Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The definition of inborn errors of metabolism includes not only enzyme deficiencies but also any condition in which perturbation of a biochemical pathway is intrinsic to the pathobiology of the disorder. Inborn errors of metabolism can be classified as disorders that result in the accumulation or deficiency of small molecules, thereby leading to intoxication disorders; the abnormal accumulation or deficiency of complex molecules such as glycogen, sphingolipids, or glycosaminoglycans; and disorders of energy deficiency. Inborn errors of metabolism can be the result of defects in membrane transporter proteins, chaperone proteins, transcription factors, assembly factors, or other classes of proteins and nucleic acids. The more than 1400 inborn errors of metabolism include autosomal recessive, autosomal dominant, X-linked, and matrilineal (mitochondrial DNA [mtDNA]) inheritance.
The clinical phenotypes associated with inborn errors of metabolism can occur across all ages, with the more severe disorders having an onset in infancy or in utero. Clinically, a disorder can be episodic and fluctuating, stationary, or inexorably progressive, such as in neurodegenerative diseases, depending on organ system involvement. Disorders of intermediary metabolism, such as aminoacidopathies, often exhibit an acute or chronic intoxication and encephalopathy, whereas disorders of complex molecules, such as glycosaminoglycans or organelles such as peroxisomes, have a more chronic and progressive course, depending on the specific organ system that is involved. Disorders of energy production, such as mitochondrial respiratory chain disorders, may have both acute and chronic manifestations.
Inborn errors of metabolism are individually rare but collectively have an incidence as high as 1 per 800. The specific incidence of any given disorder depends in part on the severity of the condition, the presence of founder mutations in a particular population (e.g., Old Order Amish), and the degree of consanguinity in the population (e.g., Saudi Arabia). Although some inborn errors of metabolism are relatively more common ( Table 189-1 ) across diverse populations, genetic counseling and carrier screening should be tailored to different populations, such as small and/or consanguineous populations, when the frequency of disease alleles is known. In developed countries where consanguinity is rare, most affected individuals are compound heterozygous, and the clinical phenotype is generally determined by the milder allele.
| DISORDER | FUNCTIONAL DEFECT | INHERITANCE | CLINICAL PHENOTYPE |
|---|---|---|---|
| Argininemia | Arginase | Autosomal recessive | Hyperammonemia, fibrotic liver disease, progressive spasticity |
| Argininosuccinic aciduria | Argininosuccinate lyase | Autosomal recessive | Hyperammonemia, liver cirrhosis, hypertension, hypokalemia |
| Nonketotic hyperglycinemia | Glycine cleavage enzyme | Autosomal recessive (multiple loci) |
Glycine encephalopathy, seizures, apnea, severe intellectual disability |
| Maple syrup urine disease | Branched-chain α-keto acid dehydrogenase complex | Autosomal recessive (multiple loci) |
Encephalopathy, ataxia, brain herniation |
| Ornithine transcarbamylase deficiency | Ornithine transcarbamylase | X-linked | Severe hyperammonemia, occasional liver failure in females |
| Phenylketonuria | Phenylalanine hydroxylase | Autosomal recessive | Intellectual disability, hypopigmentation, seizures |
| Tyrosinemia type I | Fumaryl acetoacetate hydrolase | Autosomal recessive | Acute or chronic liver failure, tubulopathy, liver cancer |
| Tyrosinemia type II | Tyrosine aminotransferase | Autosomal recessive | Corneal lesions, hyperkeratosis of the skin, mild intellectual disability |
| Lysinuric protein intolerance | Lysine, ornithine, arginine transporter SLC7A7 | Autosomal recessive | Hyperammonemia, anemia, osteoporosis, lung disease, growth failure |
| Glutaric acidemia type I | Glutaryl-CoA dehydrogenase | Autosomal recessive | Macrocephaly, dystonia, seizures, developmental delay |
| Methylmalonic aciduria | Methylmalonyl-CoA mutase, defects in vitamin B 12 metabolism | Autosomal recessive (multiple loci) |
Hyperammonemia, metabolic acidosis, renal failure, pancreatitis, optic atrophy, and neurologic abnormalities |
| Propionic aciduria | Propionyl-CoA carboxylase | Autosomal recessive (two loci) |
Neonatal metabolic acidosis and hyperammonemia, developmental delay, dystonia, autism, cardiomyopathy |
| Fabry disease | α-Galactosidase | X-linked | Neuropathy, paresthesia, cardiomyopathy, renal failure, angiokeratoma, anhidrosis |
| Gaucher disease | Glucocerebrosidase | Autosomal recessive | Hepatosplenomegaly, anemia, thrombocytopenia, osteoporosis, fractures, neuropathy |
