Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Articular cartilage is a remarkable tissue with a unique functional architecture that supports multiplanar motion under a wide variety of loading conditions. In the absence of injury, and when allowed to function within its “zone of homeostasis,” human articular cartilage can provide a lifetime of pain-free motion. However, all too frequently, premature tissue failure occurs as a result of trauma, disease, or altered loading. Although articular cartilage has limited capacity to heal injuries that compromise the articular surface resulting from increased fibrillation friction and shear stress, new data support an ability to recover from subsurface injuries. It is important for orthopedic surgeons to understand the basic science of articular cartilage in health, as well as in response to injury and disease, so to apply new concepts of preosteoarthritis, functional tissue engineering, and precision medicine toward improving and maintaining joint health to support a lifetime of healthy movement.
Articular cartilage can be thought of as a tissue where the three main components of water, collagen, and proteoglycans (PGs) are arranged in an intricate fashion to provide incredible functional characteristics. The tissue functions under extreme loading conditions, yet it is composed primarily of water. It is also aneural and avascular, properties that likely contribute to the mechanical durability of the tissue but also hamper protective and reparative processes. Although proportionately thicker in human knees than the stifle joints of even the largest modern quadrupeds, articular cartilage is an incredibly thin tissue uniquely organized to provide extremely low-friction surfaces, permitting multiplanar motion that can resist or transmit repetitive tensile, shear, and compressive forces. In the absence of injury or disease, articular cartilage can remain intact and functional throughout life, although factors such as trauma and obesity can accelerate cartilage degeneration. As an avascular tissue, it also routinely survives for several days after donor death, and even longer with proper preservation.
Because of differential functional needs, articular cartilage varies in cellularity and thickness within the same joint and between different joints, reflecting the sensitivity of knee cartilage to kinematic changes as a result of the local mechanical environment being associated with functional loading. However, it consists of the same basic components and anisotropic structure throughout all joints. Grossly, human articular cartilage appears as a smooth, homogeneous tissue approximately 1 to 5 mm thick ( Fig. 27.1 ). When probed, healthy cartilage is firm, resists deformation, and is smooth and slick. Diseased cartilage is soft, deforms when probed, feels rough, and may contain visible surface disruptions ranging from increased surface porosity with fine fibrillations to deep fissures, clefts, and full thickness cartilage loss.
The composition and matrix structure of articular cartilage varies with depth from the surface and has been traditionally divided into four structural zones. The matrix composition has additionally been characterized into three regions based on distance from the chondrocyte. This precise arrangement of tissue components provides specific mechanical properties for the tissue as a whole.
Chondrocytes, which are the cells in articular cartilage, synthesize matrix components and respond to a variety of mechanical and biochemical stimuli to regulate cartilage homeostasis. Despite these important roles, chondrocytes account for just a small fraction of articular cartilage tissue (1% of volume). Chondrocytes are derived from pluripotential mesenchymal stem cells (MSCs) that differentiate into chondroblasts and mature chondrocytes during growth and development. Articular cartilage is avascular; thus chondrocytes must derive nutrition and oxygen from the synovial fluid by diffusion and must meet energy requirements through glycolysis. Chondrocytes within healthy, mature articular cartilage are individually surrounded by an extracellular matrix and form few cell-to-cell contacts ( Fig. 27.2A ). Despite this isolated arrangement, chondrocytes are able to respond to a variety of mechanical and biochemical signals.
