Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Osteochondroses of the foot and ankle are found in multiple locations, including the talus, tibia, metatarsals, navicular, cuneiforms, and calcaneus. The cause of osteochondrosis is varied and complex, and has been described as traumatic, constitutional, idiopathic, and hereditary. Most investigators now believe that numerous factors are responsible for these changes. For example, excessive physical demands during athletic activity may incite osteochondral changes in growing bone made vulnerable by constitutional factors. Once the process has begun, repetitive trauma or pressure may prolong recovery or contribute to deformity. Osteochondroses often heal and defects are filled with fibrocartilage, but treatment may be required to relieve pain or prevent residual deformity. Treatment of any osteochondritic condition of the foot and ankle must be individualized to facilitate a rapid, safe return to activity and to minimize sequelae of the condition.
The incidence of osteochondral lesions of the talus (OLT), at least in military recruits, has been steadily rising during the past 10 years and is 27 per 100,000 person-years. Most lesions are unilateral, but 10% of patients may have bilateral OLT. Patients with acute injuries usually present with swollen and painful ankles or feet, limiting the specificity of physical examination. Patients with chronic injuries generally report mechanical symptoms, such as locking, catching, or giving way, or a feeling of instability of the ankle joint, in addition to pain and persistent swelling. Pain may occur only with certain ankle movements during sport or strenuous activity. An OLT should be considered in any patient who presents with a history of a “persistent ankle sprain.”
Chondral injuries are frequent in patients with chronic ankle instability and ankle fractures. Chondral injuries have been found in 25% to 95% of ankles with chronic ankle instability. Taga and associates found cartilage lesions in 89% of acutely injured lateral ankle ligaments and in 95% of ankles with chronic lateral ligament instability. They concluded that the longer the time from the initial injury, the more severe the associated cartilage lesions. No correlation has been found between the amount of cartilage damage and the severity of the lateral ligament injury. In their prospective study, Stufkens and colleagues performed ankle arthroscopies in 288 consecutive patients with ankle fractures; they found talar cartilage lesions in 65%, tibial lesions in 50%, and fibular lesions in 39%. Deep lesions (>50% depth) at the anterior and lateral talus significantly increased the odds of poor long-term clinical outcome as measured by the American Orthopaedic Foot and Ankle Society (AOFAS) ankle-hindfoot scale.
Visual inspection of the foot and ankle should be performed to identify areas of swelling and ecchymosis, which are critically important in the acute setting. Alignment should be inspected from both the anterior and posterior aspects with the patient standing to determine if he or she has distal tibial varus or valgus or hindfoot varus or valgus, which may need to be corrected to improve the long-term outcome. The ankle and foot should be palpated to identify locations of tenderness; the medial and lateral corners of the talar dome should be palpated with the ankle maximally plantar flexed. A careful neurovascular examination is essential. Range of motion (ROM) in the involved foot and ankle should be compared with that of the contralateral extremity. Stability of the ankle should be evaluated with an anterior drawer test with the ankle plantar flexed and dorsiflexed, with a talar tilt test, and with inversion and eversion stress testing. Other soft-tissue or bony causes should be ruled out.