| Wilson disease | ATP7B copper transporter | Autosomal recessive | Liver failure, neurologic and psychiatric features |
| G6PD deficiency | Glucose-6-phosphate dehydrogenase | X-linked | Acute or chronic hemolytic anemia, drug-induced hemolytic anemia |
| Familial hypercholesterolemia | LDL receptor | Autosomal dominant and recessive |
Elevated LDL cholesterol levels, premature or early onset coronary heart disease, tendon xanthomas |
Understanding of the genetic architecture of human populations is incomplete but growing due to the development of low-cost DNA sequencing and other technologies. Databases such as gnomAD ( https://gnomad.broadinstitute.org/ ) that aggregate exome (the protein coding regions of the genome) and, increasingly, genome sequences obtained from hundreds of thousands of individuals from diverse populations provide valuable information on polymorphic DNA variants in humans, whether single nucleotide variants, insertion/deletion (indels) variants, or copy number variants. Most of these variants are benign, but they may be low-impact disease variants for common diseases or more rare, highly penetrant monogenic alleles. The genetic interactions of these variants affect many different biologic processes commonly referred to as the proteome, transcriptome, methylome, and other “-omics”. This complexity and the effects on the mechanisms of disease are increasingly understood because of advances in protein, lipid, and carbohydrate analytic techniques, single-cell RNA and DNA sequencing technologies, computational biology, and studies in model organisms. Epigenetic methylation of DNA and histones as well as acetylation or other covalent modifications also can lead to epigenetic disorders that typically are syndromic in nature, with effects on growth, organogenesis, cognition, and the risk of cancer. Finally, copy number variants and chromosomal rearrangements are much more frequent than de novo single nucleotide variants ( Chapter 32 ), with potential effects on gene regulation or expression over considerable chromatin distances. For example, copy number variants of cytochrome P450 enzymes in diverse populations influence rates of xenobiotic elimination.
Inborn errors of metabolism are generally monogenic disorders, with the majority exhibiting autosomal recessive inheritance due to complete or partial loss of function. However, autosomal dominant conditions can occur, either due to haploinsufficiency, dominant negative alleles, or occasionally overactive enzymes. Both recessive and dominant inheritance can be observed at a single locus, often with distinct or overlapping phenotypes. For X-linked disorders, the pattern of X inactivation can have an impact on the severity of disease, as is seen with the clinical variability of ornithine transcarbamoylase deficiency in females. Postulated genetic modifiers may explain intrafamilial variability, but identifying these modifiers has been challenging. Certain disorders, such as Tay-Sachs disease (caused by mutations in HEXA ), appear not to be influenced by environmental modifiers, whereas other conditions (e.g., glucose 6 phosphate dehydrogenase deficiency [ Chapter 147 ] or hemochromatosis [ Chapter 196 ]) are heavily influenced by diet or other environmental effects. Disorders of mitochondrial DNA can either be mendelian in inheritance or matrilineal if they involve one or more of the 37 genes encoded in mitochondrial DNA. In the latter case, the proportion of mutated mitochondrial molecules (termed heteroplasmy) and the distribution across tissues are major determinants of the severity of disease. In general, the mendelian forms of disorders of mitochondrial DNA occur more commonly in infancy or childhood, with primary disorders such as MELAS syndrome (mitochondrial encephalopathy, lactic acidosis, strokelike episodes; commonly due to c.3243A>G) occurring in older children and adults. Late-onset forms of childhood disease typically occur due to milder alleles (hypomorphs), an example being Pompe disease (acid glucosidase deficiency; Chapter 191 ), in which the classic picture is one of an infantile onset of a lethal cardiomyopathy and hypotonia, in contrast to a slowly progressive cardiomyopathy and respiratory failure in adults in whom the correct diagnosis may be missed. Similarly, propionic acidemia is typically detected by newborn screening and is symptomatic with hyperammonemia and refractory acidosis in infancy, but mild forms of the disease may manifest as isolated heart failure in older adults. Glycogen storage disorder type IV (glycogen branching enzyme deficiency, GBE1 ; Chapter 191 ) may be severe enough to cause lethal hydrops fetalis or more mild cases and cause the adult-onset neurologic disorder polyglucosan body disease, depending on the residual enzyme activity.
