Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter describes the surgical aspects of aortic valve disease, excluding congenital aortic stenosis in infants and children (see Chapter 47 ) and aortic regurgitation with either ventricular septal defect (see Chapter 35 ) or sinus of Valsalva aneurysm (see Chapter 36 ).
In 1947, Smithy and Parker at the University of South Carolina in Charleston first reported an experimental study of aortic valvotomy. During the early 1950s, Bailey and colleagues in Philadelphia used closed methods—either a dilator introduced transventricularly or a digital approach through a “poncho” sewn onto the ascending aorta—in clinical attempts to relieve severe aortic stenosis. Modest success in some patients was obtained by them and by Ellis and Kirklin.
In 1951, Hufnagel in Washington, D.C., developed a ball valve prosthesis for rapid insertion into the descending thoracic aorta. (From his work with Gross in developing the coarctation operation, Hufnagel was well aware of the risk of paraplegia with aortic clamping and therefore emphasized the rapidity of insertion of his device; see Chapter 24 .) The prosthesis could be inserted quickly because of two multipoint fixation rings, each placed around the aorta and over the end of the prosthesis lying within the aorta. Hufnagel and Harvey, Ellis and Kirklin, and others obtained fairly good palliation of severe aortic regurgitation in some patients with this device. However, upper body signs of aortic regurgitation became severe. During the early 1950s, Bailey and Likoff developed and used a number of ingenious but unsuccessful closed methods of overcoming aortic regurgitation.
A more effective approach to surgical treatment of aortic valve disease in adults began with the advent of clinical cardiopulmonary bypass in 1954 and 1955 (see Chapter 2 ). At first, aortic valvotomy and removal of calcific deposits were all that could be done. Then Bahnson and colleagues and, independently, Hufnagel and Conrad developed a single-leaflet prosthesis that was commercialized. Generally, the leaflets were used to partially replace the aortic valve, but three leaflets could be used together for total aortic valve replacement. Probably the first single-unit prosthesis for total aortic valve replacement was the polytetrafluroethylene (PTFE) sleeve prosthesis developed and first used by McGoon at the Mayo Clinic in 1961. Although this device was successful in terms of early results, competence was sometimes not achieved, leading to appreciable hospital mortality. Introduction of the ball valve prosthesis by Harken and colleagues and Starr and colleagues in 1960 and reported in 1963 established aortic valve surgery on a firm basis. Many types of prosthetic valves have subsequently appeared.
In 1956, Murray demonstrated that the aortic valve could be used as an allograft valve transplant in the descending thoracic aorta in patients with aortic regurgitation, and Kerwin and colleagues reported 6-year follow-up. The first orthotopic insertions of an allograft valve using the double-suture-line technique were performed in 1962 by Barratt-Boyes and separately by Ross using a single-suture-line technique described by Duran and Gunning. At first, cadaveric valves were collected aseptically and implanted within a few days or weeks, but for logistic reasons this technique was soon replaced by unsterile collection and sterilization by β-propiolactone, ethylene oxide, or irradiation. The allografts were then stored either in Hanks’ balanced salt solution at 4°C or frozen and dried. In 1968, because of high occurrence of cusp rupture with these techniques, antibiotic sterilization was introduced. Cryopreservation rather than wet preservation was introduced in 1975 by O’Brien and colleagues. Yacoub and colleagues and Ross and colleagues expanded the use of allografts to include combined aortic valve and ascending aorta replacement.
In 1967, Ross and colleagues introduced the pulmonary autograft for aortic valve replacement, after Lower and colleagues had shown the feasibility of the procedure experimentally in 1960. Subsequently, the pulmonary valve and trunk were introduced as autograft composite conduits (cylinders) for replacing the aortic valve and ascending aorta.
Other biological valves were introduced. Senning in Zurich replaced the aortic valve clinically with individual cusps made of the patients’ fascia lata. Because of high late postoperative occurrence of infective endocarditis, however, this method was abandoned. Use of autologous fascia lata mounted on a frame was described by Ionescu and Ross but abandoned because of late dehiscence. Allograft dura mater valves, stent-mounted and preserved in glycerol, were used for aortic valve replacement by Zerbini and colleagues in Brazil. Bovine pericardium, glutaraldehyde treated and frame mounted, was introduced by Ionescu and colleagues at Leeds, England, in 1971.
In 1965, Binet and colleagues in Paris implanted porcine xenograft aortic valves , sterilized and preserved in a special formaldehyde solution, directly into the aortic root. The valves degenerated rapidly, most likely because of suboptimal tissue preservation. This led to abandoning direct xenograft valve implantation in favor of xenograft valves mounted on a stent frame. S tent-mounted bioprostheses are manufactured to provide a standard device that is easily implanted and provides reproducible results in the aortic position. Glutaraldehyde-preserved stent-mounted porcine valves were introduced by Carpentier and colleagues in Paris in 1967.
David and colleagues revived the concept of direct insertion of nonstented porcine xenografts into the aortic root. This valve was manufactured on a limited trial basis by Hancock Laboratory and by St. Jude Medical as the Toronto SPV (stentless porcine valve).
In April 2002, Professor Alan Cribier at the University of Rouen, France, performed the first percutaneous aortic valve replacement for aortic stenosis in a 62-year-old man who was not a candidate for surgery. Cribier used the antegrade transseptal approach through the femoral vein. The second percutaneous aortic valve insertion was in a 30-year-old man with severe aortic regurgitation who had no contraindication for surgery.
Beginning in 1979, Yacoub and colleagues developed the remodeling method of aortic valve-sparing root replacement for patients with aneurysms of the ascending aorta and root (including those associated with Marfan syndrome ) and aortic dissection. In 1988, David and Feindel described an aortic valve-sparing operation (subsequently termed the reimplantation technique ) for patients with aortic regurgitation and aneurysm of the ascending aorta in which the aortic valve is reimplanted within a polyester tubular graft. These two methods have underpinned the techniques of aortic valve-sparing aortic root replacement.
Calcific aortic stenosis implies stenosis secondary to heavy dystrophic calcification of a congenitally abnormal valve ( Fig. 12-1, A and B ). Calcification is rarely present before age 20; thereafter it is slowly progressive and results in important stenosis, most often in the fifth and sixth decades of life, earlier in unicommissural than bicuspid valves, and earlier in men than women. The calcification presents as a bulky cauliflower-like mass within the cusps, maximal at sites of commissural fusion or congenital buttress formation, often extending into the anulus (left ventricular [LV]-aortic junction) and adjacent aorta. Retrograde extension of calcification into the region beneath the right noncoronary cusp commissure adjacent to the membranous septum may lead to complete heart block. The valvar orifice is slitlike, often eccentrically located and oriented in a sagittal (most often) or transverse plane, and fixed, which often results in trivial or mild aortic regurgitation. (For description of critical congenital aortic stenosis, see Morphology in Chapter 47 , Section I.)

Bicuspid aortic valve is considered the most common congenital heart anomaly, reported in 0.5% to 2% of the general population. The valve has two cusps of unequal size, the larger one containing a central raphe. The raphe results from commissural fusion (type 1). The most common pattern involves fusion of the right and left cusps and is associated with coarctation of the aorta. Rarely, the cusps are symmetric without residual commissure or raphe (type O). Even less common is two raphes (type 2), usually with a well-developed commissure between the left and noncoronary sinuses.
Among patients with a bicuspid aortic valve, structural abnormalities exist at the cellular level that are independent of hemodynamic effects. The thoracic aorta typically shows reduced fibrillin-1, and increased matrix metalloproteinases are associated with smooth muscle cell detachment, matrix disruption, and cell death. The genetics of bicuspid aortic valve is complex and likely involves multiple pathways. Mutations in the signaling and transcriptional regulator NOTCH1 and in the ACTA2 gene (which encodes vascular smooth muscle cell B-actin) are linked with bicuspid aortic valve and familial thoracic aortic aneurysms. Numerous cardiac malformations are associated with bicuspid aortic valve: coarctation, Shone syndrome, William syndrome, Turner syndrome, and hypoplastic left heart syndrome. Abnormalities of the aorta (aortopathy) are the most frequent cardiac anomalies (with a male predominance of 3 : 1). Although ascending aortic dilatation is most common, aortic root and arch involvement are also frequent.
Controversy exists regarding the contribution of genetic mutations vs. flow characteristics in the genesis of the aortopathy. Using magnetic resonance imaging (MRI), Hope and colleagues demonstrated two distinct flow patterns specific to the two most common cusp fusion types and related these to location of thinning and dilatation of the ascending aorta. Asymmetric distribution of wall stress in patients with a bicuspid aortic valve (likely superimposed on genetically conferred aortic wall weakness) has been linked with asymmetric aortic smooth muscle cell apoptosis that could be flow mediated.
Degenerative disease is often present in stenotic aortic valves of patients older than 65 years of age, and its prevalence increases with age. In a series of patients whose mean age was older than 70, prevalence of degenerative aortic valve stenosis exceeded 70%. The valve is tricuspid, without commissural fusion; the cusps are held in a closed position by deposits of diffuse nodular or eggshell calcification ( Fig. 12-1, D ). These deposits are not bulky and may also involve the sinuses of Valsalva and ascending aorta. Although degenerative (senile) aortic stenosis is presumed to be arteriosclerosis, Hoagland and colleagues found no correlation between aortic stenosis in adults over age 50 and systemic hypertension, elevated serum cholesterol, smoking, or diabetes. A more recent study of 5201 subjects older than 65, however, found that clinical factors associated with aortic sclerosis and stenosis are similar to risk factors for arteriosclerosis. Aortic valve sclerosis was present in 26% and aortic valve stenosis in 2% of the entire study cohort. In patients over age 75 the prevalence of aortic sclerosis was 37% and stenosis 2.6%. Smoking increased the risk by 35% and hypertension by 20%. Other factors associated with increased risk of aortic valve disease were high lipoproteins, elevated low-density cholesterol levels, and diabetes mellitus. Older age was directly associated with risk, with a twofold increase in risk for each 10-year increase in age.
Mitral anular calcification is common in elderly patients with calcific aortic stenosis. Presumably, both are degenerative in origin.
Rheumatic aortic stenosis is characterized primarily by diffuse, prominent fibrous cusp thickening of a tricuspid valve ( Fig. 12-1, C ), with fusion to a variable extent of one or two commissures (rarely all three). The orifice is approximately central and irregular in shape. Calcification other than a mild form is rarely present except in elderly patients, but is bulkiest at sites of commissural fusion. Rheumatic aortic stenosis is seldom if ever isolated, although at the patient's first operation this may appear to be the case. In surgical series of apparently isolated aortic stenosis, prevalence of rheumatic etiology is low compared with that when patients with important mitral valve stenosis are included.
About half of patients with so-called rheumatic aortic stenosis fail to report a history of rheumatic fever, suggesting other unrecognized inflammatory processes as the cause. However, with the decline in incidence of rheumatic fever in the United States and other developed countries, rheumatic aortic stenosis decreased from a prevalence of 30% to 18% by the 1980s (and senile degenerative disease increased from 30% to 46%).
The terms aortic regurgitation, aortic incompetence , and aortic insufficiency are used interchangeably. Regurgitation is the preferred and most descriptive term. Morphologic characteristics of aortic regurgitation depend on etiology. These characteristics are not as easily categorized as in aortic stenosis.
Basic anatomy of the aortic root is detailed in Chapter 1 . This section provides additional details about aortic root anatomy and relationships that are relevant to aortic root reconstruction and valve-sparing aortic root replacement (discussed later in this chapter). The aortic root is that part of the aorta bounded proximally by the bases of the aortic valve cusps and distally by the sinutubular junction. McAlpine conceptualizes a continuous membrane covering the ostium or opening of the LV (called the aortoventricular membrane ) that contains the anulus of the mitral valve and the aortic anulus and adjacent fibrous components. The left anterior fibrous trigone is a membrane between the left and right cusps and the ostium of the LV. The remaining structures related to the aortic root result from thickening of the aortoventricular membrane ( Fig. 12-2 ), and these are the right anterior fibrous trigone, the ventricular and atrial segments of the membranous septum, intervalvar trigone, right fibrous trigone, and fila coronaria (the portion of the aortoventricular membrane between the ostium of the LV and the left atrial attachment, which comprises about 75% of the mitral anulus). The region where the aortic valve cusps are in fibrous continuity with the anterior leaflet of the mitral valve (aortomitral anulus) is thickened at each end to form a left and right fibrous trigone. The right fibrous trigone is in continuity with the membranous portion of the septum, and these two structures form the central fibrous body. The left anterior fibrous trigone, right anterior fibrous trigone, and intervalvar trigone are also termed intercusp triangles . The membranous septum is divided into the ventricular membranous septum and atrial membranous septum by attachment of the tricuspid valve septal leaflet to the aortoventricular membrane ( Fig. 12-3 ). Attachment of the right ventricle (RV) to the aortoventricular membrane is in close relationship to the left and right anterior fibrous trigones ( Fig. 12-4 ).



