, rarely living beyond the age of 26 years. Unfortunately, the heterozygous condition is not uncommon, with total cholesterol levels ranging up to 350 mg/dL. These individuals account for 1 in 500 live births and develop atherosclerosis during early middle age. The atheromas of these patients are similar in morphology to those seen in individuals with acquired hyperlipidemia or premature atherosclerosis associated with heavy smoking.
This unfortunate natural experiment is powerful evidence that elevated LDL cholesterol is a relentless factor in plaque formation and the rapid progression of atherosclerosis to lethal consequences. Liver transplantation has been successful in retarding the progress of this type of atherosclerosis. Familial hypercholesterolemias are autosomal-dominant disorders produced by at least 12 different molecular defects of the LDL receptors. Familial abnormalities of high-density lipoprotein (HDL), a negative risk factor for atherosclerosis, also exist. In addition to LDL and HDL metabolism, surface proteins of the lipoprotein complex or apoproteins also appear to be relevant to pathogenesis. With the availability of effective therapy, a need for childhood screening for these disorders has been endorsed recently.
In the mid-19th century, von Rokitansky postulated that fibrinous substances deposited on the arterial intimal surface because of abnormal hemostatic elements in the blood and could undergo metamorphosis into atheromatous masses containing cholesterol crystals and globules. This theory posited that atheromatous lesions resulted mainly from degeneration of blood proteins (i.e., fibrin deposited in the arterial intima). Duguid resurrected this theory in 1946 with the observation that in rabbits, indwelling arterial catheters or arterial injury caused cholesterol accumulation and arterial lesions in the absence of dietary cholesterol.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here