Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although the basic principles of cardiac electrophysiology (EP) apply broadly across the age spectrum, several distinctive characteristics of the pediatric population deserve emphasis. These begin at the cellular level, with differences in the action potential and calcium handling by immature myocytes, and extend up to the gross clinical level, with technical challenges during therapeutic interventions in small hearts. All aspects of pediatric arrhythmia management can be further complicated by the presence of congenital heart defects.
Our understanding of developmental EP is evolving rapidly but is still far from complete. , It is generally agreed that the primitive heart tube begins to contract in a peristaltic fashion from its atrial to ventricular poles by approximately 20 to 25 days of gestational age and that the cells generating this activity are distributed as several discrete ring-like clusters along the length of the tube, where action potentials with spontaneous automaticity can be recorded that display a slow-response profile similar to mature nodal cells. As the heart tube changes configuration during the process of ventricular looping, a portion of the cluster at the atrial pole is retained to form the sinoatrial (SA) node, and portions of the other clusters combine in a complex fashion to form the specialized atrioventricular (AV) conduction tissues. At the same time, a tightly orchestrated process of programmed gene expression modifies myocyte physiology to result in rapid-response action potentials in working myocardium and specific portions of the specialized conduction system. By about 5 weeks of gestational age, an intact AV conduction axis with rapid and coordinated contraction of atrial and ventricular muscle is established in the normal fetal heart. There is growing evidence that failure of apoptosis or failure of insulation for slow-response cells from certain sections of the primitive ring clusters could be responsible for focal automatic tachycardias (e.g., ectopic atrial tachycardia or outflow tract ventricular tachycardia [VT]) later in life.
The electrical behavior of immature heart cells differs somewhat from fully mature myocytes. The most obvious features are enhanced spontaneous depolarization of pacemaker cells to account for faster sinus rates and shorter refractory periods to accommodate these rates. Maximum diastolic potentials are also slightly reduced, as are transient outward rectifier potassium currents. These subtle differences are usually not of practical importance during clinical evaluation and treatment but do confirm that cellular properties change with age, which may explain why some tachyarrhythmias presenting in the fetus and newborn (e.g., chaotic atrial rhythm, accelerated ventricular rhythm) will frequently resolve spontaneously over the first year or so of life, whereas other arrhythmias (e.g., VT in Brugada syndrome) often do not fully manifest until older ages. Perhaps the most therapeutically relevant difference between infant and mature myocytes relates to cell infrastructure, specifically the sarcoplasmic reticulum, which is relatively underdeveloped in the fetus and newborn. This can make infants far more reliant on extracellular calcium for myocyte contraction and has resulted in the firm recommendation that IV calcium channel blockers be avoided in children under the age of 1 year because of a risk for hemodynamic collapse.
Maturational changes can also occur at the macroscopic level of the specialized conduction tissues. It is known from histopathologic study that the posterior extensions off the compact AV node start out very stunted but elongate progressively relative to the size of the compact node between birth and the teenage years. The fact that AV nodal reentrant tachycardia is relatively uncommon before teenage years could be linked to this structural change. Similarly, accessory AV pathways (APs), or the tissues surrounding these pathways, can change during the early years of life. More than 30% of newborns with manifest preexcitation and episodes of AV reentrant tachycardia will have spontaneous resolution of the delta wave and be completely noninducible with aggressive transesophageal atrial stimulation by their first birthday. This may relate to ingrowth of fibrous tissue along the AV valve ring or intrinsic changes in the accessory pathway itself, but in either event, the chance for spontaneous resolution has a strong influence on how pathway-mediated tachycardias are managed in very young patients.
The embryologic accidents responsible for congenital heart disease (CHD) can interfere with development of the SA node and the AV conduction tissues. The most extreme examples involve abnormalities of atrial situs with discordant connections of the atria and ventricles (so-called heterotaxy syndromes). Heterotaxy can be divided into the broad subcategories of polysplenia (also referred to as left atrial isomerism ) and asplenia (also referred to as right atrial isomerism ). In the former condition, a true sinus node may be absent and complete heart block is common. In the latter condition, there may actually be two separate sinus nodes, and occasionally, the AV conduction system will be duplicated in an arrangement referred to as “twin AV nodes” ( Fig. 114.1 ), which can support a variety of reentrant tachycardias.
