Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Aortic valve replacement (AVR) is one of the most commonly performed operations in cardiac surgery. It is not only effective in alleviating symptoms in patients suffering from aortic valve disease, but also improves survival. However, in patients with a small aortic annulus, the benefits of this operation are dependent on the surgeon's ability to avoid patient-prosthesis mismatch (PPM). PPM was first described by Rahimtoola in 1978 as: “Mismatch can be considered to be present when the effective prosthetic valve area, after insertion into the patient, is less than that of a normal human valve.” Pibarot and Dumesnil defined PPM as a prosthetic valve effective orifice area (EOA) indexed to a body surface area of less than 0.85 cm 2 /m 2 . PPM has been shown to be associated with a number of adverse outcomes, including worse hemodynamic performance, reduced left ventricular mass regression, and lower survival. If PPM is anticipated with the type of prosthesis that is being planned, the surgeon can either implant another type of prosthesis with a larger EOA, such as a stentless bioprosthesis, a new-generation mechanical prosthesis, or an aortic homograft, or he or she can surgically enlarge the aortic root to accommodate a larger prosthesis of the same type.
The well-known and documented repercussions regarding PPM have heightened the desire of surgeons to master aortic root enlargement techniques. In this chapter, we will discuss the commonly used aortic root enlargement techniques, including posterior enlarging techniques, such as the Nicks and Manougian procedures, and anterior enlarging strategies such as the Konno-Rastan aortoventriculoplasty and Ross-Konno AVR procedure. These techniques increase the diameter of the aorta with a small annulus and allow the implantation of larger prosthetic valves with better hemodynamic performance. In addition, some other surgical considerations in patients with a small aortic root will also be discussed.
It is important to evaluate adult patients for concomitant coronary artery disease with stress testing and left heart catheterization. The coronary angiogram should be carefully studied for anomalous origin of either main coronary artery, and the location of the first septal branch of the left anterior descending artery should be identified if the pulmonary autograft operation is under consideration.
The echocardiogram should be reviewed for evidence of left ventricular hypertrophy and to assess left ventricular function. Careful preoperative measurement of the aortic annulus can guide intraoperative valve sizing to avoid PPM and to identify patients in whom aortic root enlargement is likely. Concomitant subaortic stenosis should be identified because this can be addressed with myotomy/myectomy if required.
The echocardiogram can also demonstrate concomitant poststenotic dilation of the ascending aorta or a true ascending aortic aneurysm. If the pulmonary autograft operation is under consideration, the pulmonary valve should be interrogated for insufficiency or other abnormalities. It is important to note mitral valve structure and degree of insufficiency on the preoperative echocardiogram because these may be altered with aortic root enlargement.
Adult congenital patients, in particular, who may have a history of associated arch anomalies may require a computed tomography (CT) or magnetic resonance imaging (MRI) scan to ensure that these structures and potential anatomic anomalies are well defined.
A resting electrocardiogram should be reviewed to identify any preoperative conduction abnormalities. Patients should be evaluated for concomitant atrial fibrillation or other rhythm disturbances that can be addressed during the operation.
The aortic root consists of the aortic annulus, aortic cusps, aortic sinuses, and the sinotubular junction. The aortic root represents the outflow tract from the left ventricle. It provides supporting structures for the leaflets of the aortic valve and forms a bridge between the left ventricle and ascending aorta. All aortic root enlargement procedures are described based on an anatomic understanding of the coronary artery ostia, coronary sinuses, and commissures. Special attention should be paid to the relationship of these structures to the conduction system, the mitral valve and its apparatus, and the interventricular septum. In addition, the ability to distinguish the membranous portion of the septum is of critical importance.
The left ventricular outflow tract is best appreciated when viewed directly down into the aortic annulus. The aortic valve and pulmonary valve, although closely related, have different planes. The infundibular portion of the right ventricle elevates the plane of the pulmonary valve and trunk above the aortic valve, placing the pulmonary valve higher and more posterior. The aortic valve shares fibrous continuity with the anterior leaflet of the mitral valve ( Fig. 11.1 ).