The aortic root forms the outflow tract from the LV and contains the aortic valve cusps, sinuses of Valsalva, and intercusp triangles (trigones). Morphology of the aortic valve cusps reflects their exposure to the mechanical stress of diastolic pressure. They have three distinct layers. The outflow surface is the fibrosa , comprising bundles and sheets of collagen aligned in the circumferential direction. The cusp has a coaptional portion (where the collagen bundles are discontinuous) and a noncoaptional surface or cusp belly (where the collagen bundles are continuous). The ventricular surface of the cusp is composed of the ventricularis , which is another fibrous layer. It is a mixture of both collagen and elastin (although the elastin is not as important as the collagen from a biomechanical standpoint). The fibers are arranged randomly, and therefore when the ventricularis is under load, the fibers realign in the direction of the applied load and only then resist further extension. The spongiosa layer between the fibrosa and ventricularis is composed principally of glycosaminoglycans, which are responsible for energy dissipation and lubrication of the movements between fibrosa and ventricularis. The biomechanical properties of the fibrosa and ventricularis allow radial extension of the cusp to form a large coaptional area.
The sinuses of Valsalva are the bulging portions of the aortic root from which the coronary arteries arise. They accommodate the open cusps of the aortic valve and generate vortices that are important for aortic cusp closure. The base of the aortic cusp attachment forms a coronet-like structure ( Fig. 12-5 ). The tissue inbetween the attachment of the aortic valve cusps to the aortic wall is the intercusp triangle , a layer composed of circularly oriented collagen fibers. The base of two of the intercusp triangles is LV muscle, and the intercusp triangle beneath the commissure of the left and right cusps is fibrous (left anterior fibrous trigone). Attachment of the base of the aortic root is approximately 55% fibrous and 45% muscular. The intercusp triangles are exposed to ventricular hemodynamics, and they may function in part to allow each of the sinuses to act independently. An important surgical point regarding the ventricular-aortic junction is the site of attachment of prosthetic valves, which are largely circular structures. Prosthetic valves are actually attached to the anatomic ventricular-aortic junction and do not follow the cusp attachment, although this is usually regarded as the “anulus.”

A spectrum of aortic pathology may result in aortic regurgitation due to alterations in the geometry of the sinutubular junction, sinuses, and the ventricular-aortic junction. Ascending aortic aneurysms and aortic root disease may be distinct processes, or they may coexist as a blending of morphologic manifestations.
Many different pathologies may result in ascending aortic aneurysms (see Chapter 26 ). These include long-standing hypertension, arteriosclerosis, aneurysms associated with bicuspid aortic valves, and extreme forms of post-stenotic dilatation of a stenotic aortic valve. Ascending aortic aneurysms may also result from inflammatory processes causing aortitis, including rheumatoid arthritis, ankylosing spondylitis, and Reiter syndrome. Ascending aortic aneurysms and aortic root disease may arise from clearly defined genetic syndromes, including Marfan syndrome, Loeys-Dietz syndrome, Ehlers-Danlos syndrome, and filamin A mutations. Most patients with thoracic aorta disease and aortic dissections do not have a clearly defined genetic disorder, but many have an inherited predisposition to the process.
Marfan syndrome , one of the most common connective tissue disorders, is an autosomal dominant condition affecting about 1 in 3000 to 5000 people. Most patients with the typical Marfan phenotype have mutations involving the FBN1 gene that codes for fibrillin-1, an extracellular matrix glycoprotein that contributes to structural integrity of connective tissue. In a minority of cases, an FBN1 mutation is not found. Fibrillin-1 is an important component of both elastic and nonelastic connective tissue. In about 10% of Marfan phenotypes, mutations have been noted in transforming growth factor (TGF)-β receptor genes. Criteria for diagnosis of Marfan syndrome involve genetic studies, family history, and major and minor clinical manifestations. Because of the linkage between these genetic mutations and phenotypes that overlap with typical Marfan syndromes, the clinical diagnosis requires specific combinations of criteria. Histologic features of the ascending aorta media in Marfan patients include fragmentation of elastic lamellae, loss of smooth muscle cells, fibrosis, and cystic medial necrosis (a misleading term coined to describe the lacunar appearance of medial degeneration when, in fact, cystic changes and necrosis are absent).
Other connective tissue disorders that predispose to aortic aneurysmal disease and dissection include Loeys-Dietz syndrome and the vascular type of Ehlers-Danlos syndrome. Loeys-Dietz syndrome is an autosomal dominant aortic syndrome resulting from mutations in genes for the cytokine (TGF)-β receptor (TGFBR) type I or II. Arterial tortuosity and aneurysms, hypertelorism, and bifid uvula or cleft palate characterize the disease phenotype. Skeletal features are similar to those of Marfan syndrome. The aortic disease in this syndrome is particularly aggressive, and 98% of patients develop aortic root aneurysms that have a high propensity for dissection. Mean age of death with this syndrome is 26 years. In children affected with Loeys-Dietz syndrome, prominent craniofacial features are associated with more severe aortic disease. Because these patients are prone to aneurysm development in other locations, yearly MRI or computed tomography (CT) is advisable from the pelvis to the brain.
The vascular form (type IV) of Ehlers-Danlos syndrome is a rare autosomal dominant disease caused by a defect in type III collagen, encoded by the COL3A1 gene. Prominent clinical features include easy bruising, thin skin, characteristic facial features, and tendency for rupture of arteries, uterus, or intestines. The role of prophylactic aortic replacement surgery to prevent aortic rupture or dissection is less clear than for Marfan or Loeys-Dietz syndromes. Of importance, these patients typically have extreme tissue fragility, so if aortic aneurysms or aortic regurgitation require cardiac surgery, reinforcement of suture lines with felt pledgets or strips is recommended.
Anuloaortic ectasia can produce aortic regurgitation of varying but sometimes severe degree even though the cusps are normal. It is most often caused by cystic medial degeneration of the aorta and may be associated with Marfan syndrome. Even in the absence of Marfan syndrome, anuloaortic ectasia appears to be a genetic disease. It begins in the sinuses of Valsalva; at this stage, regurgitation is usually not present. With time, the process extends to involve the proximal ascending aorta, producing a symmetric, pear-shaped aneurysmal enlargement. Regurgitation now appears and progresses because dilatation of the aortic wall at the sinutubular junction separates the commissures and tightens the free cusp edges, preventing coaptation during diastole. As dilatation of the aorta progresses, central aortic valve regurgitation increases. The LV-aortic junction usually does not increase in size, even in patients with large aneurysms associated with anuloaortic ectasia. Size of the aortic valve prosthesis used in these patients (if indicated) is generally similar to that used for replacement in patients with rheumatic or other disease.
The aneurysmal process eventually involves the entire ascending aorta, but usually stops just before the level at which the brachiocephalic artery originates, although the remainder of the arch may show cystic medial degeneration. The aneurysms are thin walled with a smooth lining. Dissection may begin within the aneurysms, extending proximally and distally or remaining localized (although most acute aortic dissections occur in the absence of an aortic root aneurysm). In patients with Marfan syndrome, about 30% of those operated on for anuloaortic ectasia and aneurysm of the ascending aorta have an aortic dissection. It is limited to the ascending aorta in about half the patients and extends into the transverse and descending aorta in the rest. Frequently, dissection is unexpectedly found at operation. With proximal extension of this dissection, the commissural attachment of the valve becomes separated from the outer aortic wall such that the valve prolapses centrally, and regurgitation may abruptly increase (see Chapter 25 ).
Ascending aortic aneurysms also produce valvar regurgitation because of tightening of the free edges of the cusps that results from aortic dilatation. In syphilitic ascending aortic aneurysm, aortic regurgitation is exacerbated by a valvulitis that produces thickening and retraction of the cusp edge. Neither condition, however, is generally associated with aortic dissection.
In some patients with rheumatoid arthritis, ankylosing spondylitis, or Reiter disease, an aortitis occurs that may lead to aneurysmal dilatation of the ascending aorta and aortic valvar regurgitation. The aortitis is characterized by dense adventitial inflammatory fibrosis involving the sinuses of Valsalva and proximal aorta, especially adjacent to the commissures. The process may extend below the base of the aortic valve to form a characteristic subvalvar ridge and may involve the base of the anterior mitral leaflet or even the adjacent ventricular septum, causing conduction disturbances. Particularly in rheumatoid arthritis, the cusps may be thickened and shortened and show rheumatoid nodules histologically.
Rheumatic aortic regurgitation results from a different response of the valve to the rheumatic process than occurs when stenosis develops. Commissural fusion is minimal or absent, and the cusps are only slightly thickened. Minor calcification is present in about 10% of affected valves. The major pathologic process is cicatricial shortening of the cusps between their free edge and their anular attachment, with rolling of the free edge. As time passes, the aortic root widens in response to the regurgitation, further increasing central valvar regurgitation.
Native valve endocarditis, which may occur on a structurally normal valve or on congenitally or rheumatically deformed valves, is a common cause of aortic regurgitation. The regurgitation may result from a destroyed commissure and consequent cusp prolapse or from a perforation in the belly of the cusp. An infected pannus may appear below the cusps, or extensive destruction of the aortic root may occur, with a periaortic root abscess sometimes extending into the mitral anulus and anterior mitral leaflet. Mitral regurgitation may also develop because of perforation of the anterior leaflet by a “drop lesion” caused by the infected regurgitant stream from the diseased aortic valve.
A congenitally bicuspid or unicuspid valve can produce regurgitation from prolapse of the free edge of a redundant cusp. In such patients, the regurgitation may be aggravated by infective endocarditis or an improper valvotomy (see Chapter 47 ). Lack of support of the aortic anulus in association with ventricular septal defect may result in aortic valve prolapse and regurgitation (see Chapter 35 ).
Occasionally, aortic regurgitation may be caused by prolapse of redundant aortic cusps that are mildly thickened and myxomatous. The aortic root may be normal or dilated, usually with cystic medial necrosis, and mitral valve prolapse may also occur.
Aortic valve regurgitation may be caused by a number of physician-related interventions. Perforation of the aortic valve cusps may result from diagnostic or balloon dilatation catheters. Even with newer methods and lower doses of mediastinal irradiation, occasional cases of mediastinal fibrosis occur, with injury to the pericardium, cardiac valves, coronary arteries, and myocardium. Cardiac valve disease has also been associated with migraine medications (ergotamine, methysergide) and appetite suppressants (fenfluramine, with or without phentermine) ( Fig. 12-6 ).