Even some of the less severe forms of CHD can have associated abnormalities of the AV conduction tissues. One such condition is congenitally corrected transposition of the great arteries (L-TGA) where the compact node develops outside of Koch’s triangle in an unusual anterior location near the base of the right atrial appendage. This displaced node is often feeble and can deteriorate over time so that complete heart block is seen in as many as 5% of these patients at birth, and more than 25% by adulthood. Displacement of the AV node also occurs in patients with endocardial cushion defects (i.e., primum atrial septal defects or complete AV canal defects). These hearts do not have a proper triangle of Koch, and the compact node is consequently displaced in a posterior direction beneath the mouth of the coronary sinus. Similar to L-TGA, AV nodal function can be suboptimal in endocardial cushion defects, with complete heart block at birth in some individuals, and a significant risk for surgically induced block after repairs. Congenitally impaired AV nodal function can also be observed in some simple cases of secundum atrial septal defect among patients with Holt-Oram syndrome, an autosomal dominant disorder causing cardiac and upper-limb abnormalities that involves a mutation on the TBX5 gene.
The incidence of accessory pathways in most forms of CHD is not substantially higher than in the general population, with the notable exceptions of Ebstein anomaly and L-TGA (which can have an Ebstein-like malformation of the left-sided tricuspid valve). Patients with Ebstein anomaly have a greatly elevated risk for APs in the range of 20% to 30%. These can include both AV and atriofascicular connections that tend to be localized to the region where the tricuspid valve is most abnormal. Furthermore, when preexcitation is present in a patient with Ebstein anomaly, the chances of that patient having multiple pathways may be as high as 50%. Management of these abnormal connections can become a significant challenge in the care of Ebstein patients. ,
Apart from congenital conduction abnormalities, the hemodynamic stresses of CHD and the tissue injury resulting from surgical repairs can have a negative long-term impact on rhythm status. Fibrosis and hypertrophy, in combination with conduction barriers along incisional scars and patches, all combine to create a substrate that is conducive to the development of macroreentrant tachycardias. These occur most commonly at the atrial level, especially after surgery that involves extensive atrial baffling, such as the Mustard or Senning operations for transposition of the great arteries or the Fontan operation for single ventricle. Macroreentrant VTs can also develop in some CHD lesions, most notably tetralogy of Fallot. , Bradyarrhythmias may occur as a consequence of direct surgical trauma to the SA node and/or the AV conduction tissues.
The intrinsic rate of SA node depolarization in children is naturally faster than the adult, but it is also noteworthy that autonomic modulation of SA node activity can result in rather dramatic extremes of rate among young patients that must be differentiated from pathologic rhythm conditions. Sinus tachycardia can exceed 220 beats/min in a stressed infant, whereas abrupt surges in vagal tone can result in prolonged asystolic pauses lasting more than 30 seconds in some children with an otherwise normal SA node. This relative hypersensitivity to autonomic influence is reflected in a high degree of sinus arrhythmia at normal resting rates and in a strong predisposition to neurally medicated syncope, both of which become attenuated by adulthood.
Some incessant forms of tachycardia can result in severe degrees of ventricular dysfunction. Animal models of this phenomenon , have demonstrated that rapid pacing for extended periods will reliably depress left ventricular (LV) performance and that this depression will reverse after ceasing pacing, although the cellular mechanism underlying the process is still not entirely clear. Tachycardia-induced cardiomyopathy is well described in all age groups and can be caused by a variety of arrhythmia mechanisms, but among pediatric patients, the most common culprits are ectopic atrial tachycardia (EAT), the permanent form of junctional reciprocating tachycardia (PJRT), and focal automatic VT. Because the heart rate associated with these disorders is only modestly elevated above the expected sinus rates for a child and because young patients can remain relatively asymptomatic until late in the course of ventricular dysfunction, tachycardia may go unrecognized for some time. Consequently, many of these children are ill with congestive heart failure (CHF) at presentation. It is imperative that incessant tachycardia always be considered in the differential diagnosis of any young patient with new onset cardiomyopathy and that experienced eyes scrutinize the electrocardiogram (ECG) carefully for these curable rhythm disorders.
Inherited channelopathies and myopathies are discussed in detail elsewhere in this textbook, and their major pediatric features will be reviewed briefly later in this chapter, but it needs to be stressed here that many of these conditions can cause life-threatening arrhythmias during infancy and childhood and that family screening of young relatives is a required exercise whenever an index case is identified. Box 114.1 provides a partial list of the hereditary disorders that are most relevant to the pediatric population. Most of these are capable of causing serious symptoms at young ages, and, in fact, channelopathies have been implicated as the cause for some cases of sudden infant death syndrome (SIDS). Brugada syndrome and arrhythmogenic right ventricular (RV) cardiomyopathy may not be expressed phenotypically until adulthood, but there are well-documented anecdotes of malignant presentations earlier in life.