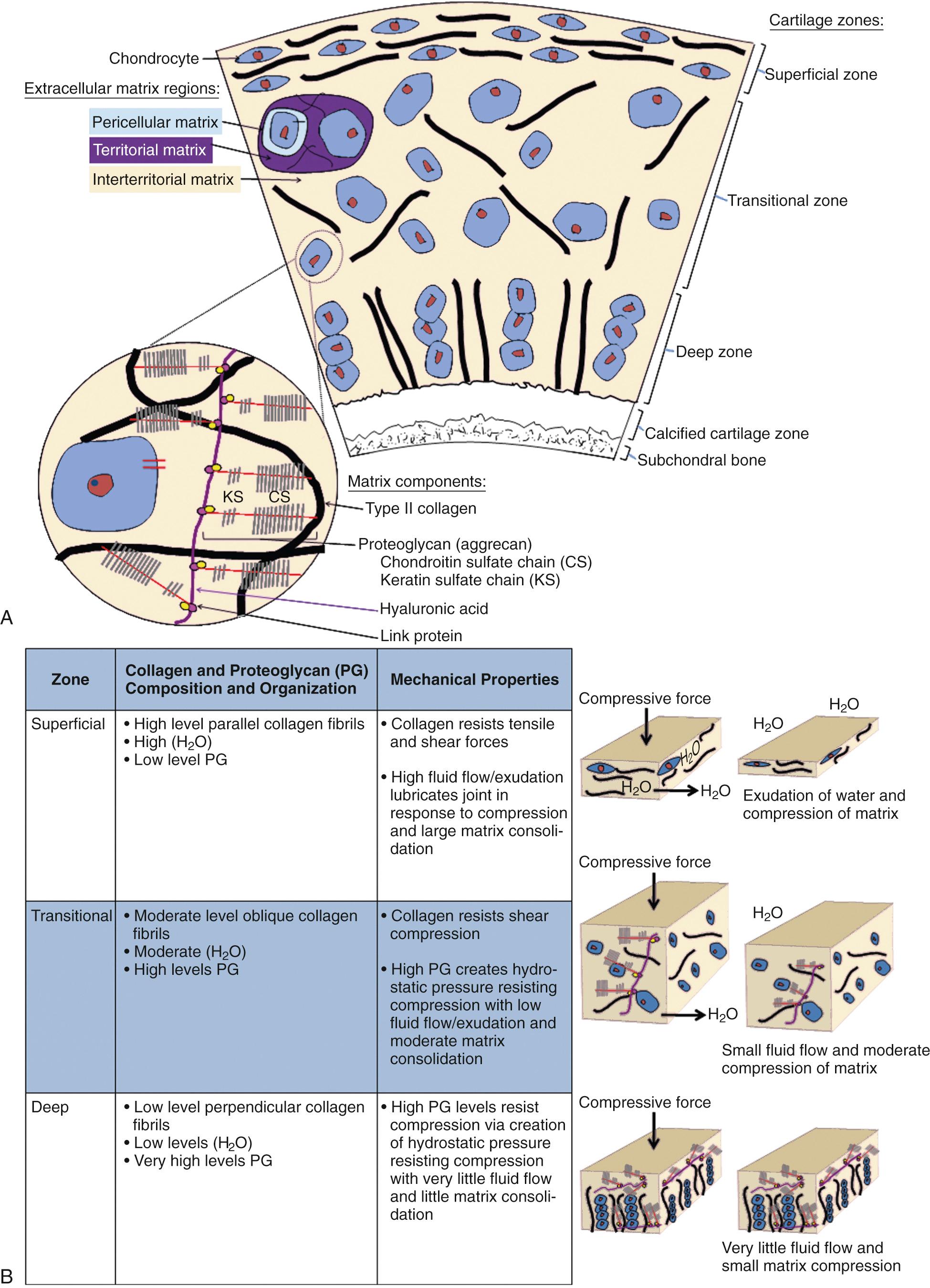
Chondrocytes differ in size, shape, and metabolic activity in the different structural zones, but all cells contain endoplasmic reticulum and Golgi apparatus for matrix synthesis. Chondrocytes synthesize the two major articular cartilage macromolecules—type II collagen and aggrecan—and organize the structure of the matrix. Specific interactions between chondrocytes and the extracellular matrix are poorly understood, but the ability to detect and respond to a variety of mechanical and biochemical factors by the chondrocyte is vital for matrix synthesis and maintenance of tissue homeostasis. A few mechanisms have been discovered, including the presence of binding proteins (integrins) and osmotically sensitive ion channels on the cell surface of chondrocytes.