A number of pathophysiologic mechanisms ( Table 189-2 ) may act individually or in combination to cause symptoms, and the organ system involvement can be cell type–specific or may affect multiple cell types and organs, either due to shared function or because of circulating toxic metabolites. The accumulation of enzyme substrates may lead to toxicity or to the increase of a toxic byproduct; alternatively, the loss of the enzyme reaction product may cause disease. Similarly, deficiencies of vitamin cofactors (e.g., vitamin B 12 , which is required for the metabolism of homocysteine [ Chapter 193 ] and methylmalonic acid) or allosteric regulators (e.g., N-acetylglutamate, needed to activate carbamoylphosphate synthetase of the urea cycle) may also mimic an enzyme deficiency.
| MECHANISM | DISORDER |
|---|---|
| Accumulation of toxic substrates through primary blockage of catabolic pathway | Organic acidurias (MMA, PA, glutaric aciduria type I) MSUD, tyrosinemia type 1 |
| Accumulation of nontoxic macromolecules through blockage of catabolic pathways | Lysosomal storage disorders (MPS, Pompe disease) |
| Energy failure through primary blockage of pathway relevant for ATP synthesis | Fatty acid oxidation defects Glycogen storage disorders Respiratory chain enzyme deficiencies |
| Impairment of post-translational glycosylation | Congenital disorders of glycosylation |
| Deficiency of end product through primary blockage of anabolic pathway | Albinism, orotic aciduria, disorders of serine synthesis, creatine deficiency |
| Lack of detoxification through primary blockage of catabolic pathway | Urea cycle defects |
| Enzyme overactivity leading to substrate depletion and/or excess product | Glutamate dehydrogenase overactivity; hyperinsulinism and hyperammonemia, PRPP synthetase overactivity; gout |
Locus heterogeneity, by which more than one gene can cause the same clinical phenotype, can also be a feature of inborn errors of metabolism. For example, defects in the synthesis of tetrahydrobiopterin (BH4) lead to elevated levels of phenylalanine, as does a defect in the chaperone protein DNAJC12 or in phenylalanine hydroxylase. Defects in catabolic pathways, such as amino acid degradation, may be more readily detected because of the accumulation of substrates, but disorders in the synthesis of metabolites, such as defects in serine biosynthesis, are increasingly recognized.
The clinical features and natural history of inborn errors of metabolism are highly diverse owing to the large number of entities and varying degrees of protein dysfunction. Neurologic manifestations, which are the most common feature, include neurodevelopmental delay, encephalopathy, seizures, neurodegeneration, and peripheral neuropathy. Psychiatric manifestations are also observed in over 100 inborn errors of metabolism. However, essentially any organ system may be involved ( Table 189-3 ). Disorders of intermediary metabolism can broadly affect acid/base balance, the excretion of nitrogen, and glucose homeostasis, or they can cause isolated neurometabolic conditions, such as glutaric aciduria type I. A subset of disorders also affects fetal development, thereby leading to a variety of birth defects such as congenital heart disease, brain malformations, limb defects, and genitourinary anomalies.