Other causes of aortic valve regurgitation include spontaneous cusp rupture, rupture caused by severe closed-chest trauma, and severe long-standing systemic hypertension with aortic root dilatation. In patients with long-standing hypertension, regurgitation may result from typical myxoid degeneration of the valve. Some instances of regurgitation are probably related to arthropathies with minimal joint involvement or to hypertension, psoriasis, giant cell aortitis, or Takayasu disease. Occasionally the etiology of regurgitation is not apparent.
The etiology and morphology of combined aortic stenosis and regurgitation are similar to those of aortic valve stenosis. In some cases, an episode of endocarditis produces regurgitation of a previously stenotic valve.
A time-related change in etiology and morphology has been observed at operation in patients with aortic valve disease. Although the overall prevalence of bicuspid aortic valves in the general population has not changed over the past 50 years, its relative frequency in the surgical population has decreased ( Table 12-1 ). In surgical patients at the Mayo Clinic the relative frequency of bicuspid aortic valves fell from 49% in 1965 to 36% in 1990. The relative frequency of patients with degenerative aortic valve disease increased greatly, however, and the prevalence of aortic stenosis doubled from 32% to 65%. Mean age at operation increased from 49 years in 1965 to 66 years in 1990 and continues to rise.
| Morphology | AS (Pure) | AS/AR (Mixed) | AR (Pure) | |||
|---|---|---|---|---|---|---|
| 1965 | 1990 | 1965 | 1990 | 1965 | 1990 | |
| Bicuspid (congenital) (%) | 49 | 36 | 20 | 17 | 17 | 14 |
| Rheumatic (%) | 33 | 9 | 61 | 17 | 47 | 14 |
| Degenerative (%) | 0 | 51 | 0 | 46 | 0 | 0 |
| Dilatation ascending aorta (%) | 0 | 0 | 0 | 0 | 19 | 50 |
| Iatrogenic (%) | 0 | 1 | 0 | 13 | 0 | 14 |
| Infective endocarditis (%) | 0 | 0 | 0 | 0 | 11 | 2 |
| Other (%) | 18 | 3 | 19 | 7 | 6 | 6 |
| Patients (%) | 32 | 65 | 39 | 10 | 29 | 25 |
a Data presented as percent of the total number of patients in each category.
Patients with aortic stenosis are usually symptomatic when first seen. They may present without symptoms, however, having been referred because of a cardiac murmur. The classic triad of effort dyspnea, angina, and syncope is present in about one third of patients. Understanding and recognizing the symptoms of aortic stenosis is particularly important because of the heavy reliance on symptoms in decisions regarding advisability of operation (see Indications for Operation, Selection of Technique, and Choice of Device later in this chapter).
Angina pectoris is present as the only symptom or is combined with others in 50% to 70% of patients. It is more common in patients with combined aortic stenosis and coronary artery disease than in those with isolated aortic stenosis. Angina in patients without coronary artery disease presumably results from an imbalance between coronary blood flow and oxygen demand in the hypertrophied LV. Angina appears to occur more frequently in patients with severe aortic stenosis than in those with less severe gradients across the aortic valve. Other morphologic and hemodynamic variables, such as LV wall thickness, wall stress, and wall tension, are similar in patients with aortic stenosis whether or not angina is present.
From 30% to 50% of patients with important aortic stenosis have frank or incipient syncope. Among the many possible causes of syncope (and sudden death) in patients with aortic stenosis, the most likely is peripheral vasodilatation from a faulty baroreceptor mechanism. Symptoms of pulmonary venous hypertension (dyspnea, orthopnea, paroxysmal nocturnal dyspnea, or frank pulmonary edema) are present in 30% to 40% of patients, either alone or with other symptoms. These symptoms are associated with increased LV end-diastolic pressure and systolic wall stress and lower cardiac output and ejection fraction (EF).
A few patients (10%) survive typical symptoms long enough for secondary RV failure to develop. These patients present with a clinical picture dominated by elevated right atrial and jugular venous pressure, hepatomegaly, cardiac cachexia, and rarely, tricuspid regurgitation. Patients often appear to have combined aortic stenosis and mitral regurgitation as well.
Diagnosis of important aortic stenosis can often be made by physical examination with reasonable certainty when, in addition to the presence of an aortic ejection murmur (usually best heard in the second right intercostal space beside the sternum and transmitted to the carotids, but also often at the apex and in the second left intercostal space), the arterial pulse is of small volume with a slow upstroke. Support for the diagnosis may be obtained from expiratory splitting of the second heart sound and from evidence of LV hypertrophy provided by the character of the apex beat and electrocardiogram (ECG). Usually the ECG provides evidence of LV hypertrophy, with or without inverted T waves in lead V 6 (the so-called strain pattern). When chest radiography or fluoroscopy also shows calcification of the aortic valve and convexity along the upper part of the LV silhouette produced by LV hypertrophy, the diagnosis of calcific aortic stenosis becomes a near certainty.
At times, physical findings are less diagnostic. Systemic hypertension or, in older patients, inelasticity of aortic and arterial walls may alter the character of the arterial pulse wave and prevent development of a clinically recognizable slow upstroke or soft, weak pulse (pulsus parvus). Absence of the aortic component may prevent assessment of the respiratory behavior of the second heart sound, whereas in patients with right or left bundle branch block, splitting of this sound is of no value as a guide to the severity of aortic stenosis. In older patients especially, the character of the cardiac apex may be unreliable as a clinical guide to presence and degree of LV hypertrophy. The ECG also may fail to show the degree of LV hypertrophy associated with severe aortic stenosis and occasionally remains normal without showing evidence of LV involvement.
In these patients, judicious use of graded exercise testing may uncover a clinically silent state of LV dysfunction and functional aerobic impairment. Exercise has generally been considered dangerous in patients with severe aortic valve stenosis because of effort syncope. Experience has shown, however, that graded exercise testing is not a risky procedure in patients with aortic stenosis who are asymptomatic, but it is not advised in symptomatic patients. Impaired exercise tolerance (as by 6-minute walk testing), occurrence of symptoms, inadequate blood pressure increase (10 mmHg · 30 watts −1 or less) or blood pressure drop (≥10 mmHg), bradycardia, arrhythmia, conduction disturbance, and ST-segment depression (≥0.2 mV) indicate impaired aerobic or LV function. This information may be helpful in deciding on operative intervention or, if continued observation is advised, recommendations concerning vocational, recreational, or sports participation. Finally, in the terminal stages of low-output heart failure, the murmur may be so faint that aortic stenosis is not suspected, particularly in adult patients in whom the heart sounds are distant either because of chest wall thickness or inelastic and voluminous lungs.
Doppler echocardiography is a reliable means of establishing the presence of aortic stenosis and is usually performed in patients suspected of having aortic valve disease. In most patients with aortic stenosis, the degree of obstruction to outflow, aortic valve peak and mean gradient, and valve area can be reliably determined. It is the main modality for serial evaluation. Maximal instantaneous gradient is obtained by applying the modified Bernoulli equation to peak aortic velocity; this may be 30% to 40% higher than gradient determined by cardiac catheterization ( Table 12-2 ). Using continuous wave Doppler, the simplified Bernoulli equation can be applied to obtain the peak instantaneous gradient: Peak pressure gradient (mmHg) = 4 × peak velocity 2 . The mean gradient across the aortic valve is obtained by planimetry of the continuous wave signal. Mean gradient is more useful clinically than instantaneous gradient. Mean pressure gradient is the arithmetic mean of the derived instantaneous gradients; it correlates well with mean pressure gradient obtained by cardiac catheterization. Aortic valve area may also be determined by echocardiography based on the continuity principle , which states that flow through a nonstenotic region of the heart should equal flow through a stenosis (assuming no regurgitation or shunt). Aortic valve area derived by cardiac catheterization is well correlated with this.
| Aortic Stenosis | |||
| Indicator | Mild | Moderate | Severe |
| Jet velocity (m · s −1 ) | <3.0 | 3.0-4.0 | >4.0 |
| Mean gradient (mmHg) a | <25 | 25-40 | >40 |
| Valve area (cm 2 ) | >1.5 | 1.0-1.5 | <1.0 |
| Valve area index (cm 2 · m −2 ) | <0.6 | ||
| Aortic Regurgitation | |||
| Qualitative | Mild | Moderate | Severe |
| Angiographic grade | 1+ | 2+ | 3-4+ |
| Color Doppler jet width | Central jet, width < 25% of LVOT | Greater than mild, but no signs of severe regurgitation | Central jet, width > 65% of LVOT |
| Doppler vena contracta width (cm) | <0.3 | 0.3-0.6 | >0.6 |
| Quantitative (Cath or Echo) | Mild | Moderate | Severe |
| Regurgitant volume (mL · beat −1 ) | <30 | 30-59 | ≥60 |
| Regurgitant fraction (%) | <30 | 30-49 | ≥50 |
| Regurgitant orifice area (cm 2 ) | <0.10 | 0.10-0.29 | ≥0.30 |
a Valve gradients are flow dependent and, when used as estimates of severity of valve stenosis, should be assessed with knowledge of cardiac output or forward flow across the valve.
Hemodynamic data derived by Doppler echocardiography or cardiac catheterization can provide a grading of degree of stenosis (see Table 12-2 ). The aortic valve area must be reduced to about one fourth its normal size before important changes occur in the circulation. The normal adult aortic valve area is 3.0 to 4.0 cm 2 . Thus, an area of less than 1.0 cm 2 is likely to produce clinical symptoms. The American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Practice Guidelines states that aortic valve stenosis is mild when aortic valve area is greater than 1.5 cm 2 (with transvalvar gradient < 25 mmHg), moderate when 1.0 to 1.5 cm 2 , and severe when less than 1.0 cm 2 (see Table 12-2 ). Because pressure gradient is flow dependent, stenosis is considered severe when mean gradient is 40 mmHg or higher and cardiac output is normal. However, when cardiac output is low, severe aortic stenosis may be present with a lower transvalvar gradient.
These hemodynamic criteria are helpful, but therapeutic decisions related to operative intervention are largely based on presence or absence of symptoms.
In patients over age 40, coronary arteriography is also performed when operation is being considered, because coronary artery disease coexists in many of these patients whether or not angina is present. At the time of coronary arteriography, systolic pressure gradient across the aortic valve is measured. Cardiac output can be measured and the valve area calculated by the Gorlin equation.
Hematologic abnormalities associated with severe aortic stenosis include impairment of platelet function and decreased levels of von Willebrand factor, which correlate with severity of stenosis. Clinical bleeding is observed in about 20% of patients with severe aortic stenosis, most often epistaxis or ecchymoses. Coagulation abnormalities usually disappear after aortic valve placement.
Patients with aortic regurgitation present more frequently without symptoms than do those with aortic stenosis, perhaps because of the more dramatic physical and radiographic findings and relatively long asymptomatic phase of regurgitation. In most patients, the dominant symptoms reflect pulmonary venous hypertension (dyspnea, orthopnea, paroxysmal nocturnal dyspnea, pulmonary edema). Angina pectoris is often part of the presenting complaint, but is the chief complaint in less than one fourth of patients and is more common in older patients. Coronary artery disease is present in about 20% of patients with angina pectoris. Syncope is rare.
In severe aortic regurgitation, the LV apex is usually displaced and overactive in character. The carotid and other pulses are jerky to palpation in moderate regurgitation and collapsing or “water-hammer” in severe regurgitation because of the wide pulse pressure and rapid rise and fall of the pulse wave. Blood pressure measured by Korotkoff sounds may reach 200 to 250 mmHg systolic and 50 to 0 mmHg diastolic. Normally, brachial or radial pulse pressure measured by an arterial needle is less than that measured by Korotkoff sounds, and central aortic pulse pressure is even less. These phenomena, including systolic amplification between central aortic and radial artery blood pressures, are related to standing waves created by the pulsatile ejection of an unusually large LV stroke volume into the aorta and remainder of the arterial tree. If cardiac output is low because of severe cardiac failure, these phenomena are minimal.
Auscultation in the aortic area reveals an early diastolic murmur that radiates toward the apex of the heart. Intensity of the murmur has been shown to correlate with degree of aortic valve regurgitation. Murmur grade 3 or greater predicts severe aortic regurgitation in 71% of patients, whereas murmur grade 1 or less predicts that aortic regurgitation is not severe in 100% of patients. Often a systolic click or ejection murmur is present as well. At the apex a mid-diastolic murmur is frequently caused by fluttering of the anterior mitral leaflet from a prominent regurgitant jet (Austin Flint murmur) . This may be difficult to distinguish from the murmur of mitral stenosis, although in the latter an opening snap is often present. When mitral stenosis coexists, the ECG usually shows P mitrale and the left atrium is enlarged, although in severe and long-standing pure aortic regurgitation, the ECG may also show P mitrale. Two-dimensional echocardiography is useful in making the distinction between mitral stenosis and merely an Austin Flint murmur ( Fig. 12-7 ). The chest radiograph confirms LV enlargement; the left atrium is usually normal or slightly enlarged. Radiographic evidence of pulmonary venous hypertension may or may not be present. Enlargement of the shadow of the ascending aorta to the right suggests an accompanying aneurysm of the ascending aorta, but an aneurysm can be present without this sign. The ECG shows evidence of LV enlargement, often with the high-peaked T waves and prominent Q waves of LV volume overload. T-wave inversion and ST-segment depression are seldom present until the LV is extremely large.