Development and maintenance of the chondrocyte phenotype is an important research topic because current and future treatments for articular cartilage damage include implantation of stem cells and chondrocytes into defects. To maintain chondrocyte cell phenotype, in vitro study of chondrocytes has shown that proliferation and expansion of chondrocytes in monolayer results in loss of cell phenotype and subsequent synthesis of type I collagen. However, culture conditions that include high cell density, cell-to-cell contact, and a three-dimensional environment appear to restore or maintain the chondrocyte phenotype assessed by cellular morphology and the production of type II collagen. Receptors for growth factors such as transforming growth factor-β (TGF-β), fibroblast growth factor (FGF), and insulin-like growth factor-1 (IGF-1) have been identified on chondrocytes and appear important for production of cartilage matrix proteins. A range of other molecules such as oxygen and common injectable anesthetics has been shown to impact chondrocyte metabolism and viability. As an avascular tissue, chondrocytes are adapted to low oxygen tensions yet are typically cultured at higher oxygen tensions. Given that sustained hypoxia in vitro increased type II collagen gene expression and PG synthesis, oxygen tensions should ideally be taken into account when chondrogenesis is desired in ex vivo applications.
Already few in number, the loss of chondrocytes adversely affects the ability of the remaining chondrocytes to adequately maintain matrix integrity. Rapid loss of articular cartilage, or chondrolysis, has been attributed to depletion of chondrocytes below a critical threshold, resulting from sustained administration of local anesthetics using pain pumps. A one-time dose of local anesthetics such as bupivacaine or lidocaine has been reported to be toxic to human chondrocytes in vitro in a dose- and time-dependent manner. In vivo work also showed chondrocyte loss after a single injection of 0.5% bupivacaine and that substantial chondrocyte loss after monoiodoacetate injection led to full thickness cartilage loss within a short period of time. Further research will continue to shed light on the complex interactions of biochemical and mechanical factors on chondrocyte and articular cartilage health.
Articular cartilage (90%) is predominantly composed of the extracellular matrix, which consists of fluid and macromolecules. The porosity of the zonal structure of the matrix determines its interaction with fluid and thus is responsible for determining the mechanical properties of the cartilage. The main functions of the matrix are to resist tensile and shear forces through the arrangement of collagen fibrils, and to resist compressive forces through alteration in hydrostatic pressure.
Water is the largest component of the extracellular matrix (80% of articular cartilage by weight). The fluid component of articular cartilage also includes high concentrations of cations, gases, and small proteins. The volume of water present in articular cartilage depends on the concentration and organization of the macromolecules—specifically, the distribution and relationships between PGs and the collagen network. The size, electronegativity, and concentration of PGs varies between cartilage zones, contributing to differences in water concentration, porosity, and permeability of the tissue. Throughout joint movement, water continually moves into and out of the cartilage to aid in distribution of compressive forces and lubrication of the cartilage surface.
The macromolecules of the extracellular matrix include collagens (60% dry weight), PGs (25% to 35%), and noncollagenous proteins and glycoproteins (15% to 20%). Type II collagen fibrils provide structural integrity and tensile and shear strength to the articular cartilage. PGs, mainly aggrecan, attract water and provide resistance to compression. The noncollagenous proteins and glycoproteins help bind chondrocytes to the matrix, stabilize matrix macromolecules, and assist in regulation of matrix homeostasis.
An exceedingly complex and incompletely described network of collagen fibrils contributes materially to the volume, shape, tensile, and shear strength of articular cartilage. Although multiple collagen types including II, VI, IX, X, and XI are present, type II collagen accounts for 90% of the collagen in articular cartilage. Type II collagen is composed of three alpha chains, which intertwine into a triple helix. These helices covalently cross-link in a lateral array to form collagen fibrils. Levels of type II collagen are highest in the superficial zone and decrease in concentration with increasing depth from the surface (see Fig. 27.2A and B ). The collagen fibril network restrains the PGs in the matrix and prevents swelling of the cartilage to greater than 450 mOsm when water flows into the tissue. This allows for the creation of high tissue pressure, which is vital for resistance of compressive forces.