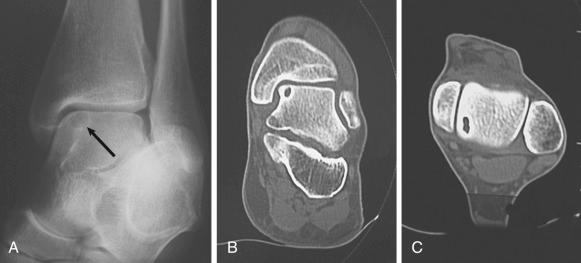
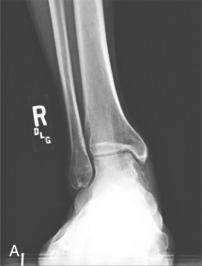
Weight-bearing anteroposterior, mortise, and lateral views of the ankle are essential. Oblique and plantar flexed radiographic views that avoid tibial overlap can show the lesion more clearly than plain films. If clinical examination or radiographs are suggestive but not diagnostic of OLT, three-dimensional (3D) imaging such as computed tomography (CT) or magnetic resonance imaging (MRI) provides more definitive information. In a study comparing MRI and CT, MRI demonstrated 96% sensitivity and 96% specificity, whereas CT demonstrated 81% sensitivity and 99% specificity, although the differences were not statistically significant. Axial and sagittal cuts can help determine the location of the lesion (anterior, medial, or posterior), as well as its depth and size ( Fig. 119.1 ). MRI can show other soft-tissue structures around the foot and ankle that can be injured concurrently with OLT, specifically the lateral and medial ankle ligaments and the peroneal tendons. In addition, MRI demonstrates bony edema not demonstrated by CT. MRI is useful for both preoperative evaluation and postoperative follow-up and has become the standard in noninvasive diagnostics. Anderson and colleagues demonstrated that low-signal intensity in T1-weighted images is an early and definitive sign of even stage 1 lesions. A high-signal rim between the osteochondral fragment and the talar bed is considered indicative of instability of the fragment, and joint fluid or fibrous granulation tissue is present as a result of the mobility of these fragments. Anderson and colleagues noted that the diameter of the lesion measured on MRI was significantly larger than that indicated on plain radiographs, which is an important factor in preoperative planning. We recommend MRI evaluation to detect changes that provide information about the detachment and viability of the fragment and help with decision-making for surgical treatment ( Fig. 119.2 ). MRI also may be useful for preoperative planning because it delineates the lesion more accurately than either plain radiography or CT. OLT that have a high-signal rim on T2-weighted images are most likely unstable. In a study of 22 ankles with OLT, Higashiyama and associates found that the low- and high-signal rims present before surgery disappeared in 100% and 77% of ankles, respectively. A decrease in or disappearance of the signal rim correlated well with clinical results; no patient in whom the signal rim persisted had a good result. It has been demonstrated that helical CT, MRI, and diagnostic arthroscopy are significantly better than history, physical examination, and standard radiography for detecting or excluding OLT. Single-photon emission CT (SPECT-CT) can evaluate OLT to help determine if the OLT was incidental or symptomatic. SPECT-CT identifies scintigraphic osteoblastic activity to combine biologic information with anatomic information. Based on these SPECT-CT images, three independent, blinded orthopedic surgeons changed their treatment plan in 52% of the 25 cases. By providing information on the subchondral plate and lesion depth, SPECT-CT may have additional diagnostic value. Verhagen and colleagues also examined the sensitivity and specificity of diagnostic arthroscopy, demonstrating 100% and 97%, respectively. In their study, diagnostic arthroscopy incorrectly identified 2 OLT (false positives), which were in fact cartilage softening caused by underlying degenerative changes. In general, diagnostic arthroscopy should not be used as the initial method for diagnosing OLT because of its invasiveness and because noninvasive 3D imaging allows for preoperative preparation.
The choice of surgical or nonsurgical treatment depends largely on the patient's symptoms, age, physical demands, level of dysfunction, and the size of the OLT. Asymptomatic or incidental osteochondral lesions should not be treated operatively. Klammer and associates concluded that asymptomatic or minimally symptomatic OLT in patients who wished to be treated nonoperatively do not clinically or radiographically worsen over time with conservative management. The mean follow-up of the 43 ankles in 41 patients in their study was 50 months. Diagnostic injections into the ankle joint can help delineate true ankle pain from other causes in the differential diagnosis. Mechanical symptoms, such as locking, clicking, catching, or functional instability, often require operative intervention. Chronic lateral instability in an ankle with an OLT should be surgically treated concurrently, which has been demonstrated to have good outcomes. Surgical treatment should not be limited to young patients or athletes only. Choi and associates demonstrated no difference in the age groups when comparing outcomes in 173 ankles with OLT treated arthroscopically. Patients were stratified into six age groups (<20, 20–29, 30–39, 40–49, 50–59, and >60 years). All age groups had statistically significant improvements in postoperative clinical outcomes at an average of 70.3-month follow-up. Even though surgical treatment has shown benefit in all age groups, some studies have shown increasing age to be correlated with poorer outcomes. In a study by Cuttica and associates, age influenced outcomes until the age of 33; after the age of 33 years, age was not correlated with worse outcomes.
