Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Radiation therapy relies on delivering energy to tissues, which ultimately disrupts cellular function through DNA damage.
The physics of high-energy photons or charged particles determine the delivery profile of energy and its biologic effects.
Historically developed by neurosurgeons, radiosurgery is the use of precise stereotactic techniques to deliver high-dose radiation in a single or few fractions.
Many radiosurgery and radiation therapy delivery systems are available today with no clear evidence of superiority of one modality over another.
Radiation therapy and, in particular, radiosurgery are critical components of the neurosurgeon's armamentarium. In general terms, radiation therapy is the use of high-energy photons or charged particles to induce specific biologic changes for the treatment of various pathologic entities including tumors and vascular lesions. Radiosurgery refers to the use of stereotactic localization techniques to deliver high doses of energy in one to five sessions (or fractions). Here we review the physics and biology that underpin modern radiation therapy and radiosurgery. From that foundation, we explore the basics of clinical radiation oncology, the history and development of radiosurgery, and finally, modern delivery mechanisms most relevant to the neurosurgeon.
Physics is an integral part of radiation therapy. German physicist Wilhelm Röntgen discovered x-rays in 1895, and within months they were being employed to treat a patient with breast cancer. Radiation therapy takes advantage of the ionizing radiation portion of the electromagnetic spectrum. The term electromagnetic radiation is used to describe perpendicular sinusoidal electric and magnetic waves carrying energy. Ionizing radiation by definition contains enough energy to excite electrons from their atoms, thus creating ions and reactive species that can damage cells. The energy is generated from a source external to the patient and passes through a portion of normal tissue to reach its intended target. Clinical radiation dose is measured in gray (Gy), which represents the energy deposited in material per unit mass of the material. Maximizing the dose of radiation to the target and minimizing the dose to normal tissue is a central goal of any radiotherapy plan.
A photon is a particle that has no mass, travels at the speed of light, and carries the energy present in all electromagnetic radiation, including microwaves, visible light, ultraviolet light, and x-rays. Photons are the most common form of ionizing radiation used in radiotherapy. The energy of the photon influences the nature of the interaction between the photon and matter. In the kiloelectron volt (keV) energy range, which is used in most diagnostic x-ray units, photons interact with matter predominantly via the photoelectric effect . A photon interacts with a tightly bound electron, which absorbs most of the energy and is ejected from the atom; it is then free to interact with other atoms in the vicinity. This interaction is highly dependent on the atomic number of the material being irradiated. Diagnostic x-ray studies take advantage of this phenomenon. Bone, for example, is high in calcium (with an atomic number of 20) and is much more likely to interact with these photons than soft tissue, which is mostly carbon, hydrogen, and oxygen (with atomic numbers of 6, 1, and 8, respectively). This differential interaction is the basis of diagnostic x-ray imaging.
At higher energies, such as the energies of most therapeutic radiation, the Compton effect predominates. A high-energy photon in the megaelectron volt (MeV) range interacts with a loosely held orbital electron, which results in ejection of the electron from the atom and scattering of the photon. The scattered photon now has a change in its energy. Interestingly, this interaction is independent of the atomic number of the material being irradiated but is dependent on electron density. Thus images generated from therapeutic energy x-rays (ie, in the megaelectron volt range) are less useful for imaging than are diagnostic x-rays, as there is little contrast between bone and soft tissue; however, this energy range is useful in radiation therapy because it is highly penetrating and can interact equally with all tissues.
There are two main sources of high-energy photons used in radiation therapy. The first source is radioactive decay, a natural process that occurs in elements with unstable nuclei and results in the emission of energy as the nucleus cascades into a stable configuration. Cobalt-60 ( 60 Co), the most common element used in radiation therapy units, undergoes a process known as beta decay to become nickel-60, which results in the emission of a high-energy photon known as a gamma ray (γ-ray). For 60 Co, the average energy of the photons generated from radioactive decay is 1.25 MeV. Radiotherapy devices that take advantage of radioactive decay use a shielded radioactive source, and a small aperture or apertures result in shaping (known as collimation ) of the beam to the desired size and shape. This is the source of radiation used in devices such as the Gamma Knife (GK).