Under physiologic conditions, type II collagen metabolism is slow and fibrils have a half-life of years. In the early stages of cartilage degeneration, degradation of collagen fibrils is observed. Enzymes called matrix metalloproteinases (MMPs) are thought to contribute to this degradation, specifically collagenases and aggrecanases. Collagenases mediate cleavage of type II collagen. Antibodies to specific neoepitopes on these fragments can be detected by synovial fluid, serum, or urine assays and are being studied for use as potential biomarkers of early cartilage degradation.
Other collagen types are less prevalent but perform important functions, including stabilization and regulation of type II collagen fibrils. Type IX collagen forms cross-links along the surface of type II collagen fibrils and interconnects the fibrils with PG aggregates. Type XI collagen binds to the interior structure of type II collagen fibrils and regulates the diameter of the fibrils. Type X collagen is localized near the calcified cartilage zone and the hypertrophic zone of the growth plate and is thought to contribute to cartilage mineralization through calcium-binding properties. Type VI collagen is located in the pericellular matrix and aids in the attachment of chondrocytes to the extracellular matrix.
PGs consist of a protein core with glycosaminoglycan (GAG) side chains. GAGs consist of long unbranched polysaccharide chains containing repeating disaccharides of amino sugars with negatively charged carboxylate or sulfate groups. Specific GAGs include hyaluronic acid (HA), chondroitin sulfate (CS), keratan sulfate (KS), and dermatan sulfate (DS). The major PG (90% of mass) in articular cartilage is aggrecan. Aggrecan has many CS and KS side chains and noncovalently associates with an HA backbone to form aggregates (see Fig. 27.2A ). HA is a long-chain nonsulfated GAG capable of binding a large number of aggrecan molecules. Link protein, a glycoprotein, stabilizes the association between HA and each aggrecan molecule.
Aggrecans play a key role in generating hydrostatic pressure. The negatively charged aggrecans attract cations, increasing the osmolality of the tissue. Water is then attracted into the tissue, decreasing the osmolality. Hydrostatic pressure created by the interaction of collagen, PGs, and water provides stiffness to the cartilage to absorb compressive mechanical loads without damage to the matrix. Displacement of water from PGs during compression of the superficial zone of matrix lubricates the joint.
Similar to collagen, degradation of aggrecan is observed in early cartilage degeneration, and aggrecanases are thought to contribute. Aggrecan synthesis and degradation can also be measured by antibodies to specific neoepitopes on fragments by synovial fluid or serum assays. Inhibitors of aggrecanases are being investigated for potential treatments for cartilage degeneration.
Smaller PGs include decorin, fibromodulin, and biglycan. The function of each PG is related to the specific core protein and GAG chains that it contains. Decorin contains DS side chains and is located at the surface of type II collagen fibrils. It is thought to inhibit the lateral growth of fibrils and contributes to their organization and stabilization. Fibromodulin has KS side chains and binds type II collagen fibrils, providing stabilization. Biglycan contains DS side chains, binds TGF-β, and interacts with type VI collagen in the pericellular matrix. Expression of smaller PG changes within zones and with mechanical stress likely contributes to cell stabilization and signaling through interaction with other proteins.
The matrix contains many additional proteins that represent a small volume of the tissue. Cartilage oligomeric matrix protein (COMP), anchorin, and fibronectin function to bind chondrocytes to the matrix. COMP binds to chondrocytes in the territorial matrix. Anchorin binds to chondrocyte surface protein, anchoring chondrocytes to collagen fibrils. Fibronectin is an adhesion molecule expressed on the surface of chondrocytes. Ongoing research is investigating the roles of these proteins, with COMP showing promise as a biomarker of cartilage degradation in early osteoarthritis (OA).
Other proteins include growth factors and cytokines, which bind to chondrocyte receptors, altering rates of matrix synthesis and degradation. Effects of these proteins depend on their concentration, cofactors, type of target cell, and number of cell receptors. TGF-β, IGF-1, FGF, bone morphogenetic protein (BMP), and platelet-derived growth factors (PDGFs) stimulate matrix synthesis and proliferation. TGF-β, FGF, and PDGFs also promote proliferation and chondrogenic differentiation of MSCs in combination with many other factors. Matrix degradation is stimulated by interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and MMPs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here