Long QT (LQT) syndromes
LQT1
LQT2
LQT3
LQT7 (Andersen-Tawil syndrome)
LQT8 (Timothy syndrome)
Other rare LQT forms
Catecholaminergic polymorphic VT
Brugada syndrome
Arrhythmogenic RV cardiomyopathy
Short QT syndrome
Hypertrophic cardiomyopathy (errors of metabolism)
Pompe disease
Danon disease
Hurler syndrome
Hunter syndrome
Glycogen storage disease
Barth syndrome
Hypertrophic cardiomyopathy (isolated familial forms)
β-Myosin heavy chain
PRKAG2 with Wolff-Parkinson-White syndrome
Other sarcomeric genes
Hypertrophic cardiomyopathy (other)
Noonan syndrome
Friedreich ataxia
Familial dilated cardiomyopathy
Left ventricular noncompaction
Muscular dystrophy
Holt-Oram syndrome
Small size is perhaps the most distinguishing feature of the pediatric patient, and it greatly impacts all aspects of arrhythmia treatment. Antiarrhythmic medication doses must be titrated carefully based on weight or body surface area ( Table 114.1 ), and oral agents that are not commercially available in solution form must be compounded to exacting specifications when prescribed for young children who cannot swallow standard pills or capsules.
| Class | Drug | Acute Intravenous Dosage | Chronic Oral Dosage |
|---|---|---|---|
| IA | Procainamide | 5–12 mg/kg load over 30 min, then infuse 20–50 μg/kg/min | |
| IB | Lidocaine | 1 mg/kg rapid bolus, then infuse 20–50 μg/kg/min | |
| IB | Mexiletine | 5–15 mg/kg/day (2 or 3 divided doses) | |
| IC | Flecainide | <6 mo: 50 mg/m 2 /day (3 divided doses) >6 mo: 80 mg/m 2 /day (2 or 3 divided doses) |
|
| II | Propranolol | 1–3 mg/kg/day (3 divided doses) | |
| II | Nadolol | 0.5–1.0 mg/kg/day (once daily dose) | |
| II | Esmolol | 100–500 μg/kg load over 1 min, then infuse 50–200 μg/kg/min | |
| III | Sotalol | 80 mg/m 2 /day (2 or 3 divided doses) (consult nomogram if age <2 years) | |
| III | Amiodarone | 5 mg/kg load over 60 min, then infuse 5–10 μg/kg/min | 10 mg/kg/day (2 divided doses) × 10 days, then 5 mg/kg/day maintenance |
| IV | Verapamil | 0.1 mg/kg rapid bolus Do not use for age <1 year |
4–8 mg/kg/day (3 divided doses) |
| — | Adenosine | 0.1 mg/kg rapid bolus May repeat with 0.2 mg/kg rapid bolus |
|
| — | Magnesium | 10–25 mg/kg magnesium sulfate Slow infusion over 30 min |
|
| — | Atropine | 0.01–0.02 mg/kg rapid bolus Maximum dose 0.4 mg, minimum dose 0.1 mg |
|
| — | Isoproterenol | 0.01–0.10 μg/kg/min infusion |
Techniques used during catheterization for diagnostic testing and ablation must also be tailored to patient’s age and size ( Fig. 114.2 ). The level of sedation must be sufficient to both quiet a child’s anxiety and ensure comfort and immobility during the critical portion of a case, oftentimes necessitating a general anesthetic. Vascular access must be planned carefully within the constraints of small vessel caliber, downsizing to 4 to 5 Fr catheters when necessary and economizing on the catheter number by combining functions. During ablation procedures, compulsive mapping is required to minimize unproductive lesions, and extreme caution is naturally required when ablating within or near the reduced dimensions of Koch’s triangle in a small heart. Cryoablation has been adopted at many pediatric centers for targets very near the normal conduction tissues, tolerating the trade-off of higher recurrence rate for improved safety in such cases. Finally, radiation exposure must be minimized in young patients who remain at risk for stochastic tissue injury over many decades after an invasive procedure. Electroanatomic mapping has largely eliminated the need for fluoroscopy and has now been adopted by all pediatric laboratories for ablation of even simple arrhythmia substrates. More detailed discussion of ablation technology for the pediatric patient can be found elsewhere in this textbook.