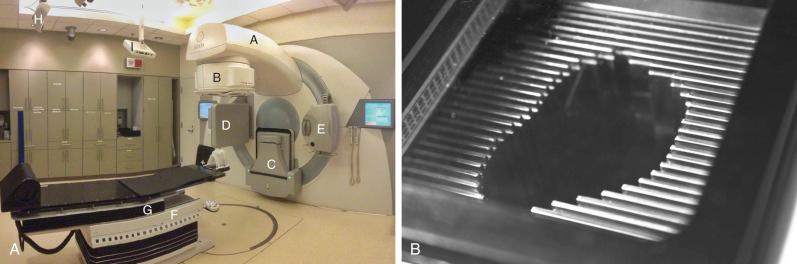
The other main source of high-energy photons is a linear accelerator, or LINAC, which uses microwaves to accelerate electrons to a high energy. These electrons are directed to collide with a high-atomic-number target , like tungsten; the resulting interactions between the target's atoms and the accelerated electrons result in the production of high-energy photons (x-rays) via a process known as bremsstrahlung , or “braking” radiation. As the electrons decelerate via their interaction with the atoms of the tungsten target, photons are produced via the bremsstrahlung phenomenon such that the law of conservation of energy is satisfied. The difference between x-rays and γ-rays is simply the site of origin: x-rays are produced by electron interactions but γ-rays are produced by nuclear decay. Unlike the γ-rays produced by 60 Co, the energy of x-rays generated in a LINAC is based on the characteristics of the machine, and the settings of the machine can be modified by the operator; most commercial units offer multiple energy options. These x-rays are produced in the arm and head of the unit (known as the gantry ) and can be shaped by a system of controllable leaves, known as a multileaf collimator (MLC), placed between the source of the x-rays and the patients ( Fig. 50.1 ).
The physical characteristics of photons determine their biologic effects. Photons deposit dose in a characteristic pattern, in which there is an initial buildup of deposited energy after entry into the patient, followed by an exponential decrease ( Fig. 50.2 ). Photons are indirectly ionizing radiation: They liberate directly ionizing radiation, electrons, which then move on and are responsible for actual deposit of energy in some vicinity of the original interaction site. The dose buildup region is due to the initial increase in the number of liberated electrons. This dose increase in counteracted by the dwindling number of primary photons in the beam, producing the dose decrease further into the tissue. The shape of this depth-dose curve is dependent on the energy of the photon involved. Higher energy photons exhibit greater skin-sparing effect, but they have a slower rate of attenuation as they pass through tissue and have a slightly larger penumbra due to higher-energy secondary electrons. In general, photons in the 1- to 6-MeV range are used for cerebral stereotactic radiosurgery (SRS). SRS is the delivery of a high dose of ionizing radiation to a target in a single or a few (usually <5) treatments.
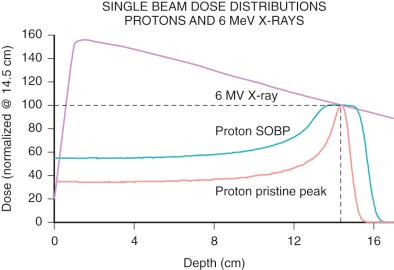
Charged particles have been used in radiotherapy over many decades, even though much less widespread than photons. Of the many ion species employed so far (protons, helium, and carbon, to name a few), protons are most widely adopted, with the number of centers offering proton therapy steadily increasing. A proton is the nucleus of a hydrogen atom. Protons are positively charged, subatomic particles of approximately one atomic mass unit. For radiotherapy purposes they are produced in an ion source by stripping electrons from molecular hydrogen gas, before being accelerated to therapeutic energies in a synchrotron or cyclotron. Charged particles are directly ionizing, meaning they deposit energy through collision with atomic electrons. As a particle travels through the medium it loses its energy in a myriad of these collisions and finally comes to a full stop. Because more energy is lost the slower the particle goes, a useful feature arises—a peak right at the end of the particle's travel (“Bragg peak”). The main advantages of protons over photons are the absence of dose beyond the target and the decrease in dose proximally (see Fig. 50.2 ). To cover lesions which extend in depth, multiple Bragg peaks, originating from protons with different initial energies, are superimposed to create a spread-out Bragg peak (SOBP). In opposition to the favorable dose characteristics of protons versus photons, there are two disadvantages: physical uncertainty and cost. Given the sharp dose fall-off, it is critical to be able to calculate and deliver proton dose precisely, which remains a continuing field of development with the goal of minimizing treatment margins. Second, in order to produce proton beams of therapeutic energies, relatively large accelerators are needed. To date, most facilities are the size of a football field, with multiple treatment rooms sharing one large accelerator. More compact technology is under development and has become available.