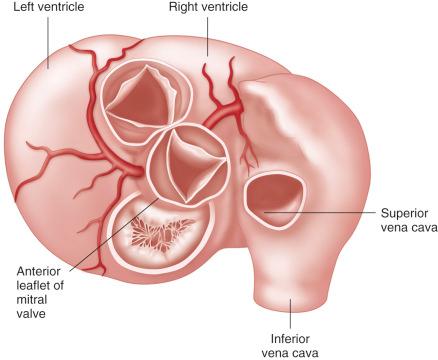
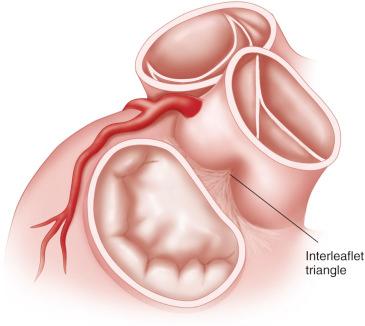
The space between the fibrous attachments of the aortic valve leaflets is termed the interleaflet triangle . These triangles are more flexible than the other segments of the aortic root ( Fig. 11.2 ). The commissure between the right and left coronary cusps is usually located directly across from the pulmonary artery, with a mirror image configuration of the pulmonary valve cusps. The commissure between the right coronary cusp and noncoronary cusp is located anteriorly and is closely related to the interventricular septum and the conduction system. The commissure between the left coronary cusp and noncoronary cusp is located more posterior and to the right. This commissure is located opposite the middle portion of the anterior leaflet of the mitral valve (see Fig. 11.2 ).
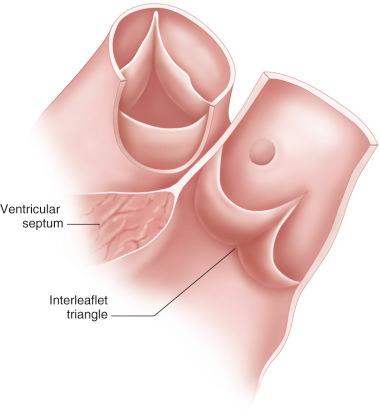
Various portions of the aortic valve are above, at, and below the true aortic annulus. The tops of the commissures rest above the aortic annulus, well into the sinus portion of the aorta. The central portions of the leaflets meet below the aortic annulus during diastole, within the left ventricular outflow tract. The true aortoventricular junction is between these two levels ( Fig. 11.3 ).
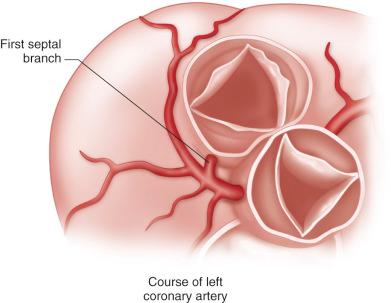
The right coronary artery arises from the right coronary sinus and courses rightward in the atrioventricular groove. The left main coronary artery is short and branches immediately into the left circumflex and left anterior descending arteries. The left circumflex artery courses laterally in the atrioventricular groove, being near the posterior mitral annulus. The left anterior descending artery lies posterior to the pulmonary artery in its proximal portion before coursing down the anterior interventricular groove. The first septal branch of the left anterior descending artery lies directly behind the posterior leaflet of the pulmonary valve ( Fig. 11.4 ).
Of paramount importance, regardless of any proposed technique, is exposure of the left ventricular outflow tract. In many cases a complete division of the ascending aorta, approximately 1 cm above the sinotubular junction, provides excellent exposure to the aortic valve and left ventricular outflow tract. Alternatively, a spiraling-type incision down into the noncoronary sinus may be used if extensive enlargement is not anticipated.
The aortic valve is excised and the annulus is débrided. A careful determination is made as to the minimum size of prosthesis that would be acceptable for the patient's body size. If root enlargement is indicated to achieve an adequately sized prosthesis, the appropriate technique is used.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here