The choice of surgical procedure depends on the size of the OLT, the location of the lesion, and whether initial nonoperative management has failed. Generally, larger OLT have been associated with worse outcomes. Uncontained lesions and lesions larger than 1.5 cm 2 have been associated with poor clinical outcomes and may need to be treated with an initial procedure more extensive than arthroscopic débridement and bone marrow stimulation (BMS). Although some studies use an area of greater than 1.5 cm 2 to indicate a “larger” OLT, more recent evidence indicates that an area of greater than 1 cm 2 or diameter of greater than 1 cm is a poor prognostic indicator in OLT treated with BMS. A 2017 systematic review concluded that an area greater than 107.4 mm 2 or a diameter greater than 10.2 mm is a poor prognostic indicator, and BMS is appropriate for lesions smaller than this size.
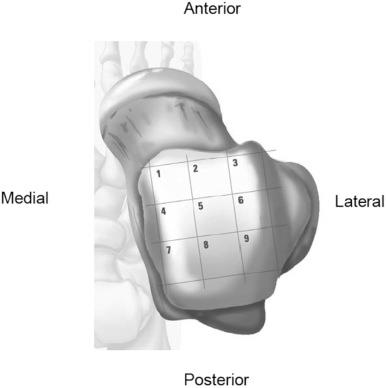
Location of the OLT also dictates surgical management. Most OLT occur in the centromedial (zone 4, 24% to 53% ) and centrolateral (zone 6, 26% to 49% ) zones of the talus ( Fig. 119.3 ). It is important to identify posteriorly oriented OLT because they may require arthroscopic transmalleolar approaches, posterior ankle arthroscopy, or open malleolar osteotomy ( Fig. 119.4 ). Careful history taking and review of operative reports can help determine the patient's surgical history and previous procedures. In patients with OLT and failed primary arthroscopic BMS treatment, osteochondral autologous transplantation (OATS) is a better choice than repeat arthroscopic BMS procedures. Postoperative visual analog scale (VAS) and AOFAS ankle-hindfoot scales were statistically superior at 24 and 12 months, respectively, in the OATS group at an average of 48 months.
Although it is reported to be successful in only about half of the patients, nonoperative treatment generally should be attempted first in patients with acute nondisplaced lesions and cystic lesions (determined by CT) with or without communication to the ankle joint. In skeletally immature patients, nonoperative treatment may be tried for chronic nondisplaced surface lesions as well. Nonoperative treatment is not recommended in adult patients with chronic symptomatic nondisplaced or in any patient with a displaced lesion.
Nonoperative treatment of acute, nondisplaced OLT generally involves an initial period of non–weight bearing with cast immobilization, followed by progressive weight bearing and mobilization to full ambulation by 12 to 16 weeks. The recommended duration of nonoperative treatment varies, with some authors recommending 6 months and others up to 12 months before operative treatment is undertaken. In patients with nondisplaced acute or traumatic OLT and MRI stage 1 or 2 OLT, we continue cast immobilization and non–weight bearing for 6 to 10 weeks. In patients with cystic or nondisplaced OLT, we prefer to try 3 to 6 months of nonoperative management before proceeding to operative treatment. Major contraindications to nonoperative management of OLT in both adults and children are acute, displaced OLT.
Based on the results of nonoperative treatment of 35 chronic cystic OLT, Shearer and colleagues concluded that (1) nonoperative management of chronic cystic OLT is a viable option with little or no risk for the development of significant osteoarthritis; (2) most lesions remain radiographically stable; (3) poor correlation exists between changes in lesion size and clinical outcome, although few patients with lesions that decrease significantly in size tend to do well, and those with lesions that increase significantly in size tend to do poorly; (4) the development of mild radiographic changes of osteoarthritis does not correlate with clinical outcome; (5) the general course of chronic cystic OLT is benign, with more than half of the patients improving to good or excellent results with nonoperative management; (6) lateral lesions tend to do better than medial ones; and (7) adult-onset lesions tend to do better than juvenile-onset lesions. Contrary to earlier reports, however, more recent investigations have determined that patient age is not an independent predictor of surgical results. Alexander and Lichtman suggested that a delay in treatment does not affect outcome, but more recent studies have questioned this suggestion. Lesions presenting more than 1 year after injury or the onset of symptoms may have a poorer prognosis.