The primary mechanism of cell death from ionizing radiation is generally thought to be via DNA damage. Ionizing radiation results in the ejection of electrons from atoms in the irradiated tissue and subsequent formation of ions. Because cells are predominantly made of water, the photons or protons are most likely to interact with water molecules, resulting in the production of reactive oxygen species, such as superoxide and hydroxyl radicals. These reactive species damage DNA and result in replicative failure and cell death. It is for this reason that radiotherapy is believed to be more effective in the presence of oxygen and that hypoxic areas of tumors may be less sensitive to the effects of ionizing radiation.
Although many different types of DNA damage can result from ionizing radiation, the double-strand break is thought of as the most critical event. Double-strand breaks are difficult for cells to repair, and the repair process can generate aberrant chromosomes that result in mitotic catastrophe, or mutations that result in reduced replicative fitness. The importance of the double-strand break is evidenced by the fact that patients with defective mutations in the ataxia-telangiectasia ( ATM ) gene, one of the key sensors of DNA double-strand breaks and an integral part of double-strand break repair, are highly sensitive to ionizing radiation damage.
The production of double-strand breaks is related to the efficiency of ionizing radiation in transferring its energy to the tissue. This concept is quantified as the linear energy transfer (LET) of differing radiation modalities, and it results in differences in the relative biologic effectiveness (RBE) of different types of radiation. For example, photons produced by 60 Co are considered to have low LET and have an RBE of 1. However, the LET varies with both energy and with the type of particle used in irradiation. Neutrons, which are large, uncharged particles, have a very high LET and therefore cause more complex genetic damage, resulting in a higher RBE. Protons at therapeutic energies, although of similar mass to neutrons, do not exhibit a similarly high LET. Protons used in radiotherapy are assigned an RBE of 1.1 in clinical practice compared to photons produced by 60 Co. This means that for a given absorbed dose, protons will have a 10% greater biologic effect. To avoid confusion, proton therapy doses are typically reported as gray (relative biologic effectiveness) or Gy (RBE), which takes this correction factor into account.
Although DNA damage is known to be the primary mechanism of action of radiation, the cellular target of radiotherapy is more controversial. For malignant tumors, which are highly proliferative and often have impaired DNA repair, the target is thought to be the cancer cells themselves, as they struggle to repair the DNA damage inflicted by ionizing radiation. Furthermore, their rapid progression through the cell cycle results in more potential checkpoints that can trigger cell death. Thus cancer cells are more sensitive than normal cells to radiation. This effect is seen clinically, as radiation of malignant tissues often causes clinical or radiographic regression of the lesion, whereas adjacent normal tissue is preserved.
In contrast, with benign diseases, the cells are not as proliferative and may be in resistant phases of the cell cycle. These observations have led some to speculate that benign tumors are relatively radioresistant. However, in clinical practice, radiation appears to induce a quiescent state, which corresponds to radiographic and clinical stability, suggesting that benign tumors also respond to radiotherapy. It is possible that these tumors undergo DNA damage that limits their replication, but the biology of this process remains less clear.
Although tumor cells have been thought to be the primary target of radiotherapy, many have suggested that radiotherapy has an effect on vascular endothelium, which mediates the primary mode of cell death, especially at the higher doses used in SRS. Irradiation of B16 melanoma cell explants in mice with doses of 15 to 20 Gy resulted in waves of endothelial cell apoptosis 1 to 6 hours after irradiation. The endothelial response was critical to tumor control, as endothelial-specific mutation of Bax, a critical regulator of apoptosis, rendered these explants insensitive to doses of 15 Gy. Thus endothelial cell death may lead to direct hypoxic necrosis of the tumor or may secrete signaling molecules that result in tumor cell death. However, other studies have shown that at even higher radiation doses (>20 Gy), the mode of death in an irradiated gastrointestinal tract appears to become independent of endothelial cell apoptosis, suggesting that the dose-response relationship is complex and may be differentially regulated in different tissues. These differences are the subject of much investigation and may become more important as combination chemotherapy or targeted therapy is considered.
These observations correlate with data from radiosurgical experiments exposing normal rat brain or tumor explants to radiosurgical doses of ionizing radiation. For example, examination of human acoustic schwannoma xenografts after irradiation with 10 to 40 Gy showed a significant decrease in tumor vascularity, as well as significant intramural vascular hyalinization. Furthermore, irradiation of rat brains with 15 to 30 Gy resulted in changes in local blood flow, leukocyte/endothelial interaction, formation of aneurysmal structures, and thrombus formation. These data suggest that vascular changes by radiosurgery may be an important component of its clinical effect as evidenced in the treatment of arteriovenous malformations with a single high dose of radiation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here