| ORGAN | CLINICAL SIGN | DISORDER |
|---|---|---|
| Eye (cornea) | Corneal clouding, corneal dystrophy | MPS (Hurler, Maroteaux-Lamy, Sly disease), Fabry disease |
| Skeletal system | Ochronosis, black urine | Alkaptonuria |
| Connective tissue | Carpal tunnel syndrome, contractures | MPS I, II, VI, and VII |
| Central nervous system | Ataxia, intellectual disability, seizures, peripheral neuropathy | Respiratory chain enzyme deficiency, congenital disorders of glycosylation |
| Heart | Cardiomyopathy | Fabry disease, long chain fatty acid oxidation disorders, glycogen storage disease |
| Lung | Interstitial lung disease, obstructive or restrictive disease | Gaucher disease, MPS I, II, VI |
| Muscle | Hypotonia, rhabdomyolysis | Infantile Pompe disease, respiratory chain enzyme deficiency, long chain fatty acid oxidation disorders |
| Liver | Hepatomegaly/splenomegaly, fibrosis/cirrhosis | MPS I, II, VI, and VII; GSD I, III, IV, IX; Gaucher disease, Niemann-Pick disease, transaldolase deficiency |
| Kidney | Renal insufficiency, proteinuria | Cystinosis, Fabry disease, MMA |
| Skin | Angiokeratomata, hypopigmentation | Fabry disease, fucosidosis, phenylketonuria, tyrosinase deficiency |
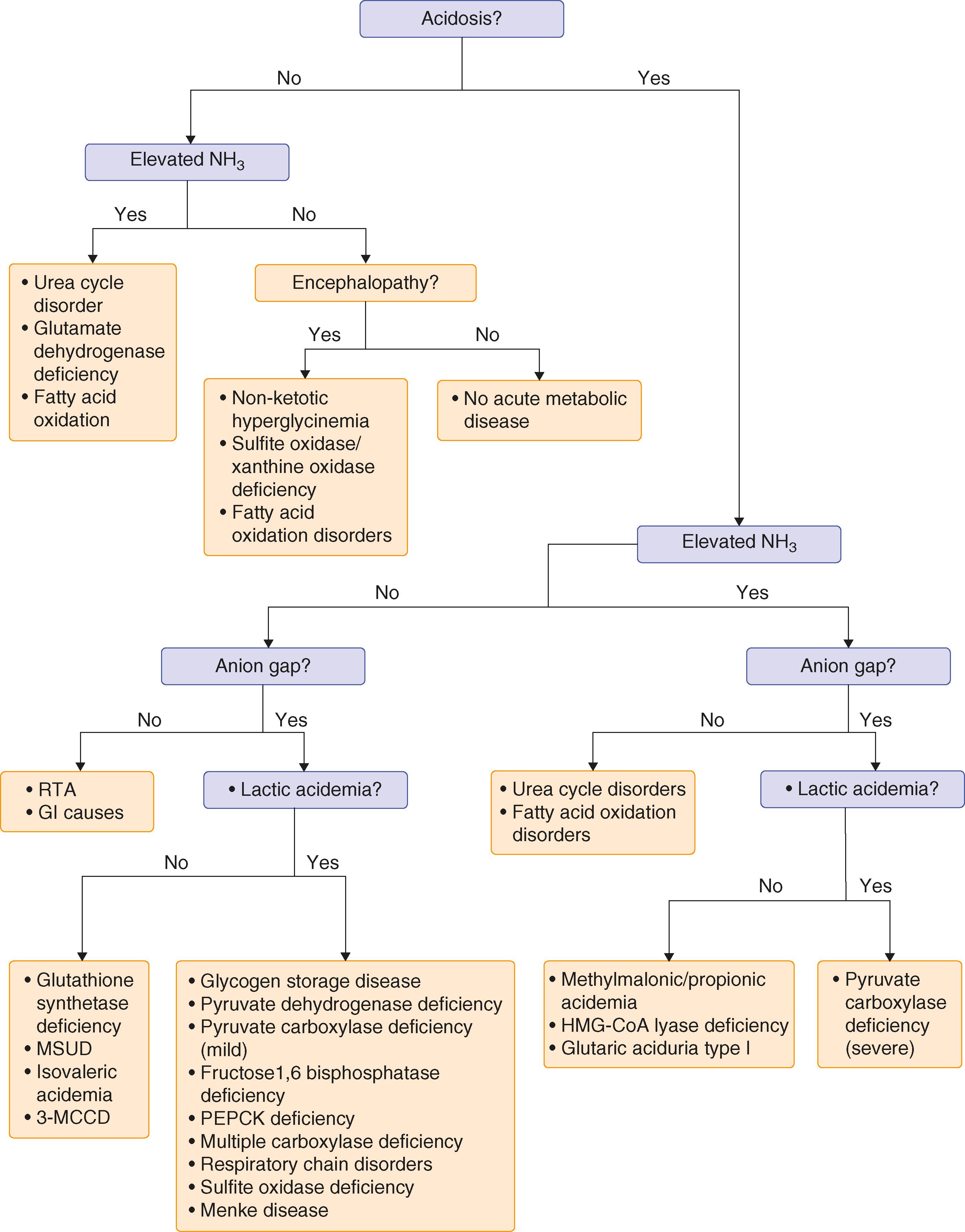
Most inborn errors of metabolism can be diagnosed by biochemical analysis of small molecules such as metabolites, peptides, and hormones in various body fluids, including serum or plasma, urine, or cerebrospinal fluid (CSF) ( Fig. 189-1 ).
DNA sequencing has largely replaced tissue biopsies for histology, histochemistry, or enzymatic analysis. Untargeted analyses of hundreds of metabolites can be performed on single samples of blood, urine, or CSF using multiple analytic platforms—an approach commonly referred to as metabolomics. This approach has been shown to improve diagnostic rates when compared with more traditional analytic testing, in part by identifying rare diagnostic compounds not typically detected by prior analytic approaches. , This technology is also useful in conjunction with DNA-based exome or genome analysis to provide metabolic confirmation of novel variants in known disease genes.