Options for open or arthroscopic treatment of OLT generally are based on one of three specific goals: (1) stimulating the bone marrow by débridement and drilling or microfracture, with or without loose body removal; (2) stimulating the development of hyaline or hyaline-like cartilage; (3) placing osteochondral autograft or allograft; or (4) securing an acute OLT to the talar dome so that it will heal in place ( Box 119.1 ). The choice between open and arthroscopic management depends on the comfort and skill level of the surgeon, as well as the nature and location of the OLT. More specifically, techniques include excision, débridement, curettage, drilling, and microfracture; internal fixation for acute lesions and bone grafting; osteochondral autograft or allograft procedures; autologous chondrocyte implantation (ACI); and retrograde drilling. The advent of orthobiologic and synthetic therapies has increased the treatment possibilities for OLT, but currently most have lower quantity and/or lower quality evidence (level IV and V).
Arthroscopic
Anteromedial and anterolateral portals
Posterior portal for posteriorly oriented lesions
Transmalleolar drilling techniques
Open
Anterior (anterior, anterolateral, anteromedial) arthrotomy
Medial or lateral malleolar osteotomies for posteriorly oriented lesions
Débridement, loose body removal
Bone marrow stimulation
Chondroplasty
Antegrade or retrograde drilling
Microfracture
Internal fixation
Headless screws, bioabsorbable devices, or Kirschner wires
Osteochondral autograft (including osteochondral autologous transplantation system)
Single plug
Double or multiple plugs (mosaicplasty)
Osteochondral allograft (bulk allograft)
Autologous chondrocyte implantation (traditional ACI, CACI or MACI)
Particulated juvenile articular cartilage
Adjunctive hyaluronic acid with bone marrow stimulation
Adjunctive platelet-rich plasma with bone marrow stimulation
Other orthobiologic and adjunctive treatments (see text)
ACI , Autologous chondrocyte implantation; CACI , collagen-covered ACI; MACI , matrix- or membrane-induced ACI.
First-line treatment for most smaller, chronic, symptomatic lesions includes arthroscopic lavage, débridement, and BMS. Arthroscopic lavage and débridement alone may benefit by removing catabolic cytokines and loose bodies from the ankle; however, this combination alone is no longer recommended since the addition of BMS with curettage, drilling, and microfracture has been associated with better results.
Marrow-inducing reparative techniques, such as abrasion, drilling, and microfracture, aim to stimulate chondroprogenitor cells within the underlying marrow. Penetration of the subchondral plate allows the release of these cells into the defect. These stem cells populate the fibrin clot in the talar defect and produce a fibrocartilaginous matrix composed of chondroblasts, chondrocytes, fibrocytes, and an unorganized matrix that protects the surface from excessive loading. The disadvantage of these reparative techniques is the weaker mechanical properties of the type I collagen fibrocartilage matrix, which lacks the normal biomechanical and viscoelastic characteristics of type II hyaline cartilage.
Fair grade evidence (grade B recommendation) supports BMS as a first-line treatment for the index procedure for chondral and osteochondral lesions or as a repeat procedure after failed arthroscopic management. Recent evidence supports superior results of OATS procedures instead of repeat arthroscopic procedures with BMS after failed initial arthroscopic management. Most authors use a less than 1.5-cm diameter as the boundary for a small OLT ; however, this boundary varies from 0.8 cm to 2.0 cm. Other authors use an area of greater than 1.5 cm 2 to define a large OLT. Arthroscopic results appear to be superior to those of open procedures. A 2017 systematic review reported treatment with BMS had an 82.1% success rate in 10 studies (1053 patients) ; this was consistent with a 2010 systematic review that included 18 studies (388 patients) reporting an 85% success rate (range 46% to 100%). OLT associated with cyst formation have been successfully treated with BMS alone (without bone grafting), achieving good to excellent results in 74% of patients. The authors also noted no difference in postoperative AOFAS ankle-hindfoot scales between cystic and noncystic small OLT. When the overlying cartilage is intact, antegrade or retrograde drilling can effectively stimulate a response. Fair (level IV) evidence with consistently positive results is available to warrant a grade B recommendation for antegrade or retrograde drilling of OLT with intact overlying cartilage. Although antegrade (transtibial) and retrograde (transtalar) drilling are technically easy, both can result in cartilage damage and osseous necrosis. Some authors advocate retrograde drilling of symptomatic OLT with the cartilage and subchondral bone intact.