Pacemaker and implantable cardioverter-defibrillator (ICD) procedures are likewise influenced by patient size. Very young children may not be candidates for transvenous hardware and often require epicardial systems until they have grown sufficiently to accommodate conventional leads. Device implant in children is further complicated by lead attrition during lifelong therapy, which often entails extractions and replacements that can eventually narrow or occlude important vessels. Maintenance of a well-functioning system over many decades can be challenging. ,
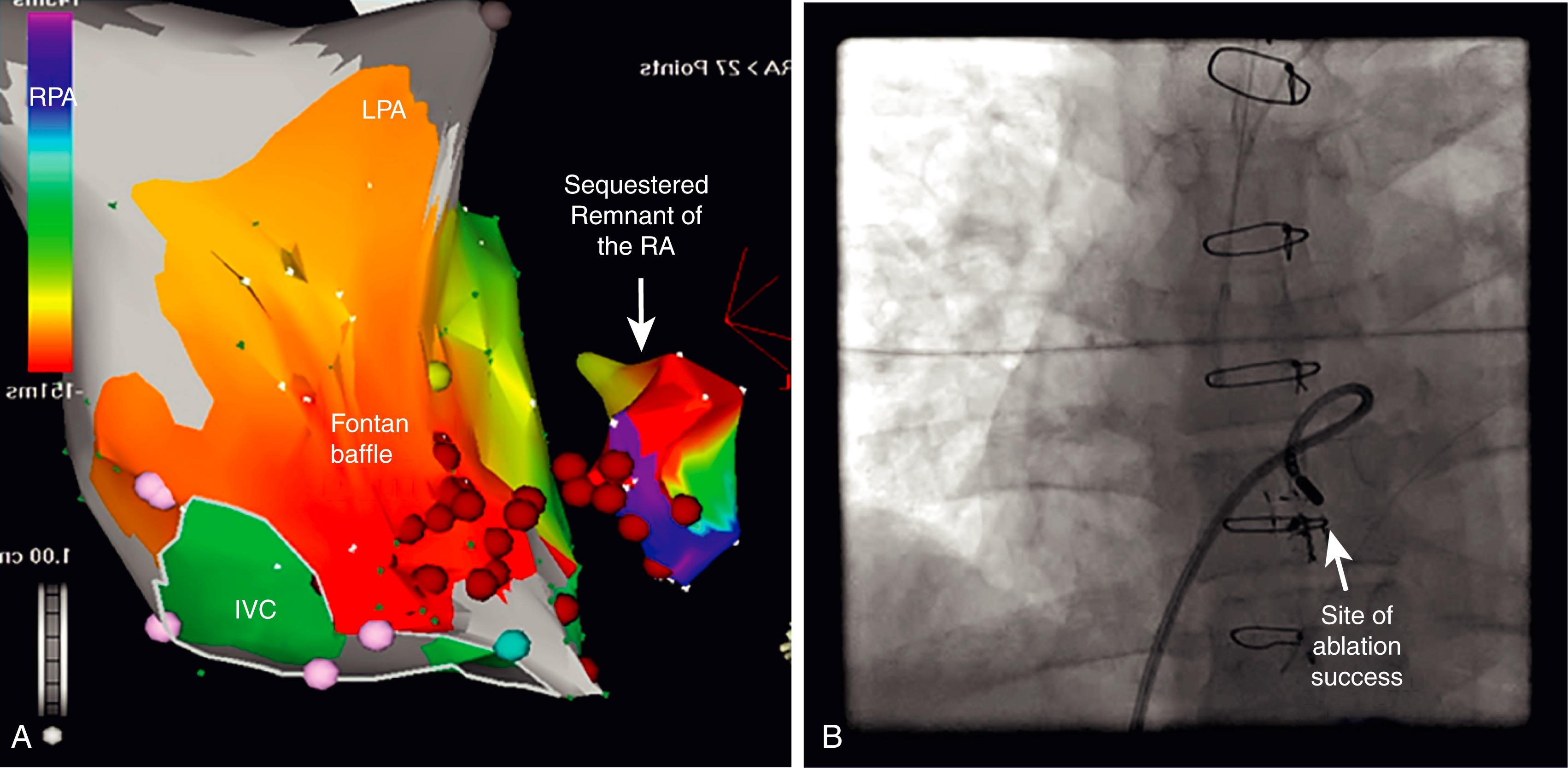
The distorted anatomy that accompanies complex CHD can also confound invasive procedures in both children and adults. It is important that the operator has a clear understanding of the specific defect and of the surgical patching and vascular redirection involved with its repair ( Fig. 114.3 ). High-resolution registration of anatomy with echocardiography, cardiac magnetic resonance imaging (MRI), angiography, and/or intracardiac echo is essential for all ablation procedures as well as device implantation in CHD patients.
An interesting branch of pediatric EP is management of fetal arrhythmias. Because a direct fetal ECG cannot be recorded, alternate methods must be used to decipher abnormal rhythms. Magnetocardiography is one such technique, but the equipment for this procedure is not widely available. A more practical approach is fetal echocardiography, where M-mode recordings of atrial and ventricular wall motion are used in conjunction with Doppler flow analysis ( Fig. 114.4 ) to provide a functional construct of rhythm status. The data obtained by this method are remarkably reliable.