DNA-based molecular confirmation ( Chapters 32 and 33 ) is warranted to provide genotype-phenotype information that is useful in clinical prognostication and treatment, genetic counseling, identification of other potentially affected family members, carrier screening, and prenatal or preimplantation diagnosis. Next-generation sequencing is performed as part of diagnostic gene panels, exome sequencing, or genome sequencing. Gene panels have the advantage of avoiding variants in genes unrelated to the clinical phenotype and are typically less expensive. Exome and genome sequencing cast a large net and are developing into first-line testing. Ideally, a “trio” (parents and patient) should be subject to testing due to the observation that in about 50% of solved cases, the variant in the disease gene is a de novo change not seen in the parents. Examining parents also allows for the “phasing” of two variants in the patient, that is, determining if any two variants in a gene are on the same or opposite chromosomes. Exome sequencing in adults leads to a lower but still significant diagnostic rate compared to pediatric cases, and reanalysis of exome or genome data yields additional diagnoses as more disease genes are identified.
In some settings, however, biochemical analyses may be followed by testing for enzyme activities in tissues or specific cells such as dried whole blood, lymphocytes, leukocytes, fibroblasts, or liver or muscle tissue. Selected metabolic tests used for the diagnosis of inborn errors of metabolism include analysis of amino acids in plasma, urine, and CSF; organic acids in urine and occasionally plasma; specific acylcarnitine species in plasma and dried blood spots; analysis of total and free carnitine in plasma and urine; succinylacetone (a diagnostic metabolite observed in tyrosinemia type I) in dried blood spots and urine; purines and pyrimidines in plasma and urine; sterols and bile acids in plasma and urine; and the pyrimidine intermediate orotic acid in urine.