Diagnosis of aortic valve regurgitation can usually be made on the clinical findings, but other abnormalities in the aortic root allowing a rapid aortic runoff (e.g., ruptured sinus of Valsalva aneurysm, large patent ductus arteriosus with pulmonary valve regurgitation) cannot be entirely eliminated without special studies. Color flow Doppler echocardiography firmly establishes the diagnosis.
Doppler echocardiography can be used to quantify aortic valve regurgitation (see Table 12-2 ). Measurements are made of regurgitant volume (volume regurgitated per heartbeat) and of regurgitant fraction (proportion of total ejection of the LV). These measurements are highly dependent on technical experience, with overestimation the rule at first. Size of the jet visualized by color Doppler echocardiography may not represent the degree of aortic regurgitation. It is possible to measure the vena contracta , which is the size of the regurgitant jet within the regurgitant aortic valve orifice. This measurement correlates well with effective regurgitant orifice size. The width of the vena contracta just below the flow convergence is measured using the parasternal long-axis view. Vena contracta of 7 mm or greater uniformly favors severe aortic valve regurgitation, whereas measurements of 5 mm or less correspond to less regurgitation. The degree of aortic regurgitation may also be quantified by cineangiography using an aortic root contrast injection in the right anterior oblique projection, but this method is difficult and often unreliable.
Coronary arteriography is indicated in patients over age 40.
Although many patients with severe aortic stenosis have mild regurgitation and a few patients with severe regurgitation have some stenosis, a small group of patients have virtually balanced lesions. Their symptoms are generally similar to those associated with aortic stenosis. This group may have a particularly unfavorable prognosis because there is both volume and pressure overload on the LV.
The natural history of adults with aortic valve disease is incompletely known, although it is evident that severity of the stenosis gradually increases. Synthesis of four echocardiographic studies indicates that once moderate aortic stenosis is present (jet velocity by echocardiography > 3.0 m · s −1 ), the average rate of progression in mean pressure gradient is about 7 mmHg · y −1 , an increase in jet velocity of 0.3 m · s −1 · y −1 , and a decrease in valve area of 0.1 cm 2 · y −1 . Aortic stenosis appears to progress more rapidly in patients with degenerative disease than in those with congenital or rheumatic etiology. A complicating factor is that some degree of stenosis may have existed in childhood, often with associated regurgitation. The natural history in these patients may be more favorable than when the disease develops de novo and more rapidly later in life.
Medical therapy has generally been regarded as ineffective in preventing or retarding disease progression in aortic stenosis. Research over the past decade indicates that aortic valve disease of the elderly is not just a passive “wear-and-tear” process, but an active inflammatory process with histologic changes similar to arteriosclerosis. An evaluation of statin therapy (based on its efficacy in arteriosclerosis stabilization) in the Simvastatin Ezetimibe Aortic Stenosis and Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression prospective randomized trials failed to identify a favorable effect of statins on progression of aortic stenosis. Ongoing research efforts may clarify the potential role of these and other agents in ameliorating disease progression.
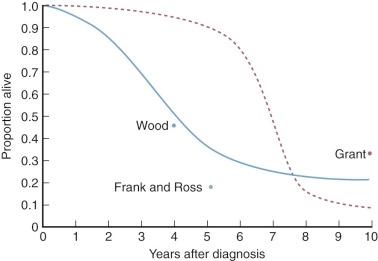
Grant reported that 35% of unoperated patients with usual symptoms of aortic stenosis are alive at 10 years. Wood stated that 46% of such patients were alive 1 to 7 years later. Frank and Ross reported that of 12 unoperated patients with severe aortic stenosis, only 18% were alive 5 years later. Based on their data, Ross and Braunwald concluded that average survival after onset of angina or syncope is 3 years and after onset of heart failure about 1.5 years. ACC/AHA guidelines suggest that after onset of symptoms, average survival is less than 2 to 3 years, with a high risk of sudden death. Thus, development of symptoms identifies a critical point in the natural history of aortic stenosis. O’Keefe and colleagues followed 50 symptomatic patients with severe aortic stenosis in whom operation was declined or deferred. Average age of these patients was 77 (60-89) years. Survival was 55%, 37%, and 25% at 1, 2, and 3 years, respectively, compared with a matched general population of 93%, 85%, and 77%. Death was from cardiac causes in all cases except one. Of 179 patients aged 83 ± 8.3 years deemed inoperable in the PARTNER IB cohort, 1-year survival was 49%.
Although it is impossible to rigorously assemble such disparate data, a likely survival curve for adult patients with severe, unoperated aortic stenosis is estimated in Fig. 12-8 . Deaths within the first 1 or 2 years are likely to be sudden, presumably associated with ventricular fibrillation (15%-20% of all deaths in aortic stenosis are sudden ) or, after a few hours or days of acute pulmonary edema, from sudden LV failure. Most unoperated patients die in the latter mode within about 5 years of diagnosis. Beyond 5 years of follow-up, some die of gradually worsening cardiac failure, with low cardiac output and gradually worsening symptoms of pulmonary venous hypertension. Moderate pulmonary artery hypertension develops in some patients who exhibit these findings; in a few, typical symptoms and signs of RV failure become prominent.
Asymptomatic patients with severe aortic stenosis usually develop symptoms within a few years of diagnosis. Otto and colleagues showed that about one fourth of initially asymptomatic patients with aortic valve stenosis had developed symptoms, half by 3 years and three fourths by 4 years. When the Doppler outflow velocities were initially 4.0 m · s −1 or greater, progression was more rapid, with three fourths of patients symptomatic by 2 years. Thus, even in initially asymptomatic patients with aortic stenosis, progression to symptoms may be rapid, and patients should be monitored closely for progressive disease. Sudden death is uncommon in asymptomatic patients with aortic stenosis, occurring in less than 1% per year. However, as noted by Freed and colleagues, failure to identify subtle symptoms of severe aortic stenosis is common, and the subsequent mortality without surgery may exceed 10% in the ensuing 1 to 1.5 years.
The LV hypertrophies progressively in the presence of important aortic stenosis, which usually develops over decades. The increase in wall thickness is usually enough to counter the high intraventricular systolic pressure and maintain normal ventricular volume. In this circumstance, LV wall stress (afterload) remains within normal range, and EF remains preserved (given the inverse relationship between systolic wall stress and EF). However, when the hypertrophic response is not adequate for progressively higher intracavitary pressures, the increased afterload can cause a decrease in EF, which is generally reversible with valve replacement.
Myocardial hypertrophy in aortic valve stenosis is caused by new myofibrils added in parallel to myocytes. No new myocytes are added, but existing myocytes become thicker, not longer, compared with normal myocytes. The hypertrophy of myocardial cells (increased myocardial cell diameter) is a determinant of both increased systolic load stress and decreased LV diastolic function found in aortic stenosis, and is also related to reduction in EF. Myocardial fibrosis exerts little effect.
Schwartz and colleagues found good systolic function and only hypertrophic myocardial cells when LV mass was less than 200 g · m −2 . When it was 200 to 300 g · m −2 , degenerative changes were present but mild. When LV mass was greater than 300 g · m −2 , systolic function was greatly depressed and multiple degenerative changes in ultrastructure were present (mitochondrial changes, disruption of sarcomeric units, nonoriented growth of fiber components, disappearance of organelles). Maron and colleagues also described these degenerative changes in detail. Krayenbuehl and colleagues found degenerative changes in the form of increased interstitial nonmuscular tissue in association with myocardial cellular hypertrophy. These changes are probably the morphologic basis for loss of inotropic (contractile) strength and irreversibility.
Thus, during the compensated phase, thickening of the LV wall keeps LV afterload (systolic wall stress) more or less normal (see “Ventricular Afterload” under Cardiac Output and Its Determinants in Section I of Chapter 5 ), preserving LV systolic function. LV compliance and diastolic function are gradually impaired, to a degree primarily related to extent of LV hypertrophy. At a more advanced stage, hypertrophy and wall thickness may increase less than LV systolic pressure (afterload mismatch ); the resulting increase in afterload impairs LV systolic function. The degree of LV hypertrophy and decrease in contractility that ultimately develop are more often the cause of declining cardiac function. As indices of systolic function (EF, end-systolic volume, LV fractional shortening, velocity of circumferential shortening) decline, cardiac output decreases gradually or acutely, and LV diastolic function decreases with a consequent increase in LV end-diastolic pressure. By this time the condition is advanced, and chronic heart failure is present.
The atrial contribution to ventricular filling is of great importance with a thickened noncompliant ventricle. As long as sinus rhythm is maintained, left atrial and pulmonary venous pressure can remain near normal. However, loss of atrial contraction with the onset of atrial fibrillation can induce rapid clinical decompensation.
The hypertrophied ventricle may have reduced coronary perfusion per gram of muscle with diminished coronary vasodilator reserve. Added myocardial oxygen demands with exercise or tachycardia may induce subendocardial ischemia and angina in the absence of coronary artery disease.
Occasionally, complete heart block develops in patients with extensive calcification of the stenotic aortic valve. It may be the result of gradually increasing pressure on the bundle of His by calcific deposits beneath the commissural area between the noncoronary and right coronary cusps. However, complete heart block sometimes occurs without calcific pressure on the bundle of His. Pressure in the LV is hypothesized to play a role. Rarely, relief of aortic stenosis relieves the heart block.
Aortic regurgitation may develop acutely or more gradually as a chronic condition. Acute onset of severe regurgitation imposes a sudden large regurgitant volume on the LV, which has been normal. There is little time to accommodate to the volume load, and LV end-diastolic and left atrial pressures increase rapidly. Tachycardia is the primary compensatory mechanism but may be insufficient, and the clinical situation may quickly deteriorate to pulmonary edema and circulatory shock.
Patients presenting with chronic aortic regurgitation have combined volume and pressure overload of the LV. Compensatory mechanisms are primarily recruitment of preload reserve and LV hypertrophy. Most patients remain asymptomatic through a long compensatory phase that may last for decades.
The natural history of patients with aortic regurgitation depends primarily on its severity. Mild or moderate aortic regurgitation appears to affect activity and life expectancy minimally. LV structure and function begin to be adversely affected, symptoms develop, and prognosis becomes more limited as severity of the regurgitation increases.
Even when aortic regurgitation becomes severe, there may be a long latent period (3-10 years), during which LV enlargement is only mild, symptoms are absent or mild, and the prognosis is good as long as the findings remain unchanged. Bonow and colleagues followed patients with chronic aortic regurgitation and normal EF. They found that 81% were alive and without need of aortic valve replacement 5 years later. Less than 6% per year required aortic valve replacement because of symptoms or LV dysfunction at rest, less than 3.5% per year developed asymptomatic LV systolic dysfunction, and less than 0.2% per year died suddenly. Vasodilator therapy using nifedipine may benefit such patients and delay surgical intervention. When important symptoms develop, however, prognosis becomes severely limited (see Fig. 12-8 ).
The probability of death increases with development of specific risk factors. Symptoms of cardiac failure, development of ventricular premature beats, marked cardiomegaly (cardiothoracic ratio > 0.6), and ECG evidence of severe LV hypertrophy all increased the risk of death in a group of 180 surgically untreated patients with isolated severe aortic regurgitation of rheumatic etiology.
When severe aortic regurgitation develops acutely, as from infective endocarditis, the natural history is much less favorable. Only 10% to 30% survive more than 1 year after onset.
Cardiac size gradually increases in the presence of important aortic regurgitation. Quantitative angiography has shown that increased LV end-diastolic volume is directly related to the magnitude of aortic regurgitant flow.
Bonow and colleagues found a higher-risk subgroup within patients who are asymptomatic and have normal LV systolic function. Progressive enlargement (dilatation) of the LV or reduction in resting EF identified by serial echocardiography heralds onset of symptoms. Patients at risk for sudden death are those with extreme LV dilatation or an LV cavity dimension of 75 mm or more at end-diastole and 55 mm or more at end-systole (normal values are ≤55 mm and ≤35 mm, respectively). As LV size and end-diastolic volume steadily increase, eventually there is loss of LV reserve, and LV end-diastolic pressure then rises rapidly.
As the LV enlarges, LV hypertrophy begins to develop. In addition, the LV undergoes an increase in mass and wall thickness, and its shape and ultrastructure change. The myocardial cell hypertrophy and increase in interstitial nonmuscular tissue found in the pressure-overloaded LV of aortic stenosis are similar in the volume-overloaded LV of aortic regurgitation. Concomitant with hypertrophy, LV compliance decreases, compromising diastolic function. Finally, LV end-diastolic and left atrial pressures become elevated, with further increases during exercise.
At some point, LV stroke work fails to respond to increased wall stress (e.g., afterload increase by infusion of angiotensin). As LV systolic function decreases, LV end-systolic dimension steadily increases, and even in asymptomatic patients the rate of increase is about 7 mm per year.
Despite these changes and because of the complex interaction between aortic regurgitation and decreasing systemic vascular resistance, LVEF response to exercise is favorable for a considerable time. Eventually, however, it declines, and systolic function may even decrease during stress. Symptoms then worsen, and the decline in LV function accelerates.
Bicuspid aortic valve disease is usually asymptomatic in childhood, although rarely the presentation and natural history may take the form of critical congenital aortic stenosis (see Chapter 47 ). The clinical manifestations relate to the functional state of the valve (stenosis, regurgitation, or both), the aortopathy (aneurysm or dissection), and the potential for endocarditis. Longitudinal studies indicate that 25% to 40% of affected patients will have cardiac events (onset of heart failure, symptomatic aortic stenosis, stroke, endocarditis, or cardiac surgery) by age 50. More than one fourth of patients who are free of important aortic stenosis or regurgitation at initial diagnosis will require cardiac surgery within 20 years. Thus, many if not most patients with bicuspid aortic valve will eventually require surgical or catheter intervention.
Among adults, cusp calcification progressing to aortic stenosis is thought to be initiated by endothelial dysfunction and inflammation, lipoprotein deposition, and fibrosis, and contributed to by turbulent flow. Calcification is frequently present by age 40, and stenosis is often then progressive.
The natural history of aortic dilatation in patients with unoperated bicuspid aortic valve has been studied by Davies and colleagues. Compared with patients having a tricuspid aortic valve, aneurysm progression is greater (0.19 cm · y −1 vs. 0.13 cm · y −1 ), nearly twice as many undergo aortic surgery, and surgery occurs at a younger age. In a study using MRI and CT, additional involvement of the aortic root, aortic arch, or both was present in more than half the patients. Those with a bicuspid aortic valve are reported to have a ninefold increase in risk of acute ascending aortic dissection compared with those with a tricuspid valve, but the incidence of dissection remains low: 0.1% per patient-year of follow-up. Despite the risk for adverse cardiac events, 20-year survival in adults without important valve dysfunction at initial observation is equivalent to that of the general population.
After the usual preparations and median sternotomy, cardiopulmonary bypass (CPB) is established at 34°C using a single two-stage venous cannula. A cardioplegia infusion catheter is positioned in the ascending aorta, and a coronary sinus perfusion catheter may be passed through a purse-string stitch in the right atrium and positioned in the coronary sinus. The cardioplegic infusion catheter, on one arm of a Y assembly on the cardioplegic infusion tubing filled with cardioplegic solution, is positioned in the ascending aorta. The other arm of the Y assembly, now clamped, also has two arms. One is connected to a second Y assembly, on each arm of which is an O-ring cannula to be used for direct cardioplegic infusion into the coronary ostia. The other arm can be attached to the coronary sinus retrograde perfusion catheter (see Technique of Retrograde Infusion in Chapter 3 ).
Perfusate temperature is lowered and adjusted to 25°C to 28°C. The ascending aorta is occluded, promptly if ventricular fibrillation occurs, to prevent LV distention. The operation may be performed without a vent; alternatively, a vent may be introduced into the left atrium from the right side through the right superior pulmonary vein and advanced into the LV. The vent catheter may be introduced before aortic occlusion if ventricular fibrillation occurs or aortic regurgitation produces ventricular distension during cooling. Following aortic clamping, antegrade cold blood cardioplegia may be infused into the aortic root provided there is no aortic regurgitation. Even the slightest leak at the aortic valve will cause ventricular filling, although a properly functioning LV vent may prevent ventricular distention. Subsequent to cardiac electromechanical arrest, cold cardioplegia may be infused into the coronary sinus. Cardioplegic arrest may be accomplished by exclusive perfusion of the coronary sinus in the presence of important aortic regurgitation. A small aortotomy should be made or the aortic root vented when coronary sinus perfusion is performed. Alternatively, in the presence of aortic regurgitation, topical and systemic cooling of the vented LV may intentionally induce ventricular fibrillation, after which the aorta is promptly clamped, an aortotomy made, and direct coronary artery cardioplegia administered.
An initial aortotomy is made about 15 mm downstream from the origin of the right coronary artery. Its precise location is very important not only for surgical exposure but also because of space for intraaortic positioning of an allograft, autograft, or prosthetic valve, ease and security of closure, avoiding damage to the right coronary artery or its ostium, and facilitating aortic root enlargement if necessary. Exposure for this incision is facilitated by the first assistant's retraction of the fat pad along the right atrioventricular groove over the aortic root. The pulmonary trunk may also need to be partially dissected from the aorta to avoid incising it. The initial incision is made directly anteriorly with scissors, facilitated by the collapsed state of the aorta. Once this small incision is made, the inside of the aortic root is visualized and a decision made as to whether an allograft valve, a pulmonary autograft valve, or a prosthesis will be used or repair performed (see “Nonreplacement Aortic Valve Operations in Adults” under Special Situations and Controversies later in this chapter).
The incision is extended. The surgeon has a choice depending on the operation to be performed:
Extend the incision transversely ( Fig. 12-9, A ). This is the most common approach and has the advantage of providing good exposure of the aortic root without distorting it. The sinutubular junction is not disturbed. Exposure at the level of the aortic valve and below into the left ventricular outflow tract (LVOT) is usually very good.