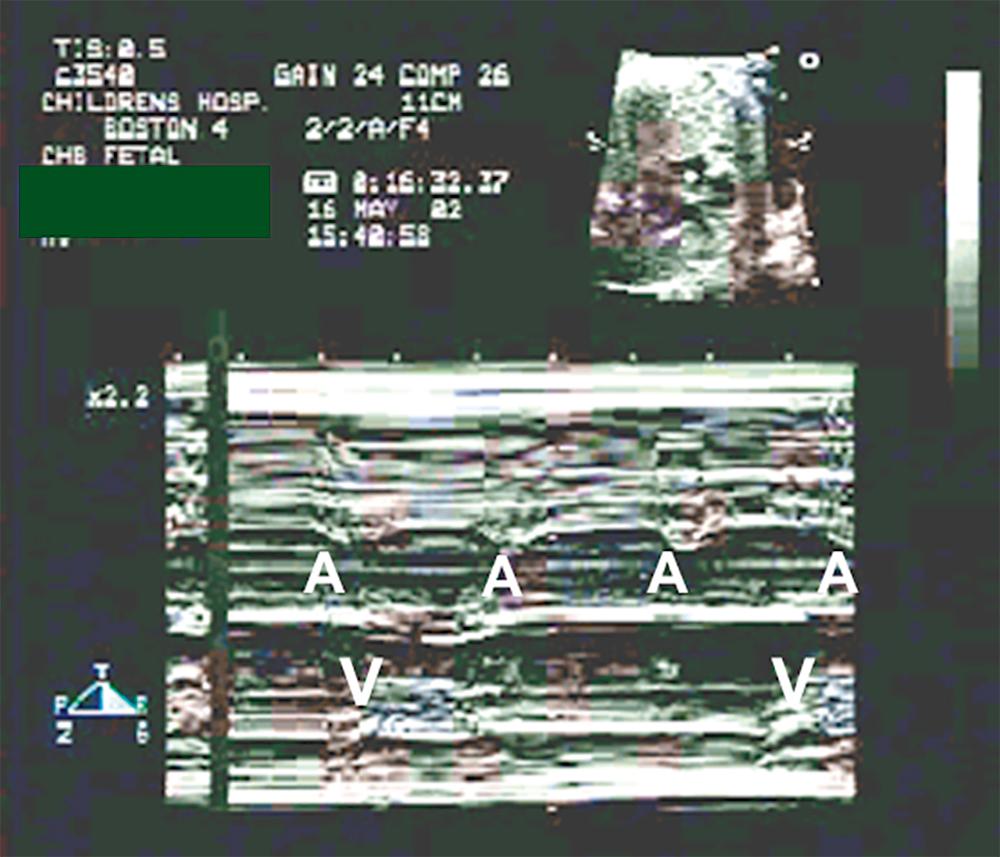
The most commonly detected fetal rhythm disturbance involves frequent atrial premature beats, which are generally benign and resolve before delivery, but when these ectopic beats occur in a bigeminal pattern (as they often do) and are sufficiently early to be blocked at the AV node (which they often are), they effectively reduce the fetal heart rate and can raise concern about a true AV conduction disturbance or fetal distress. Echocardiography will clarify the mechanism of the altered rate.
Fetal SVT is a more serious issue. The most common mechanisms are AP reentry and atrial flutter (AFL), either of which can become incessant in utero and result in cardiac decompensation with fetal hydrops, culminating in some unfortunate cases with fetal demise. Treatment can be difficult, but effective control can be achieved in many instances by passive transplacental transfer of antiarrhythmic drugs administered to the mother. Digoxin, flecainide, and sotalol are the most commonly used agents for this purpose. If the SVT cannot be controlled and the fetus remains compromised, early delivery is the best remaining option if the fetal lungs have matured sufficiently to tolerate extrauterine life.
Complete heart block in the fetus is another complex problem. As will be discussed later in this chapter, exposure of the developing heart to autoantibodies from mothers with systemic lupus erythematosus (SLE) or Sjögren syndrome can result in permanent heart block beginning at about 20 weeks of gestation. Escape rhythms in the range of 50 to 80 beats/min typically ensue and are usually sufficient to support the fetus and allow a carefully monitored pregnancy to come to term, but there are exceptions when decompensation may occur. Fetal heart block associated with CHD (heterotaxy, L-TGA, and AV canal defects as mentioned earlier) tends to be even less well tolerated. Whenever a fetus becomes compromised by heart block of any etiology, the most expeditious response is early delivery and pacemaker implant, but this again assumes the fetal lungs have reached a viable state of maturity. Successful in utero pacing of a human fetus has yet to be perfected, but promising prototype devices are now being developed.
Some form of supraventricular tachycardia (SVT) accounts for the majority of pediatric arrhythmias. Among patients with structurally normal hearts, the SVT mechanism in more than half the cases involves an AP, roughly 20% are because of AV nodal reentry, 15% because of automatic ectopic atrial foci, and the remainder because of an assortment of chaotic atrial rhythm, automatic junctional tachycardia, AFL, and atrial fibrillation (AF). The profile is somewhat different for patients with CHD, in whom AFL and other macroreentrant atrial circuits predominate. This section will not reiterate the detailed EP of these disorders, which are addressed separately elsewhere in this textbook (see Chapter 115 ) but will instead focus on the clinical presentation, natural history, and treatment approach specific to the pediatric population.