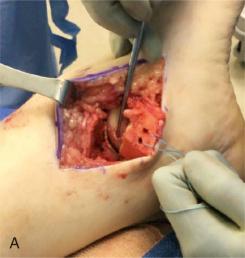
Fixation devices include permanent or bioabsorbable low-profile pins, nails, or headless screws ( Fig. 119.5 ). Acute OLT do markedly better after fixation than do chronic lesions. Lesions need to be larger than 8 mm to allow secure internal fixation.
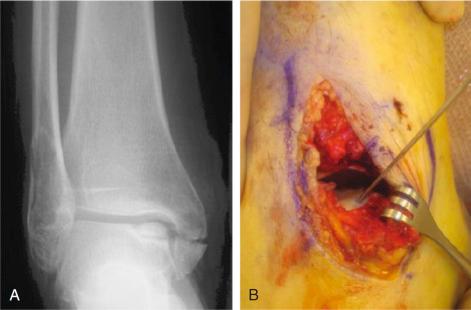
Restorative techniques usually are recommended for defects larger than 1.5 cm 2 . These techniques can include osteochondral autograft and allograft, ACI (including traditional ACI, collagen-covered ACI, and matrix-induced ACI), arthroscopic allograft or autograft implantation, and fresh osteochondral graft. Platelet-rich plasma (PRP), stem cell-mediated implants, and scaffolds also aim to restore hyaline or hyaline-like cartilage. These procedures are described in more detail later in the chapter. These procedures are generally contraindicated in bipolar (kissing) lesions that involve both the tibia and the talus, as well as in patients with advanced generalized arthritis.
Osteochondral autografting procedures such as OATS or mosaicplasty (multiple OATS plugs) require the harvest of grafts from a donor site, such as the lateral supracondylar ridge or intercondylar notch of the femur, for insertion into the OLT. These techniques generally are used for moderate to large grade III or IV lesions. Concerns about donor site morbidity have prompted graft harvest from sites other than the distal femur (e.g., the anterior talar dome) and the use of allografts.
Fresh-frozen allografting (bulk talar osteochondral allograft) is another option for large OLT, especially shoulder lesions and lesions with morphology that is difficult to duplicate using nontalar grafting. Another benefit of this technique includes avoiding donor site morbidity. Gross and colleagues listed as their indication for this procedure a lesion at least 1 cm in diameter and 5 mm deep that could not be internally fixed. Hahn and colleagues listed as the following indications for a fresh-frozen allograft procedure: (1) greater than 1 cm size on one dimension on MRI, (2) greater than 6 months from last operative treatment, (3) little or no degenerative changes in the ankle, (4) functional ROM, and (5) skeletally mature patient. The average size of fresh frozen allografts implanted by Hahn et al. was 1.9 cm × 1.4 cm (2.7 cm 2 ); in another study the average size was 3.6 cm 2 . Chondrocyte viability is a primary concern, and it is essential that the graft be harvested within 24 hours of the donor's death and be stored at 4°C. One study found a minimal decrease in viability after 14 days of storage in a serum-free modified culture medium, but after 28 days, viability was significantly decreased. Because most tissue banks require a 21-day screening process to minimize the risk of disease transmission and the grafts are not released for 3 days after screening is complete, the minimal time to implantation is 24 days. Other considerations, such as scheduling, can delay implantation even longer. Fortunately, the biomechanical properties of these allografts do not appear to deteriorate even after 28 days from procurement. Recent studies have suggested that, compared with current protocols, the storage of osteochondral allografts at higher temperatures (25°C and 37°C) can increase the window of opportunity for implantation of optimal tissue from 14 to 42 days after disease-testing clearance.
Large, chronic OLT are traditionally treated with OATS or fresh-frozen osteochondral allograft procedures. However, some authors have described surgical treatment of larger OLT with osteoperiosteal bone grafts from the distal medial tibia and treatment of larger shoulder OLT with free vascularized bone grafts from the medial condyle of the femur. These autograft options that are reported infrequently do not restore hyaline cartilage to the OLT.