Extend the incision into the posterior commissure between the left and noncoronary cusps if posterior enlargement of the aortic root is required.
Extend the incision obliquely ( Fig. 12-9, B ) into the noncoronary sinus to a point near the aortic anulus, to provide maximal exposure at the level of the aortic valve and below into the LVOT. This incision, which divides the sinutubular junction, can be extended into the anterior leaflet of the mitral valve for posterior aortic root enlargement.
Extend the incision to divide the aorta ( Fig. 12-9, C ). This incision provides optimal exposure of the aortic root because the proximal aortic structures can be easily moved and displaced inferiorly and anteriorly so that the surgeon may visualize the intact aortic root and look directly into it. This incision is best for placing aortic allografts and stentless porcine bioprostheses inside the aortic root, as well as for aortic valve replacement with a pulmonary autograft or other procedures requiring replacement of the complete aortic root. It also provides excellent exposure for routine prosthetic valve implantation. Traction stitches are placed just above the aortic valve commissures for optimal exposure of the aortic root structures, regardless of type of incision.
The aortic valve is removed ( Fig. 12-10, A ). Unless the aortic valve disease is noncalcific, a short strip of narrow packing gauze can be inserted through the valve orifice into the LV (and some foolproof system is used to ensure its removal) to trap all calcific fragments that may escape during valvectomy. Neat, complete removal of the valve, particularly when heavily calcified, without damage to the LV-aortic junction, ventricular septum, or aortic wall, is one of the operation's critical aspects. Usually an area exists in about the midportion of the right coronary cusp where an initial scissors cut can be made from the free edge to the point of cusp attachment. This incision allows entry of a knife blade to incise precisely along the attachment of the right coronary cusp toward the commissure between left and right coronary cusps. This commissure may also be calcified, but the incision can usually be carried between it and the aortic wall, often with scissors. The incision is then carried along the attachment of the left coronary cusp, stopping at a point about two thirds of the distance to the left coronary–noncoronary cusp commissure, because beyond that point, there is a tendency to carry the incision into the aortic wall or LV-aortic junction.

Returning to the right coronary cusp, the incision is extended toward the right coronary-noncoronary cusp commissure. In this area and in this commissure the calcification is often especially abundant, sometimes extending onto the underlying ventricular septum or, especially at the commissure, onto the aortic wall or underlying membranous septum. Thus, in dissecting this area, great care must be taken in deciding whether to cut through the calcific cusp attachment to the aortic wall or to go around some of the calcific material and leave it for later piece-by-piece removal. To the extent possible, one-piece removal is preferable, but perforation of the septum, LV-aortic junction, or aortic wall should not become a risk.
When the aortic valve is completely replaced by calcium deposits, or when the deposits extend into the sinus aorta or anterior leaflet of the mitral valve, it is useful to mobilize the calcified tissues by the endarterectomy technique. Using a scalpel, a shallow incision is made in the aortic intima alongside the calcific deposit. This allows insertion of an endarterectomy spatula (Freer septum elevator) to lift the intact hard deposit away from the soft underlying aortic or mitral valve tissues without fragmenting the calcified material. The incision is carried down along the attachment of the noncoronary cusp, stopping about two thirds of the distance to the commissure between the noncoronary and left coronary cusps. The latter commissural area, which is at particular risk of junctional or aortic wall perforation during valvectomy, can then be approached with excellent visibility from both sides; the incision is carried through this area with firm upward traction on the valve.
After the valve is excised, the bed is examined and any loose calcific particles removed. Any remaining fragments are grasped with forceps or small rongeur and gently enucleated with a twisting motion ( Fig. 12-10, B ).
The downstream area of aorta is irrigated and examined for any loose calcific fragments, and the valve bed is wiped with gauze and irrigated with cold saline solution to remove any tiny fragments. The LV vent is turned off so that it will not suck fragments into the inaccessible depths of the ventricle, and the gauze strip is carefully removed from the LV cavity, most likely having trapped a few small calcific fragments. The LV cavity is then vigorously irrigated and aspirated with high suction and inspected for fragments. Generally, no fragments are found. With the precautionary measures against calcific embolization complete, the LV vent is again activated.
Insertion of a prosthetic aortic valve is the most common operation for replacing the aortic valve. The anulus is sized and an appropriate-size prosthesis selected. There is no advantage in choosing an oversized prosthesis that will erode the aortic anulus. Instead, if the aortic anulus is too small to accommodate a prosthesis that will provide adequate hemodynamic performance, a supraanular device may be chosen or the anulus enlarged (see “Managing the Small Aortic Root” under Special Situations and Controversies later in this chapter).
Synthetic suture material is used, with a compressed PTFE pledget placed centrally and needles at each end. Alternating suture colors (green, white) simplifies sorting so that sutures may be held together as a group for each aortic sinus. Mattress stitches are taken through the aortic valve anulus beginning at the commissure between the left and right coronary cusps. The suture is placed through the sewing ring of the prosthesis after completing each anular pass. Stitches are placed in the right coronary anulus working clockwise toward the commissure between the right and noncoronary sinus ( Fig. 12-11, A ). Separate stitches are placed close to one another, and the space along the aortic anulus is taken beneath the pledget of the mattress stitch. The prosthesis is held away from the aortic anulus until all stitches have been placed.

The anulus of the left coronary sinus of Valsalva is then approximated to the sewing ring of the aortic valve prosthesis, working counterclockwise from the commissure between the left and right coronary cusps ( Fig. 12-11, B ). The anulus of the noncoronary sinus of Valsalva is then approximated to the valve prosthesis, working clockwise from the commissure between the right and noncoronary cusp toward the commissure between the left and noncoronary cusp ( Fig. 12-11, C ). Needles are passed through the anulus in a backhand manner. The three groups of sutures are then strongly retracted so that the prosthesis may be slid over the suture loops into the aortic anulus. Position of the occluder mechanism may be adjusted before the valve holder is removed.
Sutures are sorted and tied down in order, working first in the noncoronary sinus in a counterclockwise approach. The first suture in the left coronary sinus closest to the commissure between the left and right coronary cusps is tied to secure seating of the prosthesis directly across the anulus from those sutures already tied in the noncoronary sinus. The sutures of the left coronary sinus are tied down counterclockwise. Sutures are tied in the right sinus, working clockwise to complete the procedure ( Fig. 12-11, D ). For valve prostheses that have any part of the device projecting below the sewing ring, such that it is positioned partially below the anulus in the LVOT, the order of tying should be altered so that the prosthesis is first secured adjacent to the portions of the device below the anulus.
An alternative technique for placing the pledget stitches is used for the small aortic anulus ( Fig. 12-11, E ). Pledgets are placed below the anulus in the LVOT by passing a double-needle suture with center pledget as a mattress stitch from below the anulus and up through the prosthesis. A larger prosthesis is thereby secured above the anulus as the anulus is compressed between the pledget and device.
Continuous suture technique provides the advantage of tight approximation of the prosthesis to the aortic valve anulus, because the suture loops can slip through the tissues so that tension is equalized with heart motion. Inserting a slightly larger prosthesis may also be possible because an interrupted mattress suture technique may bunch tissues together.
The aortic anulus is divided into three segments by the commissures. During valve replacement, the anulus is further subdivided into six subsegments at the midpoint on the anulus between the commissures. Polypropylene suture (2-0) is used, with needles at each end and a compressed PTFE pledget in the center of the suture. An initial mattress stitch is placed at the center of the sinus of Valsalva through the anulus of the aortic valve and brought through the sewing ring of the prosthesis ( Fig. 12-12, A ). The prosthetic valve is held away from the anulus and positioned and retracted for added exposure. Exactly three stitches are placed between the initial pledgeted stitch and the commissure on each side of the sinus. The final stitch at each end is secured to the wound drapes by a hemostat. A loop of size 0 suture is placed around the polypropylene suture as the first suture loop is completed through the prosthesis in each subsegment. This suture loop is held by a hemostat to be used to adjust tension on the suture line.

Sutures in the right coronary anulus are placed from the center toward the commissures in the first and second subsegments. The initial stitch in the left coronary sinus passes through the sewing ring of the prosthesis opposite the last stitch of the second subsegment. Working from the center to the commissures in the left coronary sinus, the third and fourth subsegments are approximated to the prosthesis, and then the fifth and sixth subsegments are completed in the noncoronary sinus.
Traction is placed on the six size 0 silk loop sutures to pull the prosthesis into the anulus of the aortic valve ( Fig. 12-12, B ). The occluder of the prosthesis is opened, and the area below the prosthesis is checked to ensure that no loose suture loops exist in the LVOT beneath the prosthesis.
The tension suture loops are removed sequentially and the ends of the polypropylene suture pulled up tightly to approximate the sewing ring of the prosthesis to the anulus. A final check of this approximation should be made with special attention to placement of the pledget, which should be above the anulus at the point of maximal stress deep in the center of the sinus of Valsalva. The suture ends are then joined by a knot at the three commissures (see Fig. 12-12 , B ).
Replacing the aortic valve with a transplanted human aortic valve became more feasible and available to surgeons because of improved commercial cryopreservation techniques.
Because with this technique the graft is to be sewn in “freehand” using the natural aorta for support, a clear understanding of the anatomy and spatial relationships of the aortic root is essential. Important deformity of the aortic sinuses should be appreciated and corrected or the procedure abandoned in favor of conventional valve replacement or aortic root replacement techniques.
A transverse aortotomy is made initially. After assessing aortic valve morphology and anatomy of the aortic root, the incision is extended to transect the aorta (see Fig. 12-9, C ). The aortic valve is excised and anulus débrided by usual techniques. Diameter of the aortic root at the level of the ventricular-aortic junction (anulus) is determined using standard sizing devices. This dimension must be accurately measured and clearly visualized. The aortic valve allograft to be placed inside the aortic root will consume space simply because of the thickness of its wall. Therefore, it must be 1 to 2 mm smaller in internal diameter than the measured aortic anulus. This will allow some redundant aortic valve cusp to provide a larger than normal coaptation surface to accommodate the expected tissue shrinkage for several weeks after implantation.
The aortic allograft is removed from the liquid nitrogen freezer valve bank and thawed by protocol. The septal muscle is excised, with a finger placed inside the aorta to stabilize the graft, gauge the thickness of the trimmed graft, and remove endothelial cells that are antigenic.
Excess aorta is trimmed from the valve cusps, leaving a 3- to 4-mm rim of aorta beyond attachment of the cusps. Most of the sinus aorta is removed from the right and left coronary sinuses, leaving the noncoronary sinus intact ( Fig. 12-13, A ). This technique was described by Ross and colleagues in London and has been used successfully for many years.