Tachycardias involving an AP are the most common arrhythmias seen in young patients and account for the largest percentage of ablation procedures performed at pediatric centers. Tachycardia events may begin as early as fetal life or any time thereafter, but the frequency peaks during the first year of life and again during early adolescence. The functional properties of the AP determine the natural history and the management approach. A large multicenter series of young patients undergoing ablation indicated that pathways with bidirectional conduction (manifest Wolff-Parkinson-White syndrome [WPW]) are present in 59% of pediatric patients undergoing ablation, whereas APs with unidirectional retrograde conduction (concealed APs) are identified in 37%; the remaining 4% have slowly conducting concealed APs with nearly incessant tachycardia (PJRT). Pathways with unidirectional anterograde conduction (conventional AV connections and atriofascicular pathways) can also be seen in pediatric patients, but their incidence is quite low. Approximately 10% of all pediatric patients undergoing ablation will be found to have multiple APs. This occurs more commonly among those with underlying heart disease (e.g., Ebstein anomaly or cardiomyopathy) than those with structurally normal hearts.
The initial presentation for pediatric patients with manifest WPW typically involves sustained orthodromic reciprocating tachycardia (ORT). Those who are old enough to verbalize symptoms will usually be brought to medical attention promptly for conversion, according to standard pediatric guidelines, with an agent such as adenosine. Unfortunately, infants and young children who are preverbal may escape early detection and remain in ORT for hours or perhaps days, resulting in low cardiac output at presentation that may necessitate emergent electrical cardioversion or transesophageal overdrive pacing to restore sinus rhythm. Apart from the occasional case involving an atriofascicular pathway, antidromic reciprocating tachycardia is unusual as a presenting arrhythmia in pediatric patients with true WPW but can be encountered as an induced rhythm during EP study in those with multiple APs. Preexcited AF with rapid anterograde conduction over the AP is clearly the most concerning presentation for WPW at any age. AF is exceedingly rare in infants and toddlers with WPW but is certainly well documented in school-aged children and adolescents. Acute treatment options for preexcited AF in young patients include electrical cardioversion or IV procainamide.
Recommended management for young patients with WPW after acute stabilization varies according to age. The infant age group is always managed medically unless there are extenuating circumstances (e.g., impending surgery for CHD or drug-refractory tachycardia) that justify a higher than average risk ablation attempt. Oral maintenance drugs can include flecainide, sotalol, amiodarone, and sometimes β-blockers. β-Blockers are avoided in all older children with WPW for fear that they might enhance AP conduction during AF, but the rarity of AF in infants, coupled with the potential toxicity of more potent antiarrhythmic agents in this age group, is reasonable justification for considering the exception at very young ages. Older children with symptomatic WPW are recommended to undergo catheter ablation as soon as the clinician judges the risk of an invasive procedure to be lower than the risks of continued medical therapy and/or risk of potential break-through episodes of SVT. This calculation is patient-specific and institution-specific, taking into account age/size, severity of symptoms, efficacy and potential side effects of drugs in use, likely AP location based on the delta wave pattern on the ECG, and center experience. All these considerations were reviewed as part of an expert consensus document addressing ablation in children from the Heart Rhythm Society (HRS). Multicenter analysis of acute outcome data for WPW ablation in young patients indicates an encouraging acute success rate of over 95%.
Although the risk for sudden cardiac death (SCD) in all large series examining children and adolescents with WPW is numerically low, it is clear that SCD can be a sentinel event in the younger age group. For this reason, when a WPW pattern is detected incidentally on ECG in an asymptomatic child and the risk for rapid conduction of preexcited AF is uncertain, it is recommended that some attempt be made to evaluate the anterograde conduction potential of the AP with either exercise testing, Holter monitoring, transesophageal pacing, or invasive EP study. A 2012 consensus document from the Pediatric and Congenital Electrophysiology Society considered persistence of the delta wave at maximum exercise heart rates, measurements of short AP refractory periods (<220–250 ms), induction of ORT, presence of multiple pathways, and short preexcited RR intervals during AF (<220–250 ms) to be indications for elective AP ablation in asymptomatic children with a WPW pattern after careful discussion with the family, whereas asymptomatic patients with only intermittent preexcitation and/or abrupt loss of the delta wave in response to sinus tachycardia during exercise testing might be followed conservatively. More recent pediatric data, however, have raised concerns regarding the predictive accuracy of noninvasive risk assessment. Poor concordance has now been demonstrated between intermittent preexcitation and data obtained by invasive EP testing in young patients. Moreover, a recent multicenter study of children with WPW who suffered life-threatening events found that nearly 25% of the cases failed to meet the generally accepted criteria for “high-risk.” Data such as these have resulted in a slightly more aggressive approach to ablation in asymptomatic young patients discovered to have a WPW pattern on electrocardiogram, although careful consideration must still be given to age, anticipated pathway location, family attitude, and institutional experience before any final decision on the matter.