Strategies for treating inborn errors of metabolism ( Table 189-4 ) include: (1) dietary interventions to reduce the deleterious substrate, (2) administration of vitamin cofactors to enhance residual enzyme activity, (3) provision of a missing cofactor, (4) chaperone therapy to stabilize an unfolded or mistargeted protein, (5) direct enzyme replacement therapy, (6) inhibiting an upstream enzyme to reduce a substrate, (7) small molecule inhibition of a toxic metabolite, (8) replacement of an affected organ, (9) stem cell transplantation, (10) “read-through” of premature termination codons, (11) gene replacement therapy, or genetic editing. For example, in patients with primary hyperoxaluria (characterized by oxalosis, nephrocalcinosis, nephrolithiasis, and kidney failure), lumasiran, which reduces oxalate formation by blocking glycolate oxidase, can lead to normal or near-normal oxalate levels. Other therapeutic approaches, including modified mRNA therapies and gene correction strategies (e.g., CRISPR), are currently in clinical trials.
| LEVEL | THERAPEUTIC APPROACH | DISORDER |
|---|---|---|
| Gene | Solid organ transplantation | Urea cycle defects, maple syrup urine disease, tyrosinemia type I |
| Stem cell transplantation | Adrenoleukodystrophy, mucopolysaccharidosis I | |
| Gene therapy | Mucopolysaccharidoses I and II, VI, VII, phenylketonuria, urea cycle disorders | |
| Stop codon read-through therapy | Glycogen storage disease 1A | |
| Modified mRNA | Duchenne muscular dystrophy, cystic fibrosis, ornithine transcarbamylase deficiency, methylmalonic acidemia | |
| Enzyme | Recombinant enzyme replacement | Gaucher disease, Pompe disease, Fabry disease; mucopolysaccharidoses I, II, IVa, and VI, phenylketonuria |
| Chaperone | Fabry and Pompe diseases | |
| Substrate | Substrate reduction Substrate inhibition |
Phenylketonuria, maple syrup urine disease Gaucher disease, Tay-Sachs disease, Niemann-Pick type C |
| Substrate diversion | Urea cycle disorders |
A curative approach is preferred whenever possible, such as may be achieved with various transplantation or gene delivery strategies. In conditions with central nervous system manifestations, however, the blood-brain barrier may limit the effectiveness of larger molecules, such as enzymes, that are not directly administered to the brain parenchyma.
Unfortunately, curative interventions often are not possible or are potentially hazardous. Individuals with inborn errors of metabolism often require a multidisciplinary team of biochemical geneticists, internists, genetic counselors, and other subspecialists.
The therapeutic goal for nutritional therapy under expert guidance is to correct the metabolic imbalance by reducing the accumulation of substrate, promoting protein synthesis through anabolism, and preventing episodes of metabolic decompensation in conditions such as urea cycle disorders or organic acidurias. Appropriate medical foods are low in protein or have a specific composition of amino acids or fatty acids. In addition, supplementation of a deficient product may be needed, as in the case of most urea cycle disorders in which provision of citrulline or arginine is required.
Intravenous enzyme replacement therapy with glucocerebrosidase is the treatment for type I Gaucher disease ( Chapter 191 ). Enzyme replacement therapy ( Chapters 191 and 192 ) is also available for Pompe disease (α-glucosidase), mucopolysaccharidosis type I (α-iduronidase; Hurler/Scheie disease), mucopolysaccharidosis type II (α-iduronate sulfatase; Hunter disease), mucopolysaccharidosis type IVa (galactosamine-6-sulfatase; Morquio disease), mucopolysaccharidosis type VI (arylsulfatase B; Maroteaux-Lamy disease), and Fabry disease (α-galactosidase). Enzyme therapies also exist for late infantile neuronal ceroid lipofuscinosis type 2 (CLN2) caused by deficiency of tripeptidyl peptidase 1 (administered directly into the brain) and phenylketonuria (a recombinant phenylalanine ammonia lyase that is pegylated to improve its stability and administered daily as a subcutaneous injection). A variety of other enzyme products are in different phases of clinical trials.
Another approach is to reduce the accumulation of toxic substrate by inhibiting the reverse enzyme reaction that leads to its synthesis. This strategy can reduce glucosylceramide levels in Gaucher disease type I ( Chapter 191 ).
Enzyme therapy can also use small molecules to increase endogenous enzymatic activity. For example, individuals with tetrahydrobiopterin-responsive phenylketonuria have improved tolerance to phenylalanine and lower blood and tissue levels of phenylalanine when they take pharmacologic doses of tetrahydrobiopterin.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here