The allograft is implanted in anatomic position. Three stitches are used to attach it to the outflow tract. The first suture is 3-0 or 4-0 polypropylene, placed using two small (17-mm) strong half-circle needles. This suture is chosen for high needle strength and low tissue drag. The suture is placed through the patient's aortic outflow tract below the medial commissure between the right and left coronary sinuses and through the septal myocardium below the corresponding commissure in the graft; the stitch is placed below the anulus of the aortic valve. The other two stitches are simply stay sutures placed to assist in aligning the allograft to the patient's aortic root. These sutures will be removed because the primary suture line includes their position. They are placed beneath the appropriate commissure of the allograft and directly below the anterior and posterior commissures of the patient's aorta.
The allograft valve is lowered into position in the patient's aortic root. Commissures of the allograft are inverted through its anulus into the patient's LV so as to expose the subvalvar edge of the allograft. A knot is placed in the primary suture, and the stay sutures are tightened to align the allograft with the LVOT. Stitches are placed between the allograft and the LVOT at or below the level of the anulus, attempting to make a level suture line ( Fig. 12-13, B ). Because the aortic anulus is not circular but crescent shaped, the stitches are well below the fibrous anulus in the subcommissural region and through this fibrous tissue of the anulus at the midpoint of the aortic sinus (see “Cardiac Valves” in Chapter 1 ). The stitches below the left coronary sinus are placed first. The suture line is taken to a point below the posterior commissure. Using the opposite needle, the stitches between the allograft and LVOT are placed below the right coronary sinus and completed below the noncoronary sinus ( Fig. 12-13, B ). Alternative suture techniques are equally effective, such as using three separate polypropylene sutures to facilitate placing multiple suture bites without “snugging” the suture line to improve exposure, after which the sutures are tightened with a blunt nerve hook prior to tying. In the small aortic root, simple sutures of 4-0 polypropylene are also effective.
The commissures of the allograft are pulled out of the LV so that the allograft assumes its normal position and configuration. The allograft commissures are attached to the patient's sinus aorta using continuous 4-0 or 5-0 polypropylene sutures. Separate sutures are used for each aortic sinus. The first stitch is taken deep in the sinus aorta at midposition in the sinus slightly above the aortic anulus and then passed through the allograft. Suturing proceeds along the sinus aorta toward the commissure, so as to place the allograft flat against the patient's aortic wall. The final stitch is placed at the top of the commissure, leaving the suture to be secured later ( Fig. 12-13, C ). Suturing proceeds in each aortic sinus until the allograft is completely attached. Either the right or left coronary sinus is completed first, sewing from the center point of the sinus to each of the commissures using opposite ends of the suture. The repair is completed in the noncoronary sinus by shortening the allograft aorta to approximate the height of the patient's intact noncoronary sinus. The two edges of the aorta are simply oversewn by continuous suture so that the intact noncoronary sinus of the allograft is completely enclosed within the aorta. Optionally, the space between the allograft noncoronary sinus and the underlying native aortic wall can be partially obliterated with mattress sutures that are tied outside the native aorta. The ends of the patient's aorta are then reanastomosed by continuous suture.
Intraoperative echocardiography is used to confirm aortic valve competence.
An aortic allograft may be used for valve replacement as part of a root-enlarging operation. A transverse incision is made in the patient's aorta. The incision is extended to the posterior aspect of the aorta and into the posterior commissure between the left and noncoronary sinuses. Incision into the triangular space of the commissure between the fibrous attachment of the native aortic valve opens the aortic root where there is little fibrous support so that the edges of the aortotomy will separate widely. The incision is taken to the upper edge of the anterior leaflet of the mitral valve, but it does not need to enter the leaflet tissue to provide the desired separation of the edges of the incision. The increased diameter of the patient's aortic root is measured and an appropriately sized aortic allograft chosen. The allograft is trimmed, leaving the noncoronary sinus intact. The sinus aorta is removed from the left and right sinuses, leaving a few millimeters attached to the fibrous support of the cusps. The allograft is attached to the rim of the aortic-mitral anulus (without entering the actual anterior leaflet of the mitral valve) and superior aspect of the left atrium with interrupted 3-0 or 4-0 polypropylene sutures. The stitches are placed in the corresponding mitral valve and left atrium of the allograft's noncoronary sinus. A PTFE felt strip is fashioned to slightly more than the length of the unsupported area of the separated aortotomy. The sutures of the noncoronary sinus are then tied down over the felt strip so as to incorporate it and fill in any potential defects in the suture line. The commissures of the valve are inverted into the LVOT, and the rest of the repair is completed according to the approach previously described under “Subcoronary Technique.” The allograft's intact noncoronary sinus is used to close the aortotomy and widen the aortic root.
When greater enlargement of the LVOT is required, or when there is infective endocarditis with extension of anular abscess into the anterior leaflet of the mitral valve, the incision in the aorta is extended across the mitral valve anulus into the middle of the valve's anterior leaflet. Anular abscesses are thoroughly débrided and much of the anterior leaflet of the mitral valve removed ( Fig. 12-14, A ). The anterior leaflet of the mitral valve is left attached to the aortic allograft and used to widen the LVOT or repair defects in the patient's mitral valve ( Fig. 12-14, B ). The defect in the roof of the left atrium is closed with a patch taken from the aorta of the allograft or with bovine pericardium.

An aortic allograft may be used to replace the patient's aortic root completely when gross deformity is caused by infection or congenital anomaly, or it may be used to enlarge the root. Many surgeons use this technique routinely because aortic valve competence is virtually assured due to retention of the valve relationships within the intact aortic root of the allograft.
An initial transverse aortotomy is made ( Fig. 12-15, A ). After decision to proceed with aortic root replacement, the patient's aorta is transected ( Fig. 12-15, B ). The sinus aorta is removed except for a rim surrounding the ostia of the coronary arteries. The aortic valve is removed and an appropriately sized aortic allograft selected. The allograft is used intact and in natural anatomic orientation, with only the excess of septal myocardium and the anterior leaflet of the mitral valve removed. Size match is not nearly as important as it is for freehand subcoronary valve replacement, but if the aortic anulus is more than 3 mm larger in diameter than the largest available aortic allograft, the patient's aortic root should be narrowed to approximate the size of the allograft. This can be done conveniently by placing a pledgeted mattress stitch through the aortic anulus alongside the commissures so that when tied, the intercusp triangle below the commissure is obliterated (see “Method for Reducing Diameter of Dilated Aortic Anulus” later in this chapter).

The allograft is attached to the LVOT at the aortic anulus and below the commissures by simple interrupted stitches of 3-0 or 4-0 polypropylene ( Fig. 12-15, C ). The key to a hemostatic proximal suture line is use of a PTFE felt collar. The felt is approximately 5 mm wide and long enough to encircle the allograft. The allograft is slipped over the sutures into the desired position in the LVOT. The sutures are then tied down, incorporating the felt strip within the suture loops ( Fig. 12-15, D ).
An alternative hemostatic method is to incorporate a double-suture-line technique in which the first suture line uses three separate polypropylene continuous sutures (one for each sinus), placing the sutures as in the interrupted technique. They are gently tightened with a nerve hook and tied. A second suture line incorporates the cut edge of the aortic wall adjacent to the anulus and the adventitia of the allograft immediately above the prior suture line.
The coronary ostia on the allograft are removed to create openings 5 to 10 mm in diameter ( Fig. 12-15, E and F ). The coronary arteries are anastomosed to the allograft by continuous 4-0 or 5-0 polypropylene suture using a small needle (exact size depends on thickness of the tissues). The left coronary anastomosis is created first ( Fig. 12-15, E ), followed by the right coronary anastomosis ( Fig. 12-15, F ). Repair is completed by end-to-end anastomosis of the distal end of the allograft to the patient's aorta ( Fig. 12-15, G ). Continuous stitches of 3-0 or 4-0 polypropylene are used.
The aortic valve allograft may be inserted as a cylinder within the aortic root, using a technique described by O’Brien and colleagues.
Buttons of sinus aorta are excised, including the ostia of the left and right coronary arteries of the allograft. The resultant opening may need to be extended proximally because the allograft valve now lies a little downstream from its native position. This technique has the potential disadvantage of too much open space between the allograft and native aortic walls.
In a slight modification of the cylinder technique, the center of each sinus is removed before inserting the cylinder of allograft, leaving an intact strut of aortic wall over each sinus. This approach maintains the integrity and special relationships of the allograft until the majority of the second (downstream) suture line of each sinus is complete and the bar over that sinus excised.
Several manufacturers supply porcine xenograft stentless aortic valves. The devices most frequently used are manufactured by St. Jude Medical and Medtronic ( Fig. 12-16, A ). The St. Jude Medical device (Toronto SPV) is supplied for subcoronary valve replacement with the sinus aorta removed from all three sinuses of Valsalva. The device is covered with a thin polyester mesh. Proper insertion depends on near-normal diameter relationships of the aortic anulus and the sinutubular junction. The Medtronic device (Freestyle) is presented as the entire untrimmed aortic root.

The implant technique is similar to aortic valve replacement with aortic allografts. The aorta is divided about 1 cm above the sinutubular junction, or a transverse aortotomy is made in the same location. The proper size of xenograft is chosen using the sizing devices supplied by the manufacturer. The diameter of the aortic anulus is measured, and the same-size xenograft is chosen for implantation. Downsizing is not required because the xenografts are sized according to outside diameter of the device, in contrast to aortic allografts, which are sized according to inside orifice diameter. Some surgeons choose a xenograft 1 or 2 mm larger than the anulus because these flexible stentless bioprostheses fit easily into the aortic root. However, no advantage exists to greatly oversizing the prosthesis, because oversized xenografts increase the gradient across the valve.
The Medtronic Freestyle bioprosthesis requires trimming before implantation (see Fig. 12-16, A ). The sinus aorta is removed from the right and left sinuses of Valsalva. The noncoronary sinus remains intact to fix the position of two of the three commissures of the valve. The lower edge of the aortic root of the xenograft is covered with polyester cloth to assist with implantation and to prevent shrinkage when xenograft septal myocardium is absorbed. This cloth limits how deeply the sinus aorta may be excised, especially in the right coronary sinus. Care should be taken not to disrupt the cloth covering.
The aortic valve is excised in the usual manner, and all calcific deposits are removed from the aortic anulus and sinus aorta. Exposure is enhanced by traction sutures placed at the sinus rim above each commissure. Markings on the cloth covering the lower edge of the xenograft correspond to each commissure. Continuous 3-0 polypropylene suture is used to attach the lower edge of the xenograft to the patient's aortic anulus. A small needle with a taper-cut design is best to allow easier penetration of the polyester fabric. The suture line is started in the intercusp triangle below the commissure between left and right sinuses.
The prosthesis is held away from the anulus as suture loops are placed ( Fig. 12-16, B ). A heavy suture (0 or 2-0) is passed around every third suture loop to act as a tension-adjusting loop suture. These tension loops are secured by a small hemostat. Suturing proceeds below the right sinus to the commissure below the right and noncoronary sinuses. Stitches are placed mostly through the thick fibrous hinge of the aortic valve (anulus), attempting to level the plane of the suture line rather than strictly following the semilunar plane of the valve hinge ( Fig. 12-16, C ). From the midpoint of the right coronary sinus to the commissure between the right and noncoronary sinuses, the stitches must be through the anulus, not below and into the myocardium, to protect the conduction system from injury.
Suturing continues below the left sinus of Valsalva until below the commissure between the left and noncoronary sinuses. A separate suture is used for noncoronary sinus repair. Tension loops are placed around every third stitch. The prosthesis is seated in the aortic root by sequentially pulling up on the tension loops to approximate the fabric of the xenograft to the aortic anulus. Alternatively, the inflow suture line may be simple interrupted stitches placed with the xenograft held away from the anulus (see Fig. 12-16, B ). Many surgeons prefer the interrupted stitch technique because it is similar to implanting prosthetic devices and avoids the possibility of a purse-string effect.
The sinus aorta of the xenograft is attached to the patient's sinus aorta by continuous stitches of 4-0 polypropylene ( Fig. 12-16, D ). The initial stitch is placed in the patient's sinus aorta just below the right coronary ostia. The suture line comes very close to the coronary ostia to achieve proper fit of the xenograft tissues to the patient's sinus aorta. This is especially true for the right coronary sinus of the xenograft, which is covered with cloth quite high on its external surface. The commissure between the right and left sinuses of the xenograft must be carefully located so as not to distort the xenograft. Suturing continues beneath the left coronary artery to the top of each adjacent commissure ( Fig. 12-16, E ).
The noncoronary sinus repair is accomplished by trimming the xenograft to the same height as the patient's noncoronary sinus aorta. The two edges of tissue are approximated by direct suture (see Fig. 12-16, E ). Sufficient space usually exists in the patient's noncoronary sinus of Valsalva to accommodate the xenograft without distortion. If the diameter (length) of the aorta at the sinutubular junction in the noncoronary sinus is greater than that of the sinutubular junction of the xenograft, the discrepancy can be compensated for by placing stitches more closely on the xenograft than the patient's aorta. The repair is completed by anastomosis of the aorta or closure of the transverse aortotomy.
The Medtronic Freestyle device may be implanted as a complete aortic root replacement ( Fig. 12-16, F ). This method is employed when the aortic root is distorted or when it is dilated enough that support for the xenograft as a subcoronary valve implant will be inadequate. The aorta is divided just above the sinutubular junction. The left and right coronary arteries are mobilized, retaining a generous rim of sinus aorta around the coronary ostia, and the noncoronary sinus aorta is excised. The device is implanted as a complete root. The inflow suture line is placed as for subcoronary implantation using continuous 2-0 polypropylene. The suture line is started in the intercusp triangle below the commissure between left and right sinuses. Suture loops are placed through the anulus of the aortic valve below the right sinus, then the left, then the noncoronary sinus. Tension loops are placed around the primary suture every third suture loop. Suture loops may be placed around a PTFE felt strip for added suture line hemostasis. Alternatively, simple interrupted sutures may be employed.
The device is firmly attached to the aortic valve anulus by tightening the suture loops. The ligature on the left coronary artery of the xenograft is cut away to create an opening into the graft. The patient's left coronary artery is anastomosed using continuous 4-0 or 5-0 polypropylene suture. Although the left coronary artery always fits neatly to the position of the graft's left coronary artery, the right coronary artery may not fit properly to its counterpart because coronary arteries in the porcine aortic root are closer together than in humans. It is usually necessary to create another opening into the right coronary sinus of the graft at an appropriate location to prevent kinking of the patient's right coronary artery. The position is usually to the right of the right coronary artery of the porcine root, although it may be placed on part of the base of the porcine right coronary artery. Proper location of the right coronary artery is facilitated by filling the RV with blood from the pump-oxygenator. Any evidence of myocardial ischemia after aortic root replacement indicates need for revision of the location of the coronary anastomosis or for coronary artery bypass grafting.
The aortic valve may be replaced with the patient's own pulmonary valve (pulmonary autograft) and a pulmonary allograft used to replace the pulmonary valve. This operation was devised by Ross and carries his name. The operation has the advantage of placing autogenous tissue in the high-pressure aortic position that theoretically should last indefinitely, assuming the procedure is technically correct. (See “Selection of Technique and Choice of Device for Isolated Aortic Valve Replacement” later in this chapter). The allograft tissue is placed in the low-pressure pulmonary position, where even if it should fail, valve regurgitation is tolerated for a long time.
CPB is established using two cannulae for venous drainage. A right-angle cannula is placed directly into the superior vena cava at the pericardial reflection. The other is placed in the inferior vena cava through the right atrium at the diaphragm (see “Preparation for Cardiopulmonary Bypass” in Section III of Chapter 2 ). Alternatively, a single two-stage venous uptake cannula may be used, placing the second-stage opening low in the right atrium near the inferior vena cava orifice. Vacuum-assisted venous return provides more safety and reserve when the single-cannula technique is used, reducing the risk and consequences of air lock, which may occur in a gravity drainage system when the RV is opened (see “Vacuum-Assisted Venous Return” in Section I of Chapter 2 ). Oxygenated blood is returned to the ascending aorta.
The aortic root is explored through the usual transverse incision. Once it is determined to proceed with the pulmonary autograft operation, the ascending aorta is divided, the aortic valve removed, and the entire sinus aorta excised except for a generous rim around the coronary ostia ( Fig. 12-17, A ). Traction stitches on the aorta above the coronary arteries are helpful. Only the fibrous connection of the aortic cusps remains for attachment of the pulmonary autograft. Traction stitches are placed above each commissure. Diameter of the aortic anulus is measured using Hegar dilator sizers.