Management of concealed APs in pediatric patients is more straightforward because the risk for preexcited AF is absent. Acute stabilization of ORT because of a concealed AP is similar to that described for WPW syndrome, but there are more options for long-term management, including periodic use of vagal maneuvers (if episodes are infrequent and well tolerated), and additional drugs such as β-blockers or (assuming age >1 year) calcium channel blockers. The majority of patients with significant symptoms will usually undergo elective ablation at some point, but as long as episodes can be managed with simple vagal maneuvers or relatively benign medications, this is deferred at most centers until the patient has grown to a body weight of 25 kg or more.
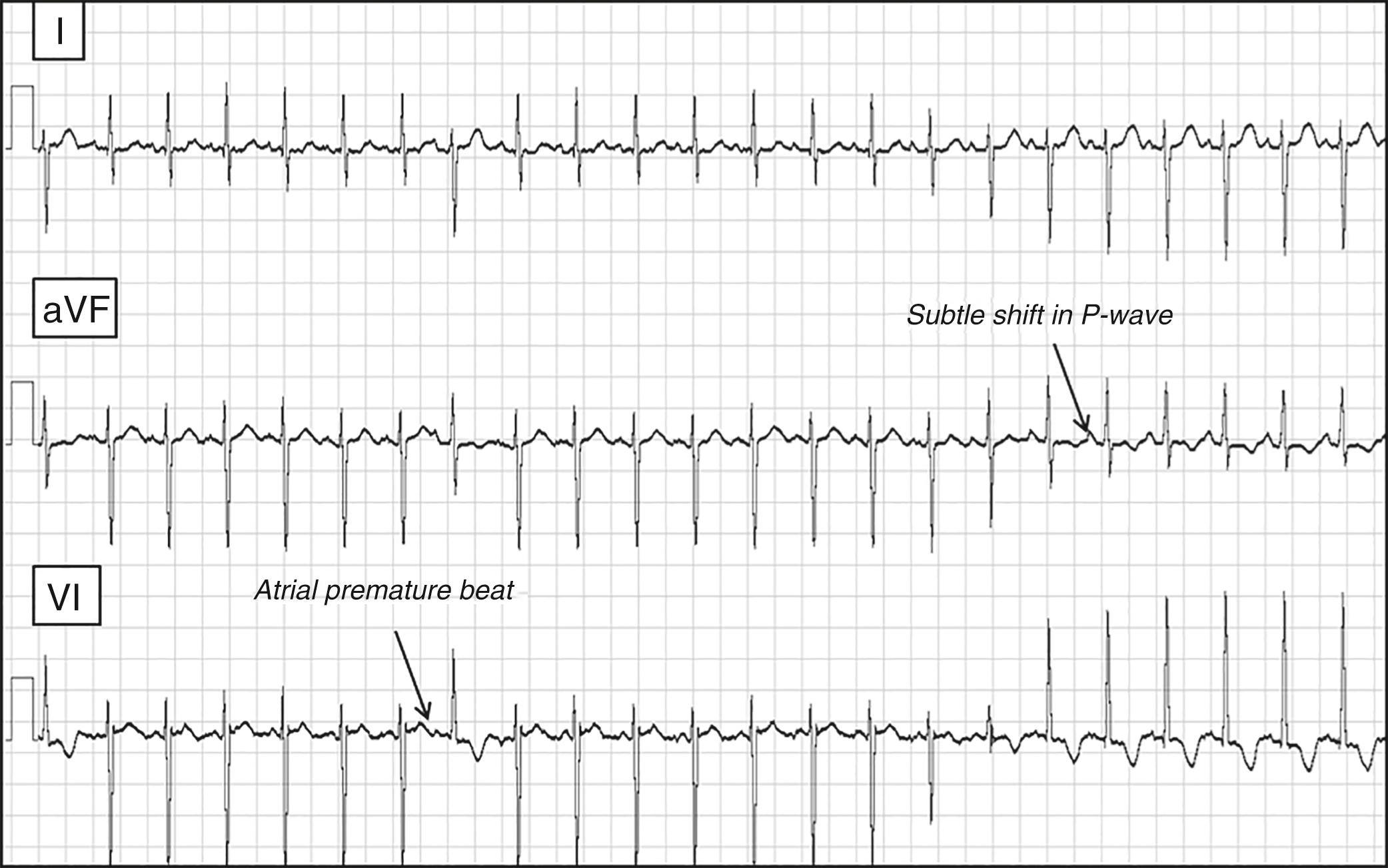
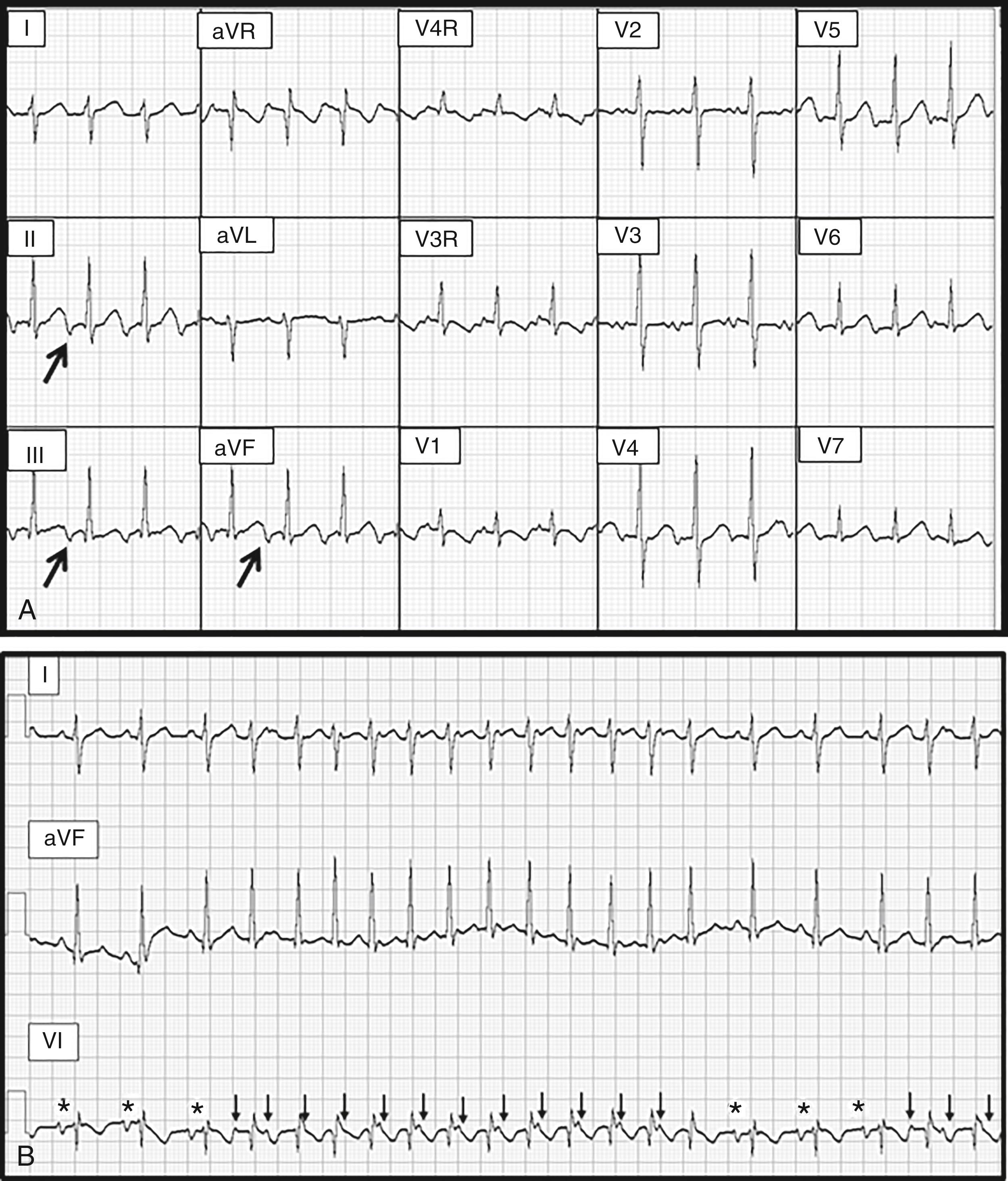
An important subclass of concealed AP is the slowly conducting variety that can result in PJRT. These pathways are usually located in the posteroseptal area and have remarkably long conduction times with decremental properties that can result in nearly incessant ORT ( Fig. 114.5 ). Acute therapy with adenosine will terminate reentry, but ORT will resume promptly after only a beat or two of sinus rhythm. Flecainide is one of the few drugs with proven efficacy in this setting. The pattern and age of presentation for PJRT is variable because rates are relatively slow compared with conventional ORT and may not cause symptoms until tachycardia-induced myopathy develops. , If detected early when ventricular function is still well preserved, a trial of flecainide can be considered as a temporizing measure until the patient is judged to be old enough to undergo elective catheter ablation. Once cardiomyopathy has developed, however, urgent catheter ablation is the most reasonable course of action regardless of age.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here