ACI is best suited for large and well-contained stage 3 or 4 defects, large lesions with extensive subchondral cystic changes, and lesions in which previous operative treatment has failed. According to Ferkel and Hommen, the ideal patient for ACI is between 15 and 55 years old and has no malalignment, degenerative joint disease, or instability of the joint. First-generation, traditional ACI involves harvesting 200 to 300 mg of autologous chondrocytes from the distal femur, growing the cells in vitro for 2 to 5 weeks, and then implanting them into the defect. An autologous periosteal flap is harvested and sewn over the implanted cells and sealed with fibrin glue. First-generation ACI (traditional ACI, periosteal ACI) is technically challenging, involves periosteal harvest, and has complications including periosteal hypertrophy, delamination, and graft failure. Second-generation, collagen-covered ACI (CACI) involves a bioabsorbable collagen membrane. Like traditional ACI, CACI involves an open procedure. Finally, third-generation, matrix- or membrane-induced ACI (MACI), involves harvesting, seeding, and proliferating chondrocytes on type I/type III collagen membranes and implanting the membranes into OLT. Other types of matrix have been used in MACI techniques, including a mixed thrombin and fibrin gel matrix.
Because of concerns about donor-site morbidity after harvest from the distal femur, other donor sites have been suggested. Giannini and associates and Kreulen and associates used detached talar osteochondral and chondral fragments, while Lee and associates used the cuboid surface of the calcaneocuboid joint as a source of chondrocytes.
Newer orthobiologic and synthetic therapies include particulated juvenile allograft cartilage (PJAC) implantation; allograft cartilage extracellular matrix (ECM); autologous matrix induced chondrogenesis (AMIC); BMS with adjunctive hyaluronic acid (HA), PRP, platelet-derived growth factor (PDGF), bone marrow aspirate (BMA) or cells, or mesenchymal stem cells (MSCs); and synthetic bone graft substitute or plugs.
PJAC implantation ( DeNovo NT Natural Tissue Graft, Zimmer, Warsaw, IN) is a single-step procedure that is ideal for younger patients (<50 years old) with large (>1 cm 2 ) OLT and OLT that has failed previous microfracture. The technique for PJAC involves preparing and drying the OLT bed ( Fig. 119.6A ), placing a fibrin glue layer (see Fig. 119.6B ), laying particulated juvenile cartilage (see Fig. 119.6C ), and sealing with another layer of fibrin glue (see Fig. 119.6D and E ).
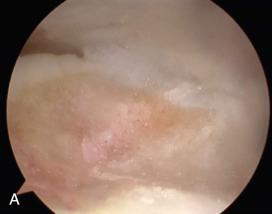
Allograft cartilage ECM (BioCartilage, Arthrex, Naples, FL) contains components of cartilage, including type II collagen, proteoglycans, and growth factors. Authors who have reported using allograft cartilage ECM in small OLT describe a single-step arthroscopic technique where they microfracture the OLT and then place the allograft cartilage ECM.
The technique for AMIC for treatment of OLT involves microfracture, placement of a porcine collagen type I/III matrix (chondro-Gide, Geistlich Surgery), and securing the collagen matrix in place with fibrin glue (Tisseel, Baxter, Deerfield, IL).
Adding adjunctive HA to BMS has been postulated to improve clinical outcomes and quantitative MRI findings because of its high viscosity and joint protective properties. Adding PRP after BMS has the theoretical effect of inducing mitogenic, chemotactic, angiogenic, and differentiation platelet functions at the OLT. Adding BMA to a scaffold boasts the advantage of a single-step, arthroscopic procedure by introducing autologous, live, iliac crest bone marrow cells to OLT. Adding MSCs to BMS introduces cadaver-donor MSCs to aid in healing OLT by MSC differentiation into chondroblasts and osteoblasts. Adding recombinant human PDGF with β-tricalcium phosphate carrier (rhPDGF/β-TCP) and sealing with fibrin glue after BMS theoretically induces mitogenic, chemotactic, osteoblastic, and chondroblastic activity in treating OLT. There is very sparse evidence at present for rhPDGF/β-TCP in the treatment of OLT.
There is inadequate evidence to support synthetic bone graft substitutes or plugs such as Tru-fit (Smith & Nephew, Andover, MA), which is made of calcium triphosphate bone substitute with poly DL-lactide-coglycolide cartilage substitute.
Although the advent of these orthobiologic and synthetic therapies has increased the possibilities in the treatment of OLT, there are few studies with higher quality evidence supporting these treatments in the current literature.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here