The pulmonary trunk is separated from the aorta up to its bifurcation. It is divided at the bifurcation, taking care not to shorten the right pulmonary artery ( Fig. 12-17, B ). The pulmonary valve is inspected from above. If it is normal, operation may proceed. If it is abnormal, the pulmonary artery is reanastomosed and an aortic allograft used to replace the aortic root.
Dissection between the medial commissure of the aorta and the pulmonary trunk comes down onto the muscle of the right ventricular outflow tract (RVOT; Fig. 12-17, C ). Thorough dissection between the aorta and pulmonary trunk, especially low onto the RV in the plane between the RVOT and the infundibular septum, makes removing the pulmonary trunk much easier.
The anterior intercusp triangle below the anterior commissure is identified as a reference point for opening the RVOT. A small right-angle clamp is placed precisely in the anterior intercusp triangle and pushed through the anterior wall of the RV. The small opening is carefully enlarged. The pulmonary trunk is separated from the RV, looking inside frequently to maintain appropriate length of the ventricle below the pulmonary valve ( Fig. 12-17, D ). Incision of the RVOT continues on the right side and posterior to the extent of previous dissection ( Fig. 12-17, E ).
The critical and unique part of the Ross operation is separating the pulmonary trunk from the RVOT above the infundibular septum. When done correctly, this part of the procedure makes the entire operation safe. Anatomy of the subpulmonary infundibulum is described in “Right Ventricle” under Cardiac Chambers and Major Vessels in Chapter 1 . Injury to the underlying first septal branch of the left anterior descending coronary artery during dissection of the pulmonary trunk is a major source of morbidity in this operation. A shallow incision of the endocardial surface is made to join both ends of the partly excised pulmonary trunk ( Fig. 12-17, F ). The incision extends completely across the remaining undivided RVOT. The angle of the scalpel is immediately changed to shave off the ventricular myocardium almost parallel to the endocardial surface. This shaves off the pulmonary trunk without injuring the underlying first septal branch. This part of the operation should be performed deliberately and under precise control. The septal excision eventually separates the pulmonary trunk completely, leaving the left main coronary artery and the first septal branch behind and protected by a generous layer of tissue. The septal artery is occasionally seen in the myocardium.
The separated pulmonary trunk is placed in the pericardial sac for immediate use. The trunk is not removed from the operating field, to prevent its misplacement or inadvertent mixture with the allograft. At this point it is convenient to measure the dimensions of the pulmonary anulus using standard prosthetic valve sizers and to choose a pulmonary allograft for preparation for later use.
The pulmonary autograft is attached to the unmodified LVOT, using interrupted stitches of 3-0 polypropylene ( Fig. 12-17, G ). Size discrepancy between the pulmonary autograft anulus and aortic anulus should not exceed 2 mm. A larger discrepancy indicates the need to adjust the anulus of the aorta to match that of the pulmonary autograft (see “Method for Reducing Diameter of Dilated Aortic Anulus” later in this chapter). The autograft is marked below each of the commissures for orientation. The anterior commissure of the autograft is oriented to approximate the commissure between the right and noncoronary sinuses. Suturing proceeds below each sinus, separating the stitches into three groups. Stitches are placed through the strong fibrous tissue of the aortic anulus, keeping the plane of repair as level as possible. A strip of PTFE felt about 5 mm wide is used to fix the diameter of the proximal suture line and to ensure hemostasis between the sutures ( Fig. 12-17, H ). A strip of autogenous pericardium may also be used. A “supported” repair is favored to prevent dilatation of the pulmonary autograft at the proximal suture line. Some surgeons prefer a continuous suture line using absorbable stitches without prosthetic material, especially for children in whom growth of the pulmonary autograft is desired. However, the accuracy of carefully spaced individual stitches and fixation support of the anulus seem best in adult patients. The felt or pericardial strip is placed within the suture loops so that it is incorporated as the sutures are tied down. The pulmonary autograft is partially inverted into the LVOT as the sutures are tied to ensure that tissue approximation is accurate and that the support ring is to the outside rather than interposed between the tissues of the autograft and the aortic anulus.
An opening is made into the left coronary sinus of the autograft using a scalpel ( Fig. 12-17, I ). Only minimal sinus tissue is excised, because the delicate pulmonary artery dilates and separates readily. The sinutubular junction of the autograft is preserved to maintain proper relationships of the commissures and cusps.
The left coronary anastomosis proceeds, working on the outside of the pulmonary autograft ( Fig. 12-17, J ). Continuous stitches of 5-0 polypropylene are used. As a practical matter, it is best to perform the right coronary anastomosis after completing the aortic anastomosis so that the pulmonary trunk can be distended by temporarily removing the aortic clamp ( Fig. 12-17, K ). The right coronary anastomosis typically fits high in the right sinus or even above the sinutubular junction on the anterior wall of the pulmonary trunk. It may also be advisable to anastomose the pulmonary allograft to the bifurcation of the pulmonary trunk before the aortic anastomosis if the medial aspect of the pulmonary anastomosis might be obscured by the overlying aorta ( Fig. 12-17, L ). Often the diameter of the aorta is larger than that of the pulmonary trunk, but the pulmonary trunk is usually sufficiently long that a bevel can be created to take up any size discrepancy. With gross aortic enlargement or aneurysm, the entire ascending aorta may be removed and the pulmonary trunk extended with a prosthetic graft (see “Method for Extending Pulmonary Autograft” later in this chapter).
The cryopreserved pulmonary allograft is trimmed minimally. An end-to-end anastomosis of the allograft to the pulmonary bifurcation is performed using continuous stitches of 4-0 polypropylene (see Fig. 12-17, L ). Retracting the LV inferiorly and placing the graft in the space between the pulmonary bifurcation and the RV makes it easier to perform the anastomosis of the posterior wall of the pulmonary arteries. It is sometimes preferable to perform this anastomosis before constructing the aortic anastomosis. The proximal end of the allograft trunk is anastomosed to the RVOT (see Fig. 12-17, L ). Continuous stitches of 3-0 polypropylene are used. Needle penetration must be through only part of the thickness in the infundibular septum to avoid injury to the underlying first septal branch of the left anterior descending coronary artery. A potentially weak point on the anastomosis at the transition from the infundibular septum to the medial aspect of the RV free wall may be reinforced with a PTFE pledget worked into the suture line. The completed repair produces a remarkably normal anatomic appearance ( Fig. 12-17, M ).
The pulmonary allograft may be tailored to achieve greater length, reducing the chance that the graft will be too tight between the RVOT and pulmonary artery bifurcation. The entire left pulmonary artery may be retained on the graft. The right pulmonary artery is removed and closed by direct suture using 5-0 polypropylene to lengthen the graft ( Fig. 12-18, A ). The left pulmonary artery is cut back to the bifurcation to create a large circumference for anastomosis. Making the pulmonary allograft long or even slightly redundant may be important in maintaining proper allograft length, because myocardium below the pulmonary allograft valve is resorbed and replaced with contracting scar.

When the patient's LVOT at the level of the anulus is more than 2 mm in diameter greater than the pulmonary autograft, the patient's anulus may be narrowed to fit the autograft, taking up this variance in anular diameters. Interrupted mattress stitches of 2-0 braided suture reinforced with PTFE pledgets are placed through the fibrous tissue that supports the aortic cusps alongside each of the commissures. The stitches are placed across the intercusp triangle so that the triangle is obliterated after tying down the suture ( Fig. 12-18, B ).
When the ascending aorta is greatly dilated or aneurysmal, it is advisable to remove the affected aorta. A polyester tubular graft with the same diameter as the pulmonary autograft's distal end at the sinutubular junction is selected for replacement of the ascending aorta as an interposition graft ( Fig. 12-18, C ).
Evidence indicates that the pulmonary trunk may dilate up to 30% in diameter and length when exposed to systemic arterial pressure. Schmidtke and colleagues found that the pulmonary trunk in the aortic position as a freestanding root assumed a diameter of 41 mm at the sinus level, compared with 32 mm when the autograft was placed within the natural aorta. The pulmonary trunk may also dilate in the clinical setting of bicuspid aortic valve with dilated ascending aorta. Fixing the diameter of the sinutubular junction of the pulmonary autograft may be desirable and may prevent or correct pulmonary autograft regurgitation. A segment of tubular polyester vascular prosthesis equal to, or 1 to 2 mm greater than, the diameter of the pulmonary autograft sinutubular junction (10% larger) is placed on the outside of the autograft to fix its diameter at its normal dimension. The polyester segment is attached to the pulmonary autograft with a few simple stitches to prevent migration ( Fig. 12-18, D ). A PTFE felt strip works equally well, but the diameter of the finished band is more difficult to control. Surgeons not wanting to fix the diameter of the pulmonary autograft at the sinutubular junction may find that narrowing the graft with a felt strip corrects possibly important autograft regurgitation ( Fig. 12-18, E ). Because of the known tendency for the pulmonary autograft to dilate, some have advocated reinforcing the entire autograft with bovine pericardium, polyester, or as an intraaortic cylinder within the native aortic root.
Replacement of the aortic valve and ascending aorta, en bloc, is most frequently performed for anuloaortic ectasia and ascending aortic aneurysm accompanied by aortic valve regurgitation. Occasionally, en bloc replacement is performed for infective endocarditis of the aortic root with extensive abscess formation and in the setting of acute or chronic aortic dissection with aortic valve regurgitation, for which the technique of distal anastomosis may be somewhat different.
The early steps of the en bloc operation differ somewhat from those of simple aortic valve replacement. The arterial cannula may need to be placed in the common femoral artery (see “Cardiopulmonary Bypass Established by Peripheral Cannulation” in Section III of Chapter 2 ). When the ascending aortic aneurysm stops short of the brachiocephalic artery origin, the distal ascending or proximal transverse arch can be cannulated in the usual manner.
A limited dissection is done, separating the right and contiguous portion of the pulmonary trunk from the back of the aorta and the aneurysm. This approach affords safe placement of the aortic occlusion clamp just proximal to the brachiocephalic artery. The procedure involves clean surgical separation of the right and contiguous portion of the pulmonary trunk from the back of the aorta and from the aneurysm, if possible.
CPB is established at 34°C using a single two-stage venous cannula and left atrial vent. The perfusate temperature is lowered to bring body temperature to 28°C. The aorta is occluded just proximal to the brachiocephalic artery. The ascending aorta is opened transversely in its midportion, stay sutures are applied, and cardioplegia is given.
The patient's aortic root is disconnected from the LVOT and left atrium–mitral valve complex. The sinus aorta surrounding the coronary arteries is retained. The remaining sinus aorta is excised, leaving only fibrous aortic valve attachments, which are normal and uninvolved with the disease process being treated. The ascending aorta is divided by extension of the transverse aortotomy.
When the ascending aorta is replaced along with the valve, sizing of the allograft valve is less critical than in the case of isolated aortic valve replacement. Aorta is usually sufficient on the allograft to replace the ascending aorta. Length of the retained ascending aorta and arch are considered when choosing the graft (see Fig. 12-14 ). The distal aorta is tailored to fit the native aorta. The distal anastomosis is performed directly or under circulatory arrest with the patient's body temperature at 18°C.
In young patients it may be desirable to use a pulmonary autograft as part of the en bloc operation. The pulmonary autograft is used as an intact pulmonary trunk to replace the aortic root as described earlier for isolated aortic valve replacement (see Fig. 12-18, C ). The abnormal dilated or aneurysmal ascending aorta is excised. A crimped polyester tubular graft, collagen coated and of the same diameter as the distal end of the pulmonary autograft, is selected for replacing the ascending aorta. This graft is anastomosed to the distal end of the pulmonary trunk. The graft is shortened and beveled appropriately for end-to-end anastomosis to the distal ascending aorta.
Replacing the aortic valve and ascending aorta as an en bloc procedure is most often done with a composite prosthesis containing a mechanical or bioprosthetic cardiac valve enclosed within a slightly larger tubular polyester graft. This operation is frequently referred to as the Bentall procedure. These grafts have a collagen or gel coating that makes them impervious to blood.
The patient is placed on CPB using a single venous two-stage cannula, with oxygenated blood returned to the femoral artery in most cases. Occasionally, the amount of ascending aorta is sufficient to allow it or the transverse portion of the aortic arch to be perfused. The ascending aorta is occluded by a vascular clamp, and a vent catheter is placed through the right superior pulmonary vein to the left atrium and LV. A vertical incision is made in the ascending aorta aneurysm. Cold cardioplegic solution is administered to the coronary sinus by a retrograde coronary perfusion cannula. Traction stitches are placed above each aortic valve commissure to expose the aortic root ( Fig. 12-19, A ). The aortic valve is excised. The coronary arteries are mobilized, retaining a generous button of sinus aorta. A limited dissection of the coronary artery is sufficient to ensure that excision of the coronary artery is complete and that the coronary button will move easily up to the composite prosthesis without kinking or creating undue tension on the artery. The remaining sinus aorta is removed.

Diameter of the anulus is calibrated, and an appropriately sized composite prosthesis, including the prosthetic aortic valve and attached crimped polyester tubular prosthesis, is selected. Stitches are placed through the anulus of the aortic valve and brought up through the sewing ring of the prosthesis using mattress stitches of 2-0 braided polyester with PTFE felt pledgets ( Fig. 12-19, B ). Suture placement is started in the right coronary sinus, working clockwise, as for typical aortic valve replacement. Repair of the left coronary sinus follows, working counterclockwise. The repair is completed in the noncoronary sinus, working clockwise.
Sutures are tied down to approximate the sewing ring of the prosthetic valve firmly to the aortic anulus ( Fig. 12-19, C ). A running suture technique is an acceptable method provided the anulus is strong and able to support the repair.
A decision is then made regarding the method of reimplanting the coronary arteries to the graft. A direct anastomosis of the coronary ostia to the graft is usually made ( Fig. 12-19, D and E ). When aortic dissection involves the coronary ostia, it is necessary to repair the dissected aorta with biological glue or reapproximate the dissected layers of aorta between PTFE felt washers ( Fig. 12-19, F ). In unusual circumstances when the coronary ostia are displaced far laterally by a large-diameter aneurysm, it may be advisable to interpose a second graft between the coronary ostia and aortic graft to prevent tension on the coronary anastomosis, which is the most frequent point of hemorrhage after repair. Irreparable coronary ostial obstruction may require a bypass graft between the aortic graft and the affected coronary artery ( Fig. 12-19, G ). The primary ostium of the affected coronary artery is closed by suture.
The graft may be shortened appropriately to facilitate anastomosis of the coronary arteries to its side. Openings are made into the side of the graft exactly opposite the coronary artery ostia using scissors or battery-operated cautery. Appropriately sized openings assist in creating the anastomosis. An adequate amount of graft should be left between the sewing ring of the prosthesis and the new coronary artery opening. The left coronary anastomosis is made first (see Fig. 12-19, D ). The coronary ostium is approximated to the side of the graft by continuous stitches of 5-0, 4-0, or 3-0 polypropylene, depending on thickness of the aortic tissues. All the suture loops around the inferior rim of the coronary ostium are placed before pulling them up so that this difficult area may be accurately approximated to the graft (see Fig. 12-19, D ). Stitches are placed in cartwheel fashion around the coronary artery ostium, with deep bites into the aorta.
The right coronary anastomosis is performed after completing the left coronary anastomosis (see Fig. 12-19, E ). Both coronary ostia are approximated to the side of the graft in a similar manner.
The distal end of the graft is then shortened to approximate the distal end of the aorta. The aorta is completely divided to allow a direct end-to-end anastomosis. A short segment of graft is cut and placed as a collar over the main portion of the graft. The anastomosis is constructed using continuous stitches of 3-0 or 4-0 polypropylene, depending on the thickness and strength of the aorta. It is convenient to bring the first stitch from the outside of the aorta so that the stitching may continue from the inside surface of the graft. Several suture loops may be placed posteriorly before pulling the graft tightly against the aorta, to ensure accurate closure of the posterior wall of the anastomosis ( Fig. 12-19, H ).
The anastomosis is continued anteriorly and is completed by including all layers of the aorta in the graft. The graft collar is used to cover the completed anastomosis for hemostasis ( Fig. 12-19, I ).
Modifications are required when coronary artery ostia are either close to the anulus or widely separated laterally by an aortic aneurysm. When coronary artery ostia are bound tightly to the aortic anulus, usually by prior operation or valve replacement, it is impossible to create an accurate anastomosis to the tubular portion of a composite graft because the valve sewing ring may impinge on the coronary ostia. In this situation, a composite valve prosthesis is constructed by placing a prosthetic valve within a tubular polyester graft so that there is a short length of tubular graft extending below and a longer length of graft extending above the sewing ring of the valve ( Fig. 12-19, J ). The graft and sewing ring of the prosthetic valve are attached by continuous suture. The extension of the tubular graft below the prosthetic valve is attached to the aortic anulus, displacing the level of the prosthetic valve cephalad above the bound down coronary ostia. Obstruction of the coronary ostia from contiguous placement of the thick sewing ring of the prosthetic valve is avoided. Ten-millimeter extension grafts from the aortic graft above the prosthetic valve to the coronary ostia complete the repair.
When the coronary artery ostia have been carried laterally and superiorly from the usual location relative to the aortic anulus by aneurysm or dilatation of the aortic root, it is necessary to create an extension from the composite prosthesis to the coronary arteries. Ten-millimeter tubular polyester grafts are attached to the coronary arteries and brought up to the anterior aspect of the composite prosthesis ( Fig. 12-19, K ).
Kunzelman and colleagues studied the relationships of the diameters at various levels in the aortic root, showing that the diameter at the sinutubular junction should be about 15% less than that at the base (anulus or ventricular-aortic junction) ( Fig. 12-20 ). Grande and colleagues showed that minor dilatation of the aortic root (5%-15%) caused increased stress on aortic valve cusps. In response to dilatation of the aortic root, strain on the valve cusps changes in order to maintain coaptation. This method of compensation fails at 30% to 50% dilatation of the aortic root, and cusp tissue is insufficient to maintain coaptation, resulting in aortic valve regurgitation. Frater demonstrated that simply adjusting the dimensions of the sinus rim or sinutubular junction can correct such regurgitation.

The remodeling operation consists of removing the sinus aorta except for a small rim of aortic tissue around the coronary ostia and a rim of about 5 mm of aortic wall above the aortic valve anulus. Commissures are positioned to achieve good coaptation of the aortic valve cusps.
Choo and Duran point out that the aortic root is dynamic, responding to pressure changes during the cardiac cycle that expand the aorta at the sinutubular junction by 35%, but the area at the base (anulus) by only 5%. Thus, they propose measuring the aortic root diameter at the base of the cusps as the most reliable method for appropriate graft sizing. The method proposed here is based on the measured diameter of the aortic anulus and simple arithmetic. When the diameter of the aortic anulus is normal, a graft is chosen that will narrow the sinutubular junction by 10% to 15%. When the diameter of the aortic anulus is enlarged, it is adjusted to normal diameter for body size using a graft diameter approximately 10% less than the desired aortic anular diameter. The geometry conveniently allows strips of the graft to support a reduction anuloplasty of five-sixths the circumference of the anulus (avoiding the conduction system) while the graft adjusts the diameter of the sinutubular junction.
David recommended that when aortic root remodeling procedures, such as the Yacoub operation, are performed in patients with Marfan syndrome, or when the aortic anulus is dilated, an aortic anuloplasty should be performed. A strip of prosthetic material is used on the outside of the LVOT below the aortic valve to correct dilatation of the fibrous components of the LVOT resulting from myxomatous changes in these tissues.
In this procedure, the aortic valve is reimplanted within a polyester tubular graft. The graft is secured to a level plane in the LVOT just below the valve, except in the one sixth of the circumference occupied by the conduction system. This fixes the diameter of the LVOT, but one may reduce the diameter if necessary. The aortic valve is attached (reimplanted) to the inside of the prosthetic graft. The graft determines the diameter of the sinutubular junction.
The reimplantation procedure has undergone a number of modifications by both David and other surgeons. However, the basic concept has been retained. Cochrane and colleagues modified the David operation to create pseudosinuses in the graft by removing three symmetric scallops from it, thereby lengthening the proximal suture line and restoring proper relationships at the sinutubular junction. The pseudosinus method (Cochrane) and sinus-tailored method (Yacoub) result in simulated cusp stresses that are closer to normal than does David's cylindrical technique. The graft is usually 30 to 32 mm in diameter, although 28- or 34-mm grafts are occasionally used.
After induction of anesthesia and before incision, intraoperative transesophageal echocardiography (TEE) is used to measure aortic root dimensions at the sinutubular junction and ventricular-aortic junction. Operations are performed on CPB using a single two-stage cannula for venous uptake, with oxygenated blood returned to a cannula placed high in the ascending aorta or arch or in the femoral artery. Hypothermic circulatory arrest is used when the aneurysm extends beyond the ascending aorta.
The aorta is divided above the sinutubular junction and the aortic valve thoroughly examined. Normal aortic valve cusps suggest the possibility of a valve-sparing operation. Diameters of the sinutubular junction and ventricular-aortic junction are measured using Hegar dilators or accurate valve sizers. Alterations of aortic root dimensions are noted and will guide the steps taken to restore dimensions to normal.
A normal aortic anulus with enlarged sinutubular junction is found in patients with aortic ectasia and aneurysm of the ascending aorta not involving the aortic sinuses. The coronary arteries are not displaced from their usual location in relation to the anulus.
A vascular graft the same diameter as the aortic anulus is selected. A 4- to 5-mm segment of the graft is prepared for placement on the outside of the aorta at the sinutubular junction ( Fig. 12-21 ). The thickness of the aortic wall when compressed within the graft will reduce the inside diameter of the aorta to restore the normal dimension, which is 15% less than the diameter of the aortic anulus. Using this short segment of graft is easier and more accurate than attempting to attach a longer graft directly to the sinutubular junction.

An enlarged aortic anulus with enlarged sinutubular junction is found in patients with anuloaortic ectasia, some of whom have Marfan syndrome. Less commonly, patients with aortic regurgitation and sinus of Valsalva aneurysm will have a normal aortic anulus. Both the remodeling and reimplantation procedures have been recommended for this condition. Both are described here.
In the remodeling procedure, the aortic sinuses are removed. The aortic anulus is reduced to a diameter appropriate for the patient's body size ( Fig. 12-22 ), generally 25 mm for the average adult male, 27 mm for a large male, and 23 mm for an adult female. A vascular graft 10% to 15% smaller than that diameter is selected; thus, for a 25-mm anulus, a 22-mm graft is chosen. Two 4- to 5-mm segments (rings) of the graft are prepared to adjust the anulus diameter. The remaining graft is tailored by making three incisions and trimming the flaps for sinus reconstruction and replacing the ascending aorta. To achieve an LVOT 25 mm in diameter at the ventricular-aortic junction, the circumference of the aorta must be reduced to 78 mm (25 · π = 78). This reduction is accomplished by anuloplasty, using the short segments of graft to size the anulus accurately and to support the repair. Anuloplasty mattress stitches are placed in the LVOT at a level plane just below the hinge point of the aortic valve. The stitches are placed beginning below the nadir or midpoint of the right coronary cusp of the aortic valve and working counterclockwise to the commissure between the noncoronary and right coronary cusps. This places the stitches on five sixths of the circumference, avoiding stitches in the one sixth of the ventricular septum that contains the conduction system. A strip of fabric to cover five sixths of the circumference and achieve a diameter of 25 mm is 65 mm in length (78 · ![]() = 65). It is convenient that a 22-mm crimped tubular polyester graft provides a strip of fabric 75 mm in length when the 4- to 5-mm segment is cut, opened, and stretched to length. From the strip of the graft, 10 mm of length is removed. The anuloplasty stitches are placed through the fabric strip and passed through the LVOT to the outside. Thickness of tissue through which the needles pass is about 3 mm. Thus, the outside diameter to be supported will be about 28 mm in diameter. The length of fabric needed to support this diameter is, conveniently, about 75 mm (28 · π ·
= 65). It is convenient that a 22-mm crimped tubular polyester graft provides a strip of fabric 75 mm in length when the 4- to 5-mm segment is cut, opened, and stretched to length. From the strip of the graft, 10 mm of length is removed. The anuloplasty stitches are placed through the fabric strip and passed through the LVOT to the outside. Thickness of tissue through which the needles pass is about 3 mm. Thus, the outside diameter to be supported will be about 28 mm in diameter. The length of fabric needed to support this diameter is, conveniently, about 75 mm (28 · π · ![]() = 73). The anuloplasty stitches are passed through the outside fabric strip. A 25-mm-diameter Hegar dilator is placed in the LVOT while the sutures are tied down. This narrows the LVOT to a calculated diameter while distributing the tension equally over five sixths of its circumference.
= 73). The anuloplasty stitches are passed through the outside fabric strip. A 25-mm-diameter Hegar dilator is placed in the LVOT while the sutures are tied down. This narrows the LVOT to a calculated diameter while distributing the tension equally over five sixths of its circumference.

The sinus aorta is reconstructed to the flap graft. Diameter of the sinutubular junction is accurately restored by the diameter of the graft chosen for the repair. Relationships just described should hold for the various graft sizes that might be chosen for reconstructing the aortic root. Intraoperative TEE is performed with the heart contracting and ejecting at normal pressure to determine adequacy of the repair.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here